Bioactive Polymeric Materials for Tissue Repair
Abstract
:1. Introduction
1.1. Objectives
2. Results
2.1. Polymeric Bioactive ACP Dental Composites
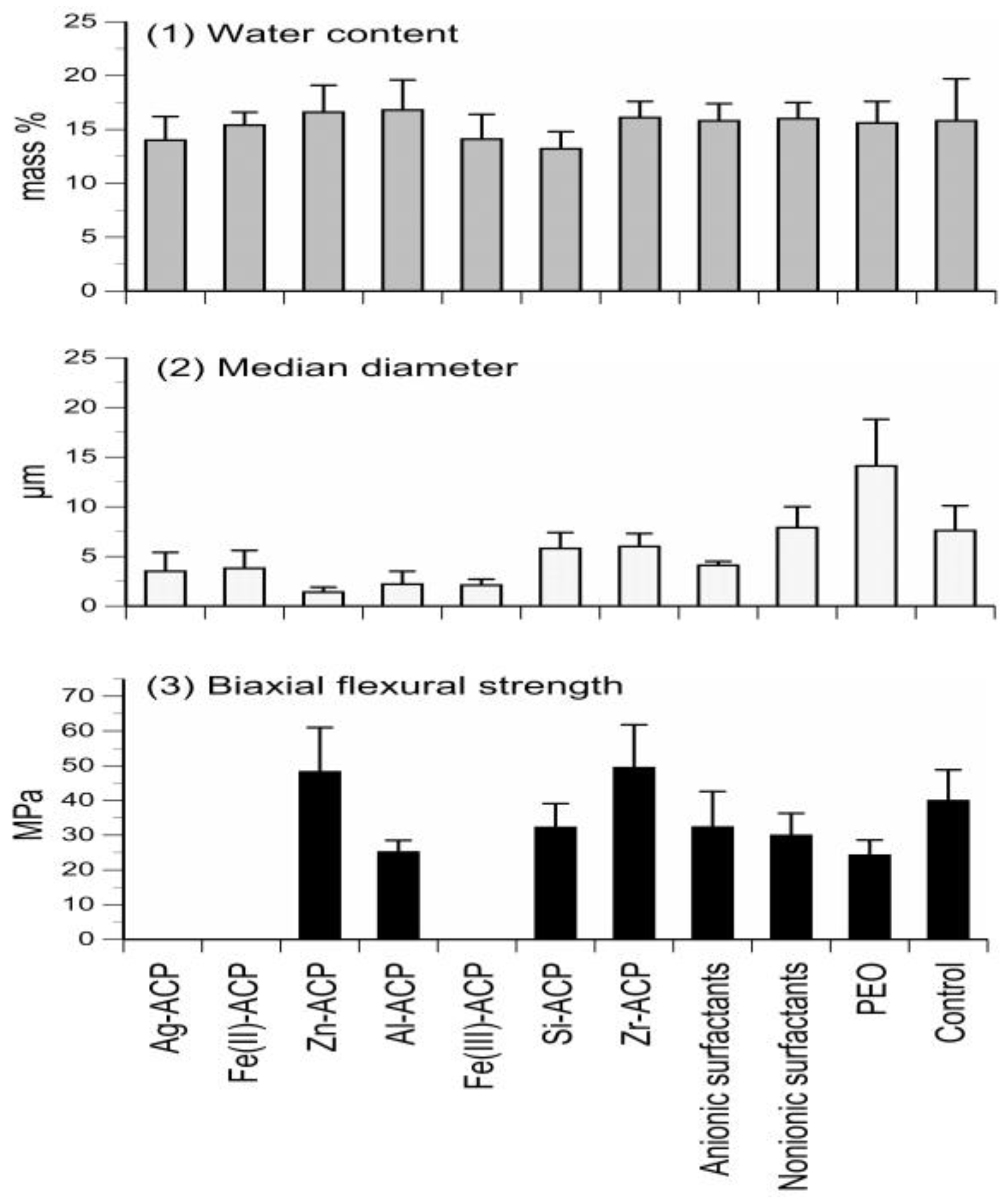
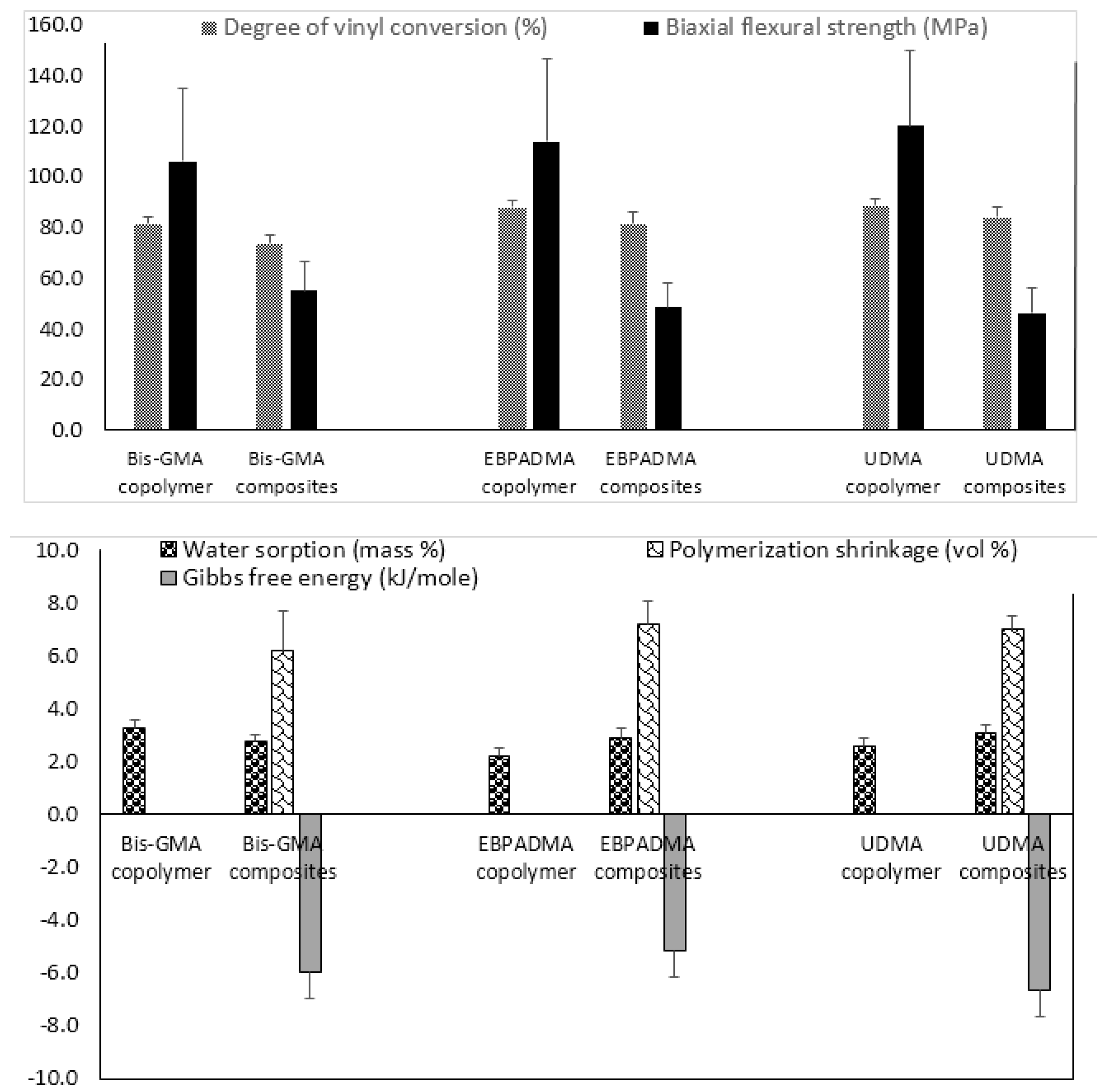
2.1.1. Physicochemical and Mechanical Properties
2.1.2. Extraction of Unreacted Monomers
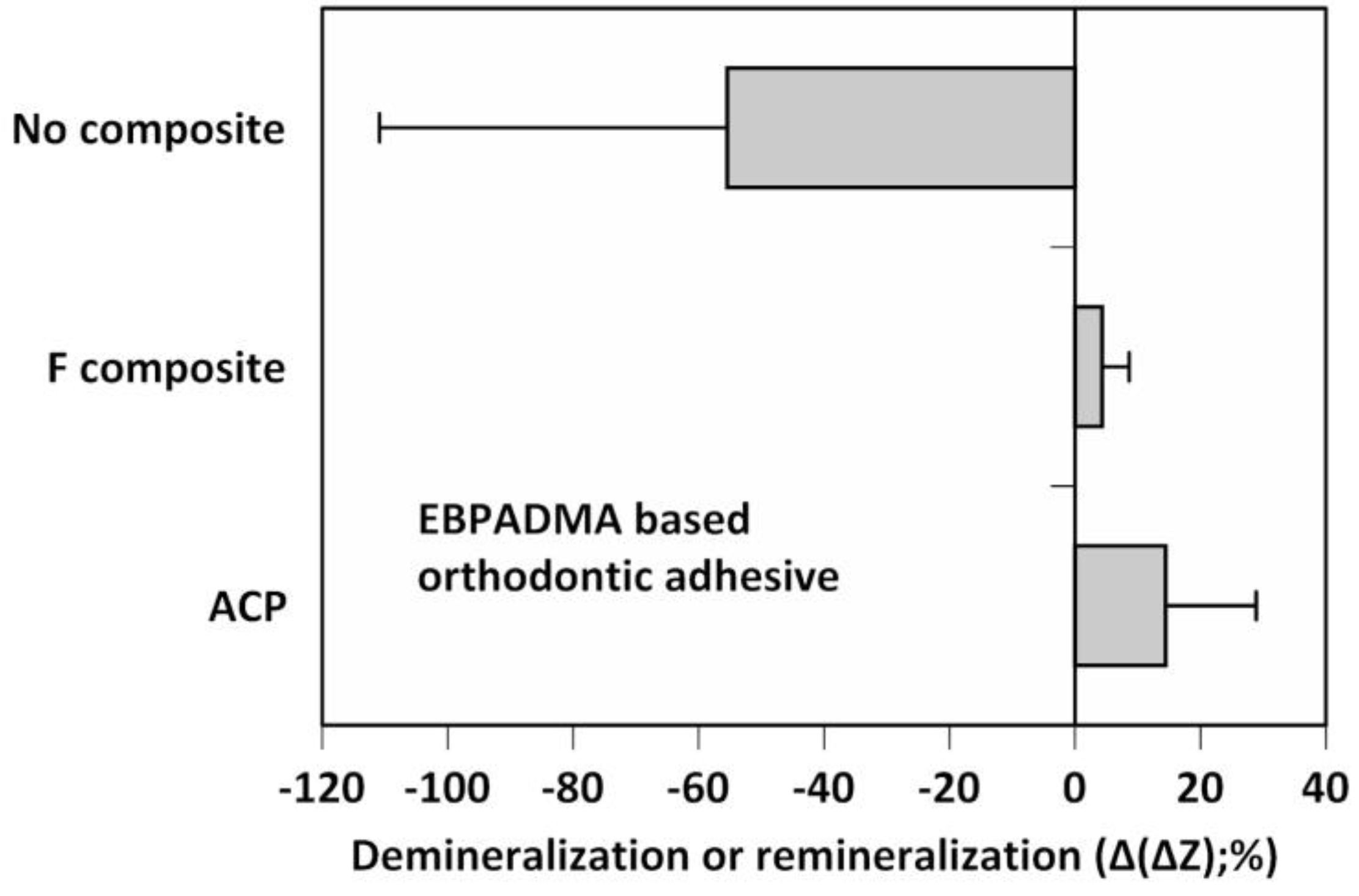
2.1.3. Remineralization Efficacy
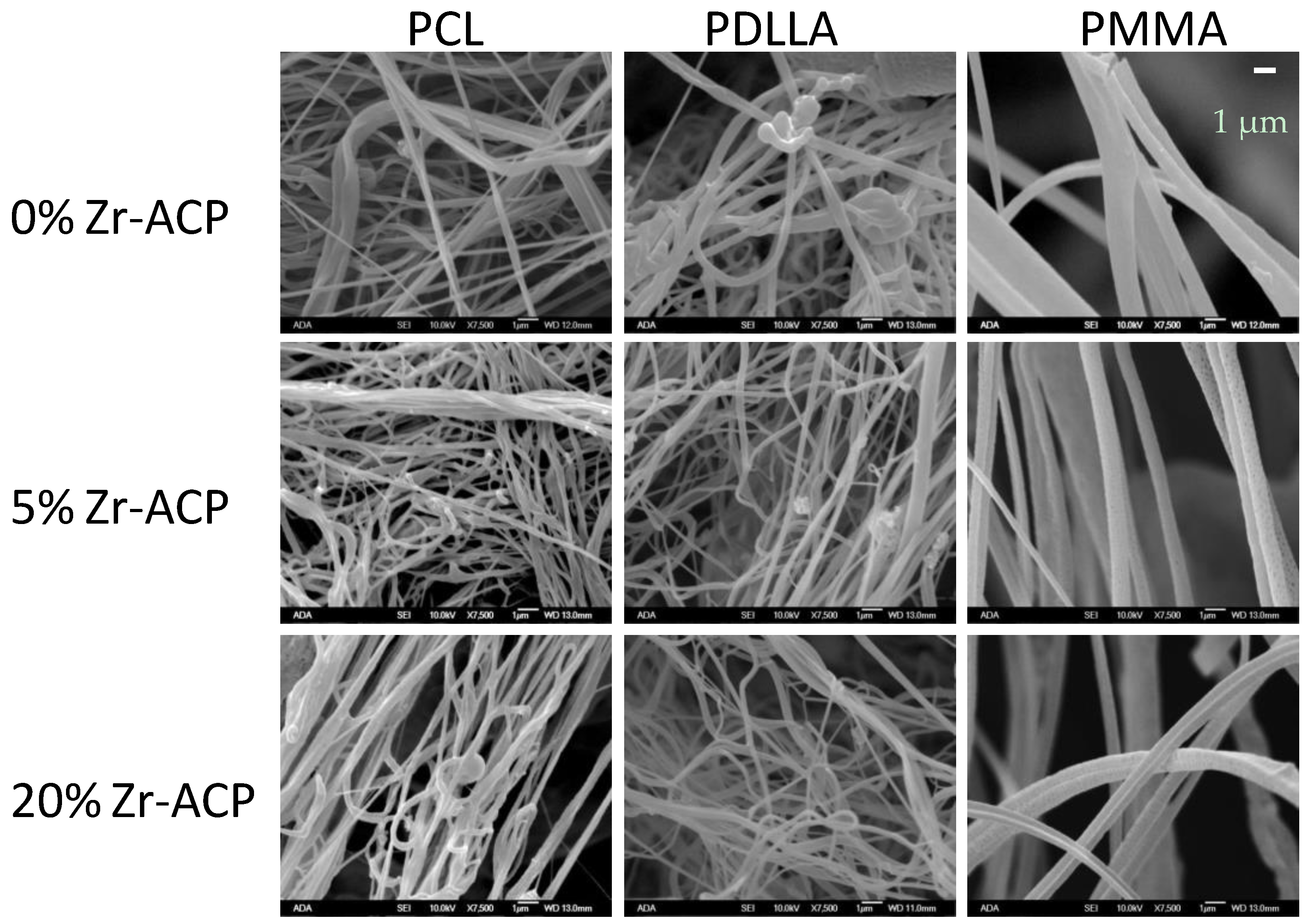
2.2. Airbrushed, ACP Nanofiber Scaffolds
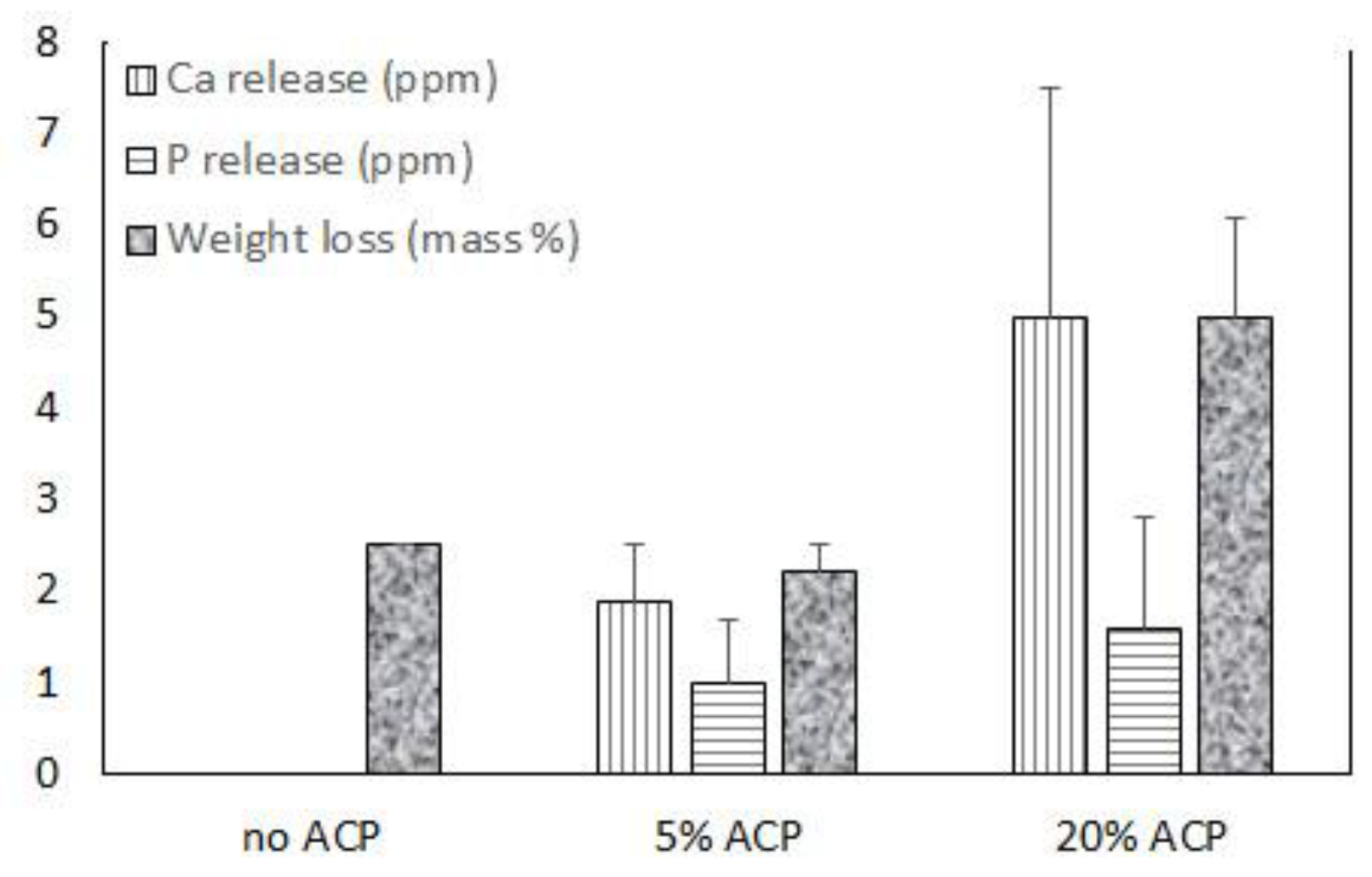
2.2.1. ACP Incorporation and Ion Release from Scaffolds
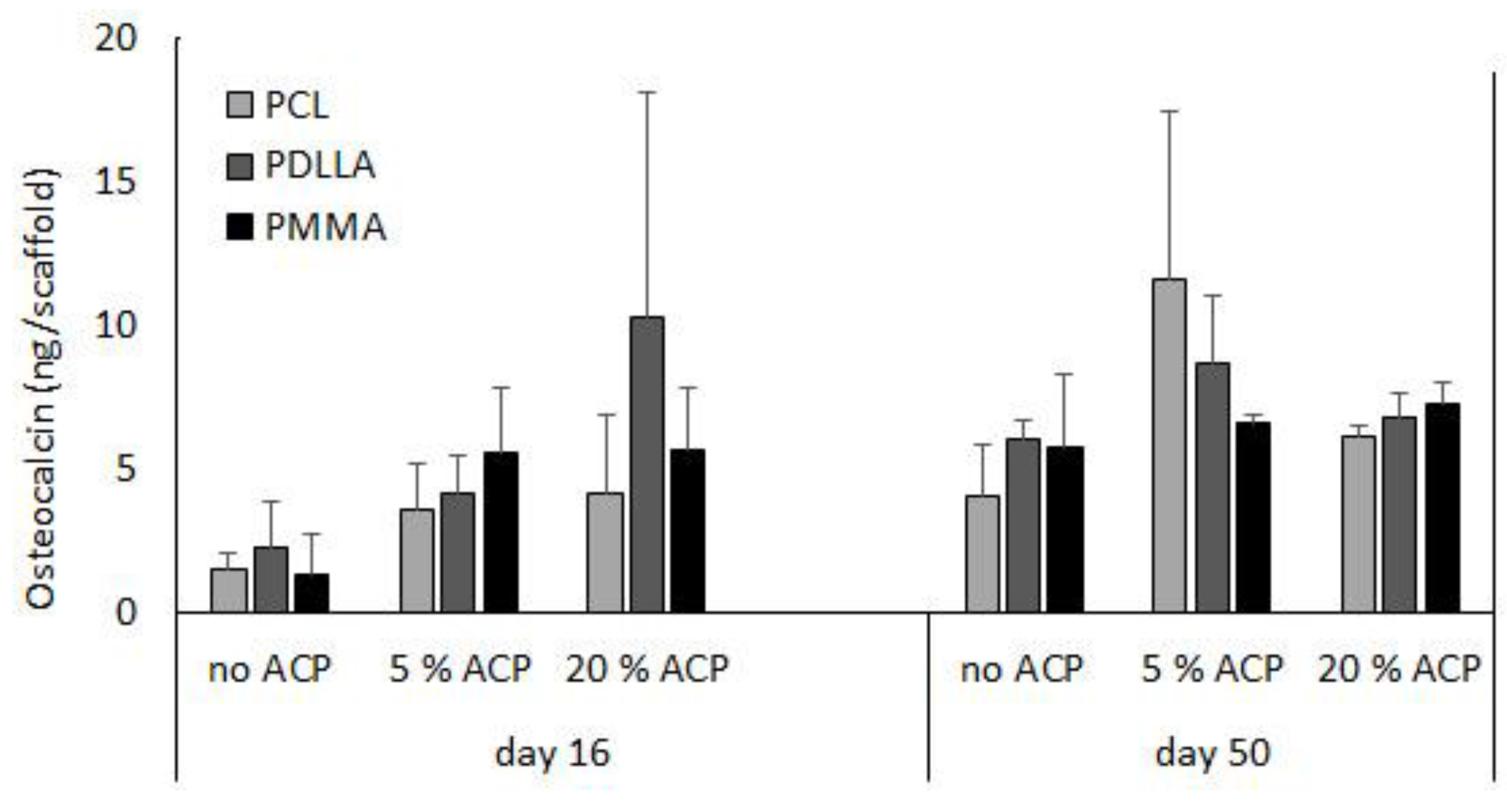
2.2.2. Cellular Responses to ACP Polymeric Nanofibers
3. Discussion
3.1. Polymeric Bioactive ACP Dental Composites
3.2. Airbrushed ACP Nanofibers
3.3. Potential for Precision Medicine
4. Materials and Methods
4.1. ACP Synthesis and Characterization
4.2. Resin Formulation and Evaluation
4.3. Composite Formulation, Physicochemical, and Mechanical Evaluation
4.4. Fabrication and Characterization of Airbrushed ACP Polymer Nanofibers
4.5. Cellular Studies
4.5.1. Double-Stranded DNA
4.5.2. Osteocalcin
Acknowledgments
Disclaimer
Author Contributions
Conflicts of Interest
Appendix A. List of Acronymns
| 1850 Irgacure | bis(2,6-dimethoxybenzoyl)-2,4,4-trimethylpentyl phosphine oxide & 1-hydroxycyclo-hexyl phenyl ketone |
| 369 Irgacure | 2-benzyl-2-(dimethylamino)-1-(4-(4-morphollinyl)phenyl)-1-butanone |
| 4265 Darocur | diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide & 2-hydroxy-2-methyl-1-phenyl-1-propanone |
| ACP | amorphous calcium phosphate |
| Ag-ACP | silver-modified ACP |
| Al-ACP | alumina-modified ACP |
| ANOVA | analysis of variance |
| BFS | biaxial flexural strength |
| Bis-GMA | 2,2-bis[p-(2-hydroxy-3-methacryloxypropoxy)phenyl]propane |
| BPO | benzoyl peroxide |
| Ca | calcium |
| CaP | calcium phosphate |
| CGI | bis(2,6-dimethoxybenzoyl)-2,4,4-trimethylpentyl phosphine oxide & 2-hydroxy-2-methyl-1-phenyl-1-propanone |
| DHEPT | 2,2′-dihydroxyethyl-p-toluidine |
| DVC | degree of vinyl conversion |
| EBPADMA | ethoxylated bisphenol A dimethacrylate |
| EHMA | ethyl-α-hydroxymethyl acrylate |
| 4EDMAB | ethyl-4-N,N-dimethylamino benzoate |
