Synchrotron-Based in Situ Characterization of the Scaffold Mass Loss from Erosion Degradation
Abstract
:1. Introduction
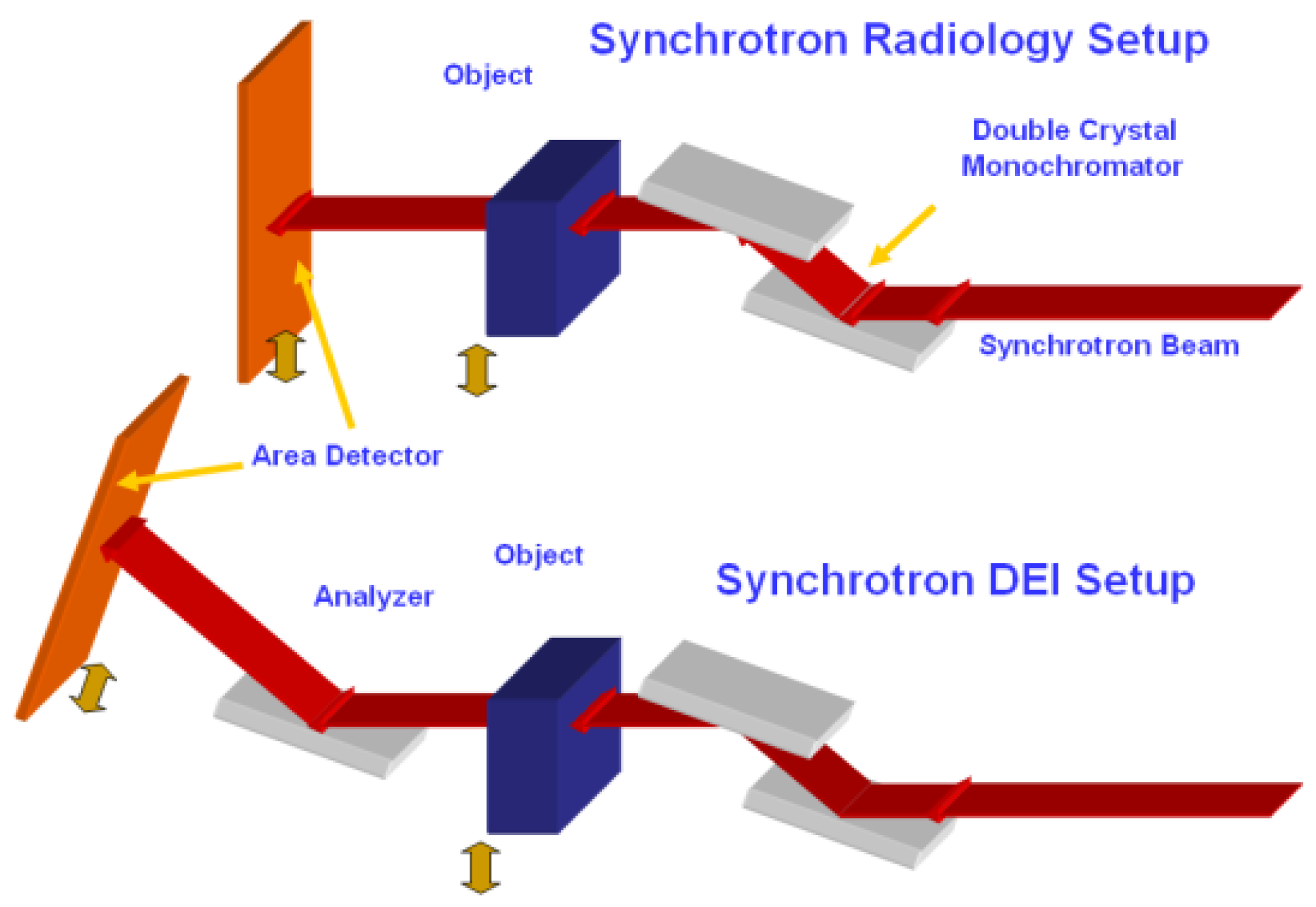
2. Experiments and Methods
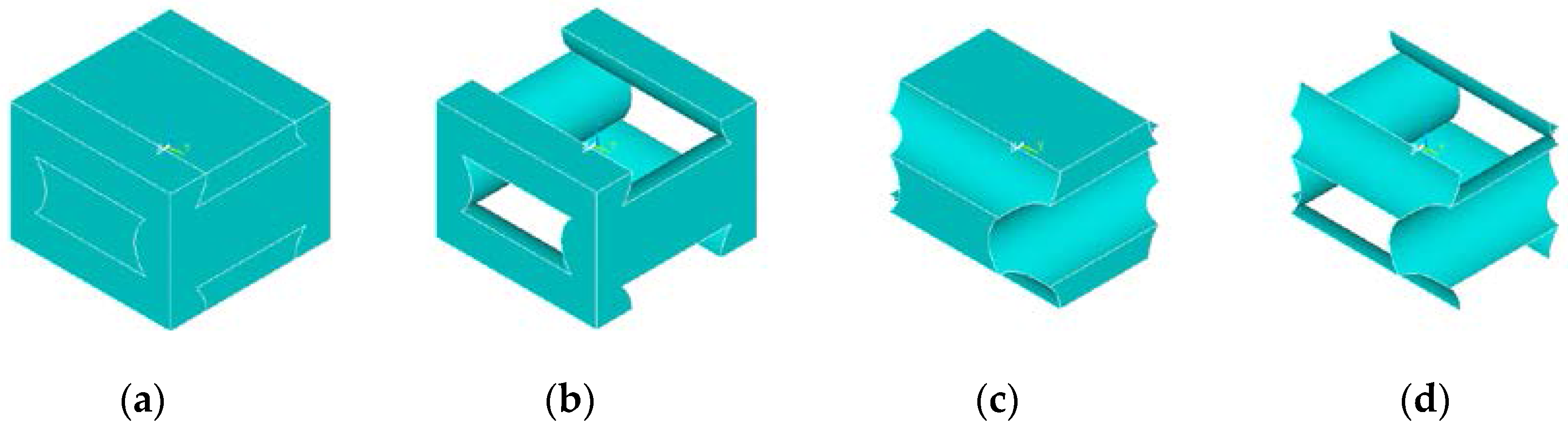
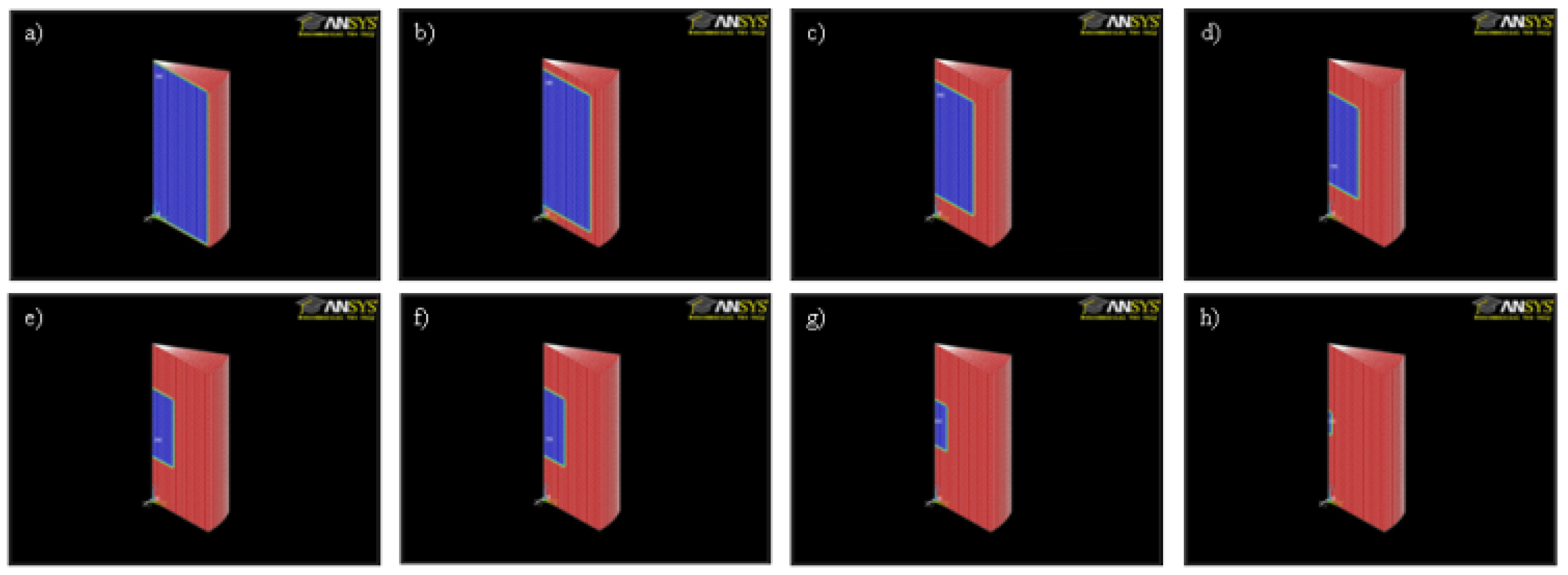
3. Modelling
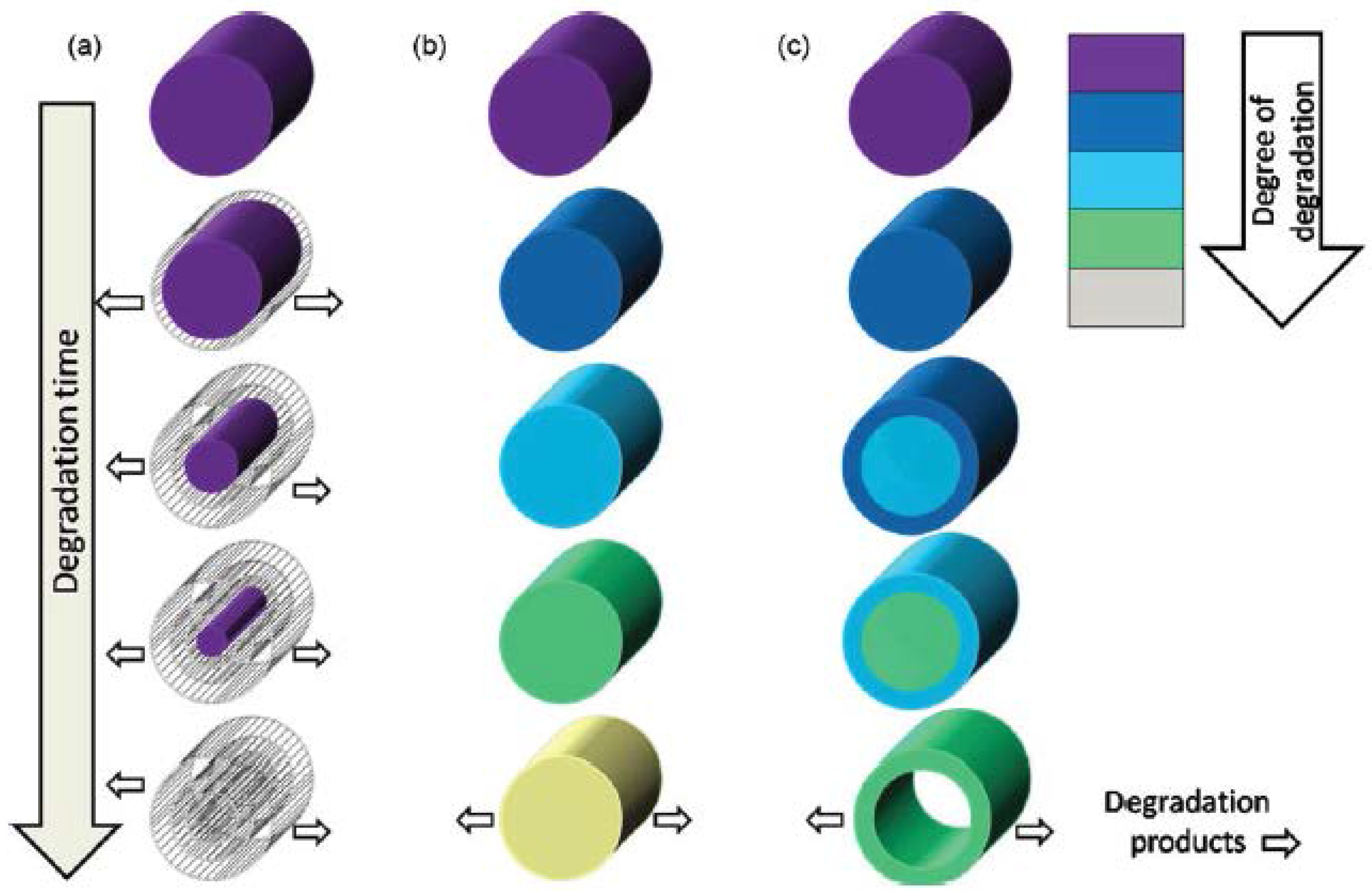
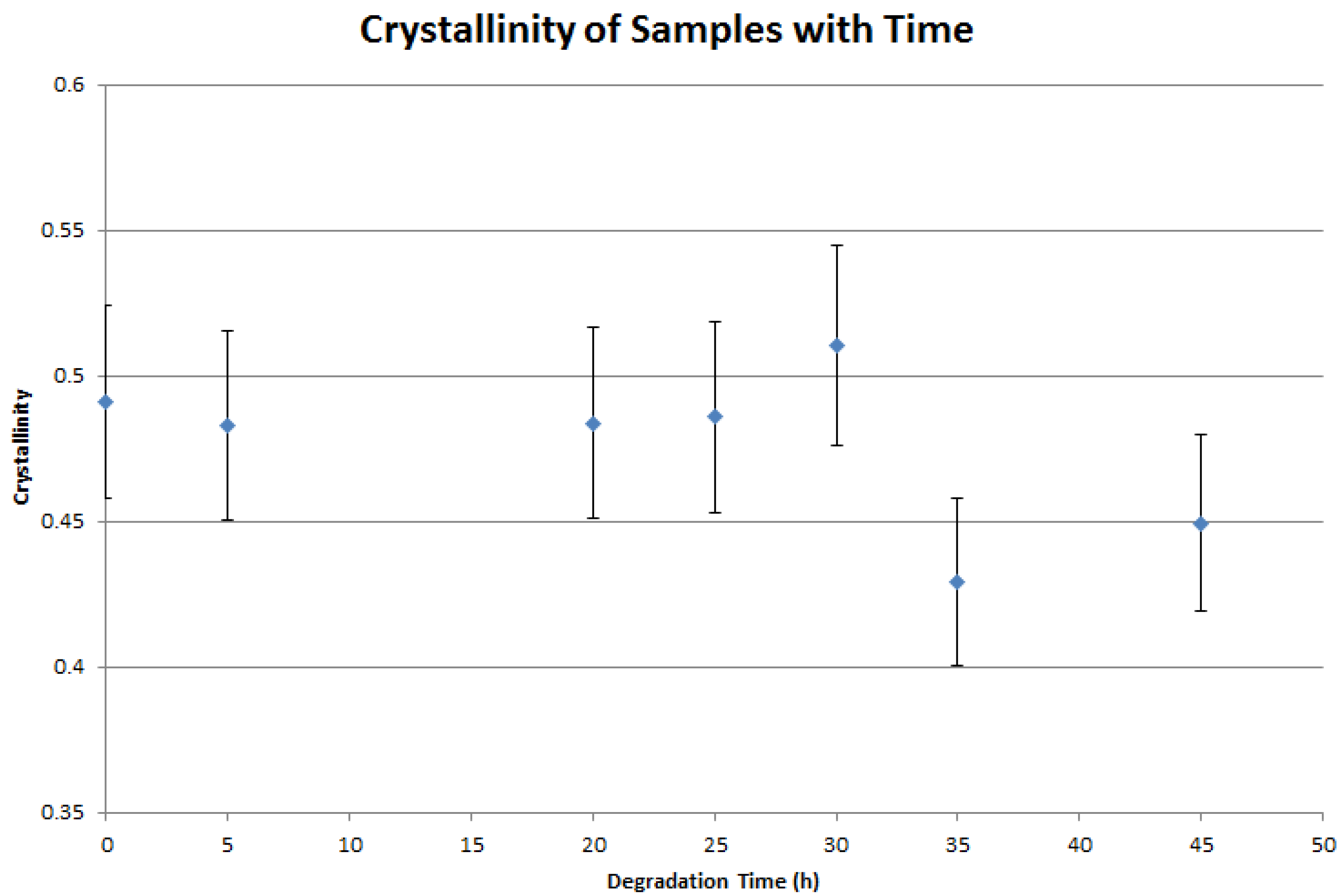
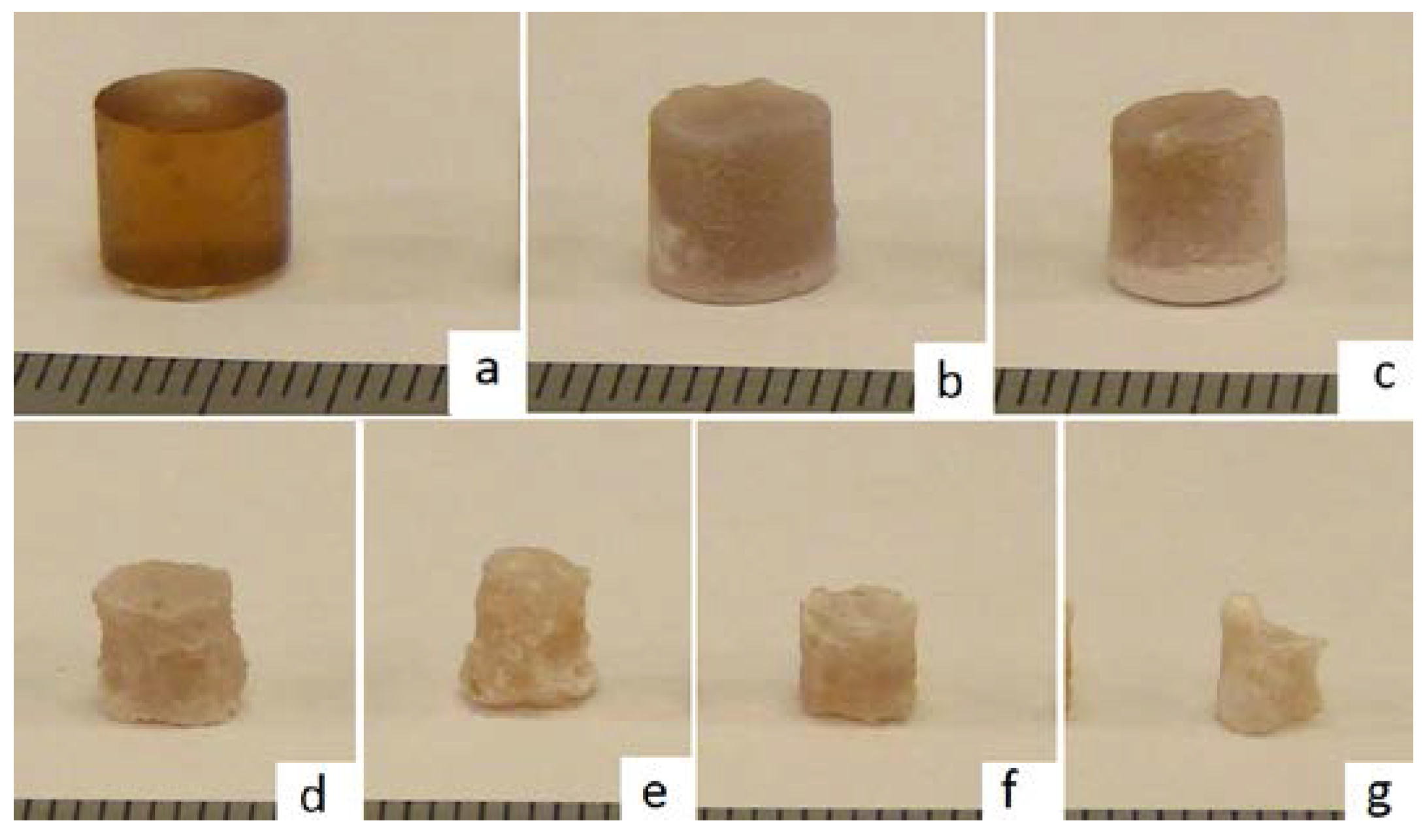
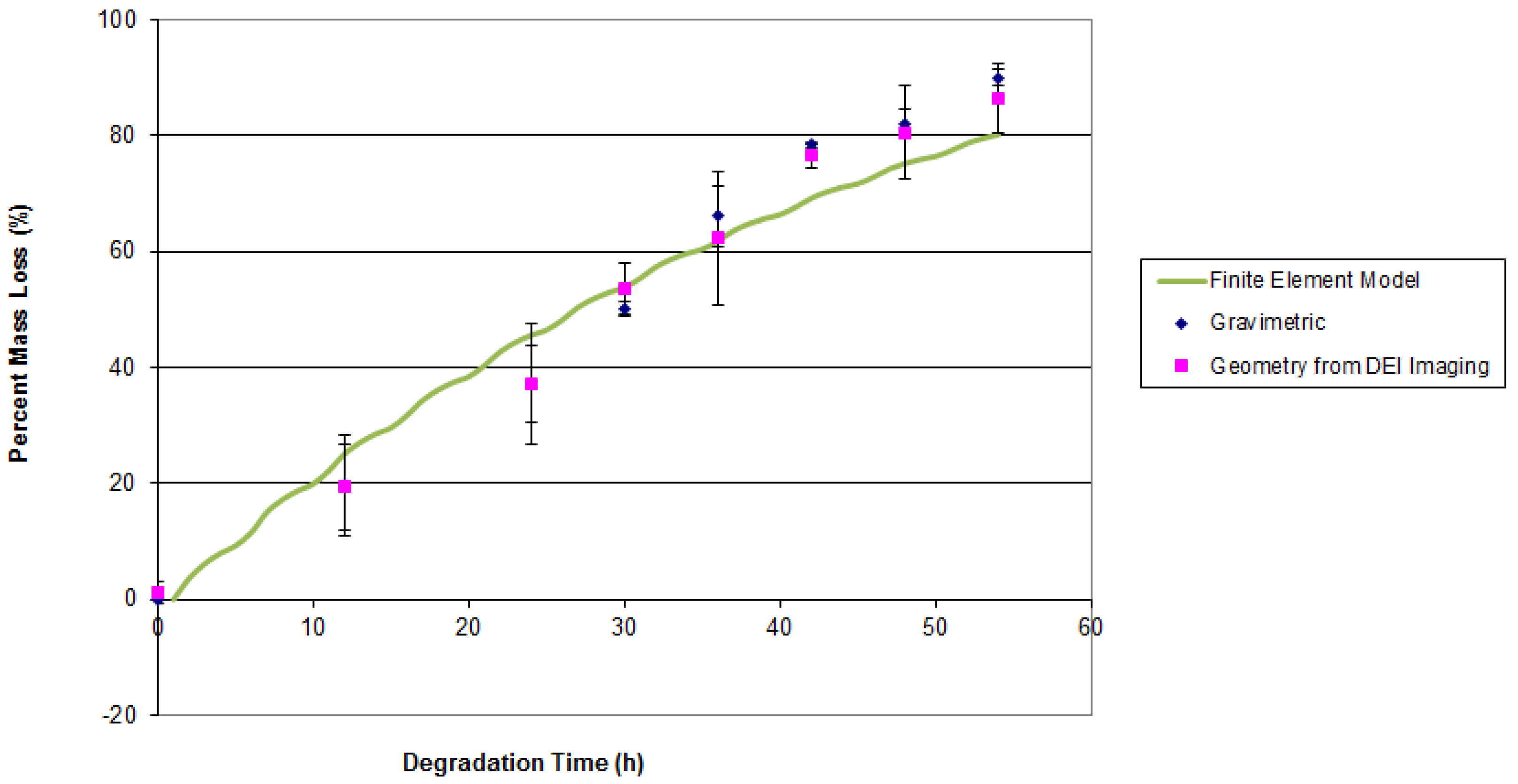
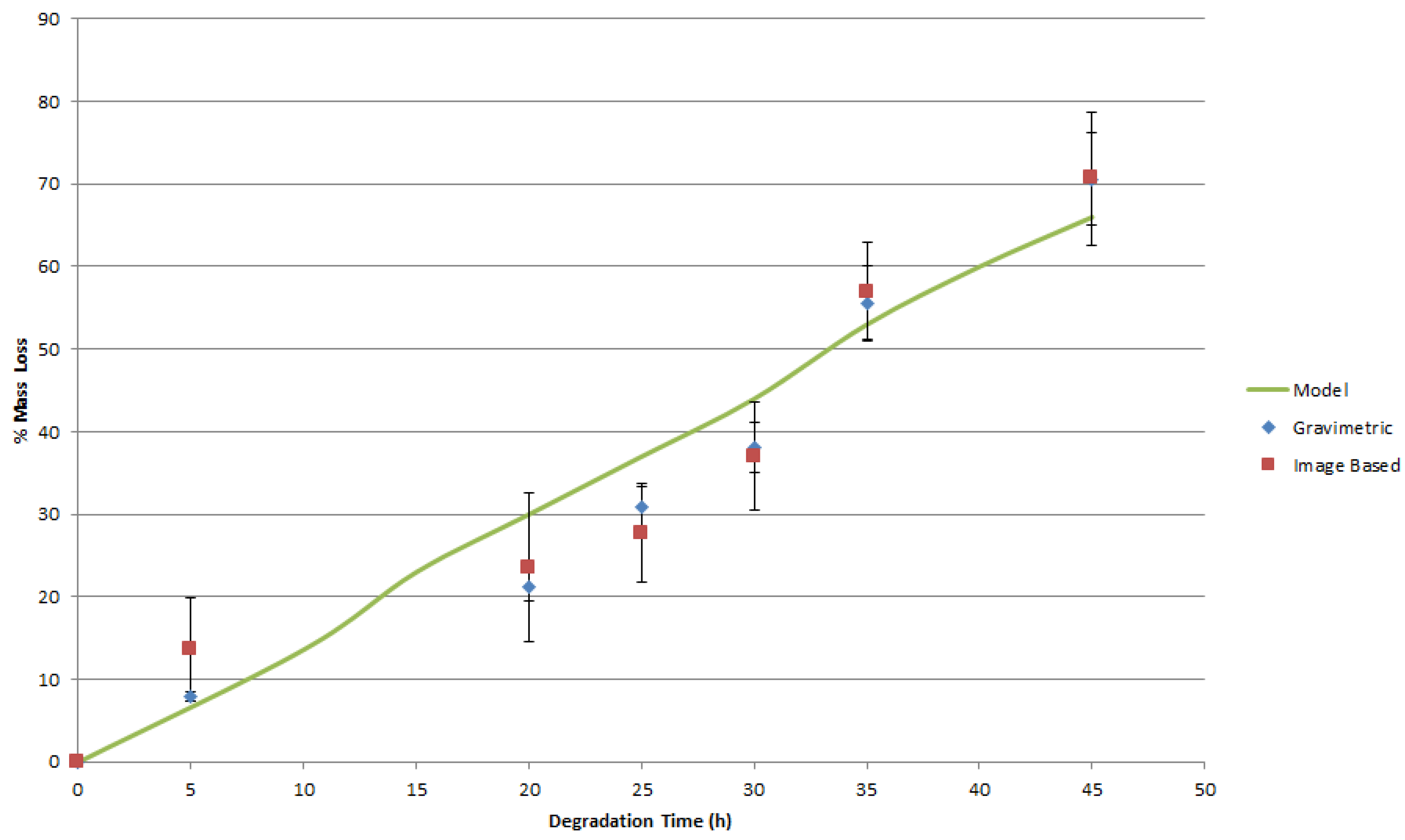
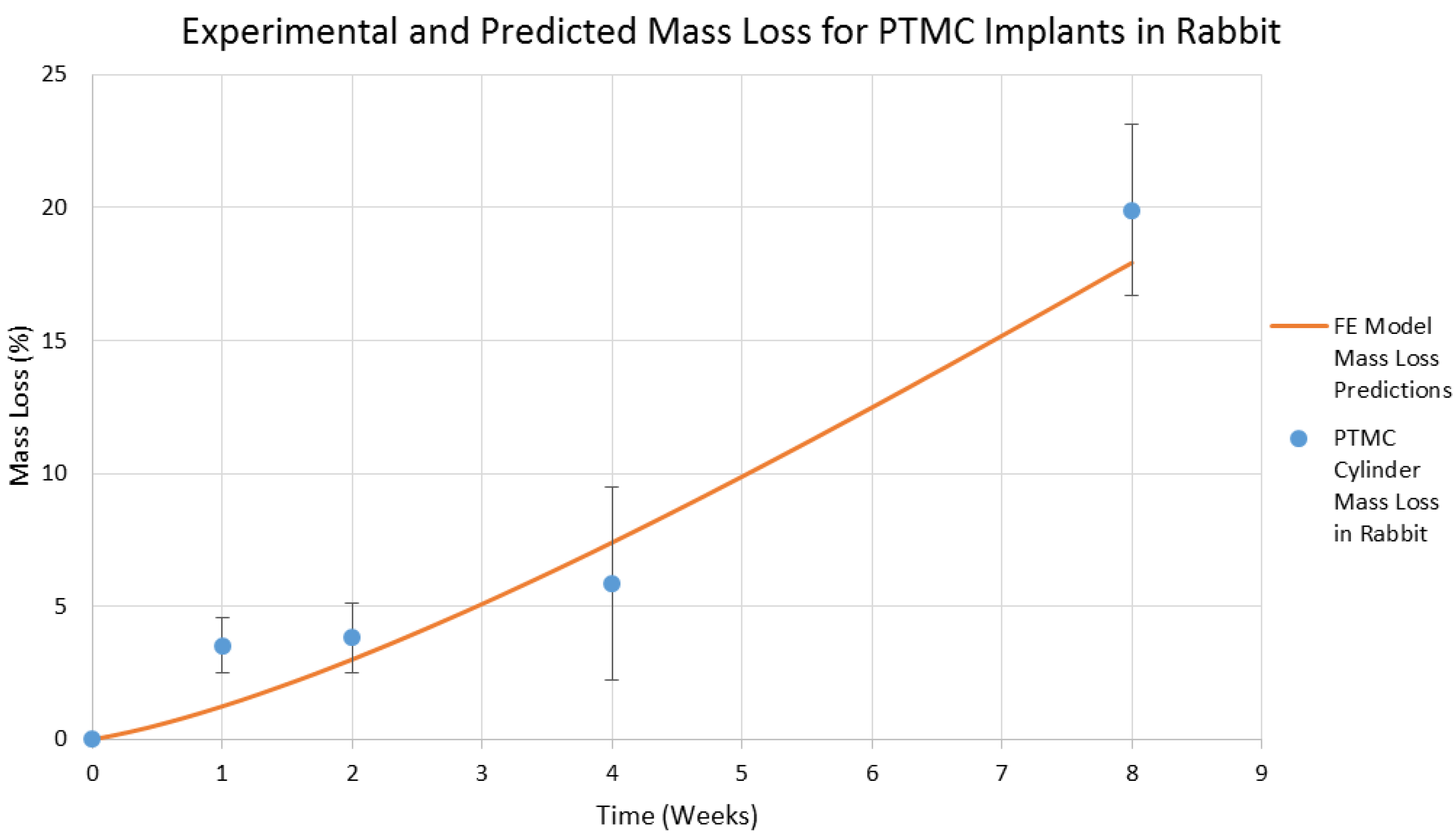
4. Results and Discussion
5. Conclusions and Future Work
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Little, C.J.; Bawolin, N.K.; Chen, X.B. Mechanical Properties of Natural Cartilage and Tissue Engineered Constructs. Tissue Eng. Part B 2011, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Zhang, W.J.; Chen, X.B. A Brief Review of the Modelling of the Time Dependent Mechanical Properties of Tissue Engineering Scaffolds. J. Biomimetics Biomater. Tissue Eng. 2010, 6, 19–33. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer: Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Han, Y.; Feng, A. Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Mater. Sci. Eng. C 2008, 28, 1462–1466. [Google Scholar] [CrossRef]
- Ibim, S.E.; Uhrich, K.E.; Attawia, M.; Shastri, V.R.; El-Amin, S.F.; Bronson, R.; Langer, R.; Laurencin, C.T. Preliminary in vivo report on the osteocompatibility of poly(anhydride-co-imides) evaluated in a tibial model. J. Biomed. Mater. Res. 1998, 43, 374–379. [Google Scholar] [CrossRef]
- Ibim, S.E.; Uhrich, K.E.; Bronson, R.; El-Amin, S.F.; Langer, R.S.; Laurencin, C.T. Poly(anhydride-co-imides): In vivo biocompatibility in a rat model. Biomaterials 1998, 19, 941–951. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Hutmacher, D.W.; Schantz, J.T.; Woodruff, M.A.; Teoh, S.H. Evaluation of polycaprolactone scaffold for 6 months in vitro and in vivo. J. Biomed. Mater. Res. Part A 2009, 90, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Holy, C.E.; Dang, S.M.; Davies, J.E.; Shoichet, M.S. In vitro degradation of a novel poly(lactide-co-glycolide) 75/25 foam. Biomaterials 1999, 20, 1177–1185. [Google Scholar] [CrossRef]