| Fe(II)-ACP | iron (II)-modified ACP |
| Fe(III)-ACP | iron (III)-modified ACP |
| FTIR | Fourier transform infrared spectroscopy |
| ΔG° | Gibbs free energy |
| HAP | hydroxyapatite |
| hBMSC HEMA | human bone marrow stromal cell 2-hydroxyethyl methacrylate |
| HmDMA | hexamethylene dimethacrylate |
| IAP | ion activity product |
| Ksp | thermodynamic solubility product |
| MEP | methacryloyloxyethyl phthalateMEP |
| Mw | molecular mass |
| n | number of specimens (or repetitive experiments) |
| NIR | near infrared spectroscopy |
| NMR | nuclear magnetic resonance |
| PCL | polycaprolactone |
| PDLLA | poly-d,l-lactic acid |
| PEG-U | poly(ethylene glycol) extended urethane dimethacrylate |
| pH | −log (molar concentration of hydrogen ions) |
| PMMA | poly(methyl methacrylate) |
| PO4 | phosphate |
| PS | polymerization shrinkage |
| R | ideal gas constant |
| SD | standard deviation |
| Si-ACP | silica-modified ACP |
| T | absolute temperature |
| TEGDMA | triethylene glycol dimethacrylate |
| UDMA | urethane dimethacrylate |
| UPHM | UDMA/PEG-U/HEMA/MEP resin |
| WS | water sorption |
| Zn-ACP | zinc-modified ACP |
| Zr-ACP | zirconia-modified ACP |
References
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Dorozhkin, S. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Pathways to biomineralization and biodemineralization of calcium phosphates: The thermodynamic and kinetic controls. Dalton Trans. 2009, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Amorphous calcium orthophosphates: Nature, chemistry and biomedical applications. Int. J. Mater. Chem. 2012, 2, 19–46. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Chem. Soc. 1998, 81, 1707–1727. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho) phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Eanes, E.D. Amorphous calcium phosphate: Thermodynamic and kinetic considerations. In Calcium Phosphates in Biological and Industrial Systems; Amjad, Z., Ed.; Kluwer Academic Publ.: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 1998; pp. 21–39. [Google Scholar]