- Gough, J.E.; Christian, P.; Unsworth, J.; Evans, M.P.; Scotchford, C.A.; Jones, I.A. Controlled degradation and macrophage responses of a fluoride-treated polycaprolactone. J. Biomed. Mater. Res. A 2004, 69, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Busini, V.; Perale, G.; Moscatelli, D.; Masi, M. A new model of resorbable device degradation and drug release—Part I: Zero order model. Polym. Int. 2008, 57, 912–920. [Google Scholar] [CrossRef]
- Pena, J.; Corrales, T.; Izquierdo-Barba, I.; Doadrio, A.L.; Vallet-Regi, M. Long term degradation of poly(3-caprolactone) films in biologically related fluids. Polym. Degrad. Stab. 2006, 91, 1424–1432. [Google Scholar] [CrossRef]
- Pena, J.; Corrales, T.; Izquierdo-Barba, I.; Concepcion Serrano, M.; Teresa Portoles, M.; Pagani, R.; Vallet-Regi, M. Alkaline-treated poly(ɛ-caprolactone) films: Degradation in the presence or absence of fibroblasts. J. Biomed. Mater. Res. Part A 2006, 76, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Yiu, C.K.Y.; Yau, J.Y.Y.; Yiu-fai, M.; Loushine, R.J.; Norman