- Costantino, P.D.; Friedman, C.D.; Jones, K.; Chow, L.C.; Sisson, G.A. Experimental hydroxyapatite cement cranioplasty. Plast. Reconstr. Surg. 1992, 90, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ehara, A.; Ogata, K.; Imazato, S.; Ebisu, S.; Nakano, T.; Umakoshi, Y. Effects of alpha-TCP and TetCP on MC3T3-E1 proliferation, differentiation and mineralization. Biomaterials 2003, 24, 831–836. [Google Scholar] [CrossRef]
- Kurashina, K.; Kurita, H.; Hirano, M.; Kotani, A.; Klein, C.P.; de Groot, K. In vivo study of calcium phosphate cements: Implantation of an alpha-tricalcium phosphate/dicalcium phosphate dibasic/tetracalcium phosphate monoxide cement paste. Biomaterials 1997, 18, 539–543. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Schlegel, K.A.; Schultze-Mosgau, S.; Kloss, F.R.; Rupprecht, S.; Kessler, P. Degradation characteristics of alpha and beta tri-calcium-phosphate (TCP) in minipigs. J. Biomed. Mater. Res. 2002, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Keeting, P.E.; Oursler, M.J.; Wiegand, K.E.; Bonde, S.K.; Spelsberg, T.C.; Riggs, B.L. Zeolite a increases proliferation, differentiation, and transforming growth factor beta production in normal adult human osteoblast-like cells in vitro. J. Bone Miner. Res. 1992, 7, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Chattopadhyay, N.; Kifor, O.; Butters, R.R., Jr.; Sugimoto, T.; Brown, E.M. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+O)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J. Bone Miner. Res. 1998, 13, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Beck, G.R., Jr.; Zerler, B.; Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S. Transient precursor strategy in mineral formation of bone. Bone 2006, 39, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Sagi, I.; Addadi, L. Structural biology. Choosing the crystallization path less traveled. Science 2005, 309, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Onuma, K.; Yamamoto, A.; Iijima, M.; Shiba, K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 16866–16870. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Metzler, R.A.; Lam, R.S.; Gilbert, P.U. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonucci, J.M. Bioactive polymeric composites for tooth mineral regeneration: Physicochemical and cellular aspects. J. Funct. Biomater. 