Weller, R.; Frank Kimbrough, W.; King, N.M. Susceptibility of a Polycaprolactone-Based Root Canal Filling Material to Degradation. II. Gravimetric Evaluation of Enzymatic Hydrolysis. J. Endod. 2005, 31, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Marini, R.P.; Schaefer, D.; Aronson, J.; Langer, R.; Prasad Shastri, V. In vivo engineering of organs: The bone bioreactor. PNAS 2005, 102, 11450–11455. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surleas, J.; Schindler, A. Aliphatic polyesters II: The degradation of poly (DL-lactide), poly (ε-caprolactone) and their copolymers in vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Doyle, C.; Tanner, E.T.; Bonfiel, W. In vitro and in vivo evaluation of poly-hydroxy-butyrate and of poly-hydroxy-butyrate reinforced with hydroxy-apatite. Biomaterials 1991, 12, 841–847. [Google Scholar] [CrossRef]
- Pistner, H.; Stallforth, H.; Gutwald, R.; Muhling, J.; Reuther, J.; Michel, C. Poly(L-lactide): A long-term degradation study in vivo Part II: Physico-mechanical behavior of implants. Biomaterials 1994, 15, 439–450. [Google Scholar] [CrossRef]
- Ho, S.T.; Hutmacher, D.W. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 2006, 27, 1362–1376. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, L.; Lesci, I.G.; Mezini, O.; Parrilli, A.; Ragazzini, S.; Rinnovati, R.; Romagnoli, N.; Roveri, N.; Scotti, R. Customized hybrid biomimetic hydroxyapatite scaffold for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.; Chub, P.K.; Leung, F.K.L.; Cheung, K.M.C.; Luka, K.D.K.; Yeung, K.W.K. Engineered polycaprolactone–magnesium hybrid biodegradable porous scaffold for bone tissue engineering. Prog. Nat. Sci. Mater. Int. 2014, 24, 561–567. [Google Scholar] [CrossRef]
- Klodowski, K.; Kaminski, J.; Nowickab, K.; Tarasiuk, J.; Wronski, S.; Swietek, M.; Blazewiczb, M.; Figiel, H.; Tureka, K.; Szponder, T. Micro-imaging of implanted scaffolds using combined MRI and micro-CT. Comput. Med. Imaging Graphics 2014, 38, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.W.; Lindsey, D.P.; Beaupre, G.S. Deriving tissue density and elastic modulus from microCT bone scans. Bone 2011, 49, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.; Dolovich, A.T.; Zheng, W.J.; Chen, X.B. Characterization of Mechanical Properties of Tissue Scaffolds by Phase Contrast Imaging and Finite Element Modeling. ASME J. Biomech. Eng. 2015, 137, 081004–081018. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Honaramooz, A.; Wiebe, S.; Belev, G.; Chen, X.B.; Chapman, D. Low-Dose Phase-based X-ray Imaging Techniques for in situ Soft Tissue Engineering Assessments. Biomaterials 2015, 82, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chapman, L.D.; Chen, X.B. Computer Tomography Diffraction Enhanced Imaging for in situ Visualization of Tissue Scaffolds Implanted in Cartilage. Tissue Eng. Part C 2013, 20, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Olubamiji, A.D.; Izadifar, Z.; Zhu, N.; Chang, T.J.; Chen, X.B.; Eames, B.F. Using SR-inline-PCI-CT to visualize 3D-printed hybrid constructs for cartilage tissue engineering. J. Synchrotron Radiat. 2016, 23, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Chapman, D.; Cooper, D.; Schreyer, D.; Chen, X.B. X-ray Diffraction Enhanced Imaging as a Novel Method to Visualize Low Density Scaffolds in Soft Tissue Engineering. Tissue Eng. Part C 2011, 17, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Olubamiji, A.; Izadifar, Z.; Chen, X.B. Synchrotron imaging techniques for bone and cartilage tissue engineering: Potentials, current trends, and future directions. Tissue Eng. Part C 2014, 20, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.; Thomlinson, W.; Johnston, R.E.; Washburn, D.; Pisano, E.; Gmür, N.; Zhong, Z.; Menk, R.; Arfelli, F.; Sayers, D. Diffraction Enhanced x-ray Imaging. Phys. Med. Biol. 1997, 42, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Dierker, J.; Joite-Barfub, S.; Sabel, M. Radiation Exposure and Image Quality in X-ray Diagnostic Radiology: Physical Principles and Clinical Applications; Aichinger, H., Ed.; Springer-Verlag: Berlin, Germany, 2012. [Google Scholar]
- Brankov, J.G.; Saiz-Herrandez, A.; Wernick, M.N. Noise analysis for diffraction enhanced imaging. In Proceedings of the 2004 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Arlington, TX, USA, 15–18 April 2004; pp. 1428–1431.