2011, 2, 271–307. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.; Skrtic, D.; Sun, J.; Tutak, W. Airbrushed composite polymer Zr-ACP nanofiber scaffolds with improved cell penetration for bone tissue regeneration. Tissue Eng. C Methods 2015, 21, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V. Classes of materials in medicine: Natural materials. In Biomaterials Science—An Introduction to Materials in Medicine; Ratner, B.D., Hoffman, A.S., Shoen, F.J., Lemons, J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 127–136. [Google Scholar]
- Kerschnitzki, M.; Kollmannsberger, P.; Burghammer, M.; Duda, G.N.; Weinkamer, R.; Wagermaier, W.; Fratzl, P. Architecture of the osteocyte network correlates with bone material quality. J. Bone Miner. Res. 2013, 28, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Kerschnitzki, M.; Wagermaier, W.; Roschger, P.; Seto, J.; Shahar, R.; Duda, G.N.; Mundlos, S.; Fratzl, P. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J. Struct. Biol. 2011, 173, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Tison, C.K.; Chatterjee, K.; Pine, P.S.; McDaniel, J.H.; Salit, M.L.; Young, M.F.; Simon, C.G., Jr. The determination of stem cell fate by 3D scaffold structures through the control of cell shape. Biomaterials 2011, 32, 9188–9196. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.M.; Sahota, J.S.; Khan, Y.; Laurencin, C.T. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J. Biomed. Mater. Res. 2001, 58, 295–301. [Google Scholar] [CrossRef]
- Popp, J.R.; Laflin, K.E.; Love, B.J.; Goldstein, A.S. In vitro evaluation of osteoblastic differentiation on amorphous calcium phosphate-decorated poly(lactic-co-glycolic acid) scaffolds. J. Tissue Eng. Regen. Med. 2011, 5, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Young, M.F.; Thomas, V.; Sun, L.; Chow, L.C.; Tison, C.K.; Chatterjee, K.; Miles, W.C.; Simon, C.G., Jr. Nanofiber scaffold gradients for interfacial tissue engineering. J. Biomater. Appl. 2013, 27, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J. Res. Natl. Inst. Stand. Technol. 2003, 108, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.R.; Hoffman, K.M.; Tutak, W. Blow-spun chitosan/PEG/PLGA nanofibers as a novel tissue engineering scaffold with antibacterial properties. J. Mater. Sci. Mater. Med. 2016, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Tutak, W.; Gelven, G.; Markle, C.; Palmer, X.-L. Rapid polymer fiber airbrushing: Impact of a device design on the fiber fabrication and matrix quality. J. Appl. Polym. Sci. 2015, 132, 42813. [Google Scholar] [CrossRef]
- Tutak, W.; Sarkar, S.; Lin-Gibson, S.; Farooque, T.M.; Jyotsnendu, G.; Wang, D.; Kohn, J.; Bolikal, D.; Simon, C.G., Jr. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials 2013, 34, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, S.E.; O’Donnell, J.N.; Skrtic, D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent. Mater. 2009, 25, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonucci, J. Design, Characterization and Evaluation of Biomimetic Polymeric Dental Composites with Remineralization Potential; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 281–318. [Google Scholar]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D.; Eidelman, N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials 2004, 25, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Dickens, S.H. Network formation and compositional drift during photo-initiated copolymerization of dimethacrylate monomers. Polymer 2001, 42, 6363–6369. [Google Scholar] [CrossRef]
- Antonucci, J.; Liu, D.; Stansbury, J. Synthesis of hydrophobic oligomeric monomers for dental applications. J. Dent. Res. 1993, 72, 369. [Google Scholar]
- Antonucci, J.M.; Stansbury, J.W. Molecular designed dental polymer. In Desk Reference of Functional Polymers: Synthesis and Application; Arshady, R., Ed.; American Chemical Society Publication: Washington, DC, USA, 1997; pp. 719–738. [Google Scholar]
- Price, R.; Rizkalla, A.; Hall, G. Effect of stepped light exposure on the volumetric polymerization shrinkage and bulk modulus of dental composites and an unfilled resin. Am. J. Dent. 2000, 13, 176–180. [Google Scholar] [PubMed]
- Guggenberger, R.; Weinmann, W. Exploring beyond methacrylates. Am. J. Dent. 2000, 13, 82D–84D. [Google Scholar] [PubMed]