- Muehleman, C.; Li, J.; Connor, D.; Parham, C.; Pisano, E.; Zhong, Z. Diffraction-Enhanced Imaging of Musculoskeletal Tissues Using a Conventional X-ray Tube. Acad. Radiol. 2009, 16, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Engineer, C.; Parikh, J.; Raval, A. Review on Hydrolytic Degradation Behavior of Biodegradable Polymers from Controlled Drug Delivery System. Trends Biomater. Artif. Organs. 2011, 25, 79–85. [Google Scholar]
- Webb, M.A.; Belev, G.; Wysokinski, T.W.; Chapman, D. Diffraction enhanced imaging computed tomography (DEI-CT) at the BMIT facility at the Canadian Light Source. In Proceedings of the 7th Medical Applications of Synchrotron Radiation Workshop (MASR 2012) Shanghai Synchrotron Radiation Facility (SSRF), Shanghai, China, 17–20 October 2012; pp. 1–5. [CrossRef]
- Ang, K.C.; Leong, K.F.; Chua, C.K.; Chandrasekaran, M. Compressive properties and degradability of poly(e-caprolatone)/hydroxyapatite composites under accelerated hydrolytic degradation. J. Biomed. Mater. Res. A 2007, 80, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.-H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108–034122. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K. Synchrotron Based Imaging for Mass Loss Characterization; Canadian Congress of Applied Mechanics: Saskatoon, SK, Canada, 2013. [Google Scholar]
- Guo, Q.; Groeninckx, G. Crystallization Kinetics Poly(e-caprolactone) in Miscible Thermosetting Polymer Blends of Epoxy Resin and Poly (e-caprolactone). Polymer 2001, 42, 8647–8655. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, J.; Han, X.; Sinka, C.; Ding, L. A Phenomenological Model for the Degradation of Biodegradable Polymers. Biomaterials 2008, 29, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Bawolin, N.K.; Li, M.G.; Chen, X.B.; Zhang, W.J. Modeling Material-Degradation-Induced Elastic Property of Tissue Engineering Scaffolds. J. Biomech. Eng. 2010, 132, 111001:1–111001:7. [Google Scholar] [CrossRef] [PubMed]
- Husmann, M.; Schenderlein, S.; Luck, M.; Lindner, H.; Kleinebudde, P. Polymer erosion in PLGA microparticles produced by phase separation method. Int. J. Pharm. 2002, 242, 277–280. [Google Scholar] [CrossRef]
- Wang, S.; Lu, L.; Yaszemski, M.J. Bone Tissue-Engineering Material Poly(propylene fumarate): Correlation between Molecular Weight, Chain Dimensions, and Physical Properties. Biomacromolecules 2006, 6, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Pan, J. A model for simultaneous crystallisation and biodegradation of biodegradable polymers. Biomaterials 2009, 30, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Bezdek, J.C.; Hathaway, R.J. Some Notes on Alternating Optimization. In Advances in Soft Computing, Vol. 2275 of Lecture Notes in Artificial Intelligence; Pal, N.R., Sugeno, M., Eds.; Springer-Verlag: Heidelberg, Germany, 2002; pp. 288–300. [Google Scholar]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Artzi1, N.; Oliva, N.; Puron, C.; Shitreet, S.; Artzi, S.; Ramos, A.; Groothuis, A.; Sahagian, G.; Edelman, E. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat. Mater. 2011, 10, 704–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bawolin, N.K.; Chen, X. Synchrotron-Based in Situ Characterization of the Scaffold Mass Loss from Erosion Degradation. J. Funct. Biomater. 2016, 7, 17. https://doi.org/10.3390/jfb7030017
Bawolin NK, Chen X. Synchrotron-Based in Situ Characterization of the Scaffold Mass Loss from Erosion Degradation. Journal of Functional Biomaterials. 2016; 7(3):17. https://doi.org/10.3390/jfb7030017
Chicago/Turabian StyleBawolin, Nahshon K., and Xiongbaio Chen. 2016. "Synchrotron-Based in Situ Characterization of the Scaffold Mass Loss from Erosion Degradation" Journal of Functional Biomaterials 7, no. 3: 17. https://doi.org/10.3390/jfb7030017