- Tilbrook, D.; Clarke, R.; Howle, N.; Braden, M. Photocurable epoxy–polyol matrices for use in dental composites I. Biomaterials 2000, 21, 1743–1753. [Google Scholar] [CrossRef]
- Pelka, M.; Distler, W.; Petschelt, A. Elution parameters and HPLC-detection of single components from resin composite. Clin. Oral Investig. 1999, 3, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Spahl, W.; Budzikiewicz, H.; Geurtsen, W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J. Dent. 1998, 26, 137–145. [Google Scholar] [CrossRef]
- Schweikl, H.; Schmalz, G.; Spruss, T. The induction of micronuclei in vitro by unpolymerized resin monomers. J. Dent. Res. 2001, 80, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.J.; Dickens, S.H. Network structure of Bis-GMA-and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Horikawa, D.; Nishida, M.; Ebisu, S. Effects of monomers eluted from dental resin restoratives on osteoblast-like cells. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Nomura, Y.; Tanaka, N.; Teshima, W.; Okazaki, M.; Shintani, H. Leachability of plasticizer and residual monomer from commercial temporary restorative resins. J. Dent. 2004, 32, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Walther, U.I.; Zaspel, J.; Hickel, R.; Reichl, F.-X. Metabolism of tegdma and hema in human cells. Biomaterials 2010, 31, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly [(lactic acid)-co-(glycolic acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Calcagni, M.; Welti, M.; Hemmi, S.; Hild, N.; Stark, W.J.; Burgisser, G.M.; Wanner, G.A.; Cinelli, P.; Buschmann, J. Proliferation of ASC-derived endothelial cells in a 3D electrospun mesh: Impact of bone-biomimetic nanocomposite and co-culture with ASC-derived osteoblasts. Injury 2014, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Gutierrez, M.C.; Serrano, M.C.; Dentini, M.; Barbetta, A.; Ferrer, M.L.; del Monte, F. In situ precipitation of amorphous calcium phosphate and ciprofloxacin crystals during the formation of chitosan hydrogels and its application for drug delivery purposes. Langmuir 2012, 28, 15937–15946. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, D.; Yan, W.; Shi, Z.; Feng, J.; Gao, Y.; Weng, W.; Yan, S. Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(l-lactic acid) hybrid materials. Biomaterials 2007, 28, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, W.; Chen, F.; Qi, C.; Lu, B.Q.; Zhang, H.; Wu, J.; Qian, Q.R.; Zhu, Y.J. Amorphous calcium phosphate nanospheres/polylactide composite coated tantalum scaffold: Facile preparation, fast biomineralization and subchondral bone defect repair application. Colloids Surf. B Biointerfaces 2014, 123, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Araki, H.; Nakamura, M.; Shimizu, Y.; Shubhra, Q.T.; Ito, A.; Tsurushima, H. Controlled superficial assembly of DNA-amorphous calcium phosphate nanocomposite spheres for surface-mediated gene delivery. Colloids Surf. B Biointerfaces 2016, 141, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Weng, W.; Cheng, K.; Du, P.; Shen, G.; Han, G.; Guan, B.; Yan, W. Preparation, characterization and cytocompatibility of porous ACP/PLLA composites. J. Biomed. Mater. Res. A 2006, 79, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Rwei, A.Y.; Wang, W.; Kohane, D.S. Photoresponsive nanoparticles for drug delivery. Nano Today 2015, 10, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Timko, B.P.; Kohane, D.S. Materials to clinical devices: Technologies for remotely triggered drug delivery. Clin. Ther. 2012, 34, S25–S35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Bomba, H.N.; Zhu, Y.; Gu, Z. Mechanical force-triggered drug delivery. Chem. Rev. 2016, 116, 12536–12563. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Liu, D.W.; Skrtic, D. Amorphous calcium phosphate based composites: Effect of surfactants and poly(ethylene oxide) on filler and composite properties. J. Dispers. Sci. Technol. 2007, 28, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Skrtic, D. Physicochemical and mechanical evaluation of cation-modified ACP acrylic resin composites. Polym. Prepr. 2006, 47, 113–114. [Google Scholar]
- Antonucci, J.M.; Skrtic, D. Bioactive and biocompatible polymeric composites based on amorphous calcium phosphate. In Integrated Biomaterials for Medical Applications; Ramalingam, M., Tiwari, A., Ramakrishna, S., Kobayashi, H., Eds.; Scrivener Publishing: Salem, MA, USA, 2012; pp. 67–119. [Google Scholar]
- Lee, S.Y.; Regnault, W.F.; Antonucci, J.M.; Skrtic, D. Effect of particle size of an amorphous calcium phosphate filler on the mechanical strength and ion release of polymeric composites. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 11–17. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials. Standard Test Method for Biaxial Strength (Modulus of Rupture) of Ceramic Substrates; ASTM-F394-78; American Society for Testing and Materials: West Conshochoken, PA, USA, 1996. [Google Scholar]
- Davis, C.; O’Donnell, J.; Sun, J.; Skrtic, D. Fabrication and Evaluation of Bioactive Dental Composites Based on Amorphous Calcium Phosphate; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 55–100. [Google Scholar]




| Constituent Monomer and/or Photoinitiator (mass %) | Bis-GMA Resins | EBPADMA Resins | UDMA Resins |
|---|---|---|---|
| Bis-GMA | 36.5–68.4 | - | - |
| EBPADMA | - | 16.8–49.6 | - |
| UDMA | - | - | 34.8–92.4 |
| TEGDMA | 0–49.5 | 0–67.2 | 0–50.2 |
| HEMA | 0–29.2 | 0–30.4 | 0–29.5 |
| HmDMA | 0–46.6 | 0–49.3 | 0–47.3 |
| EHMA | 24.0–34.0 | - | - |
| PEG-U | - | - | 0–29.1 |
| MEP | - | 0–5.0 | 0–2.9 |
| Zr-DMA | 0–1.0 | - | - |
| BPO | - | - | 0–2.0 |
| Camphorquinone | 0–0.2 | 0.2 | 0.2 |
| CGI | 0–3.0 | - | - |
| 4265 DAROCUR | 0–0.8 | 0–0.8 | 0–0.8 |
| DHEPT | - | - | 0–1.0 |
| 4EDMAB | 0–0.8 | 0–0.8 | 0–0.8 |
| 1850 IRGACURE | 0–1.0 | 0–1.0 | 0–1.0 |
| 369 IRGACURE | 0–1.5 | 0–1.5 | 0–1.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bienek, D.R.; Tutak, W.; Skrtic, D. Bioactive Polymeric Materials for Tissue Repair. J. Funct. Biomater. 2017, 8, 4. https://doi.org/10.3390/jfb8010004
Bienek DR, Tutak W, Skrtic D. Bioactive Polymeric Materials for Tissue Repair. Journal of Functional Biomaterials. 2017; 8(1):4. https://doi.org/10.3390/jfb8010004
Chicago/Turabian StyleBienek, Diane R., Wojtek Tutak, and Drago Skrtic. 2017. "Bioactive Polymeric Materials for Tissue Repair" Journal of Functional Biomaterials 8, no. 1: 4. https://doi.org/10.3390/jfb8010004





