Pre-Clinical Cell-Based Therapy for Limbal Stem Cell Deficiency
Abstract
:1. Cornea and Limbal Stem Cells
2. Limbal Stem Cell Deficiency
3. Treatment Approaches for Limbal Stem Cell Deficiency
| Author, Year, (Reference) | Cell Source | Methods | LSCD Model | Follow-up Time | Evaluation | Results |
|---|---|---|---|---|---|---|
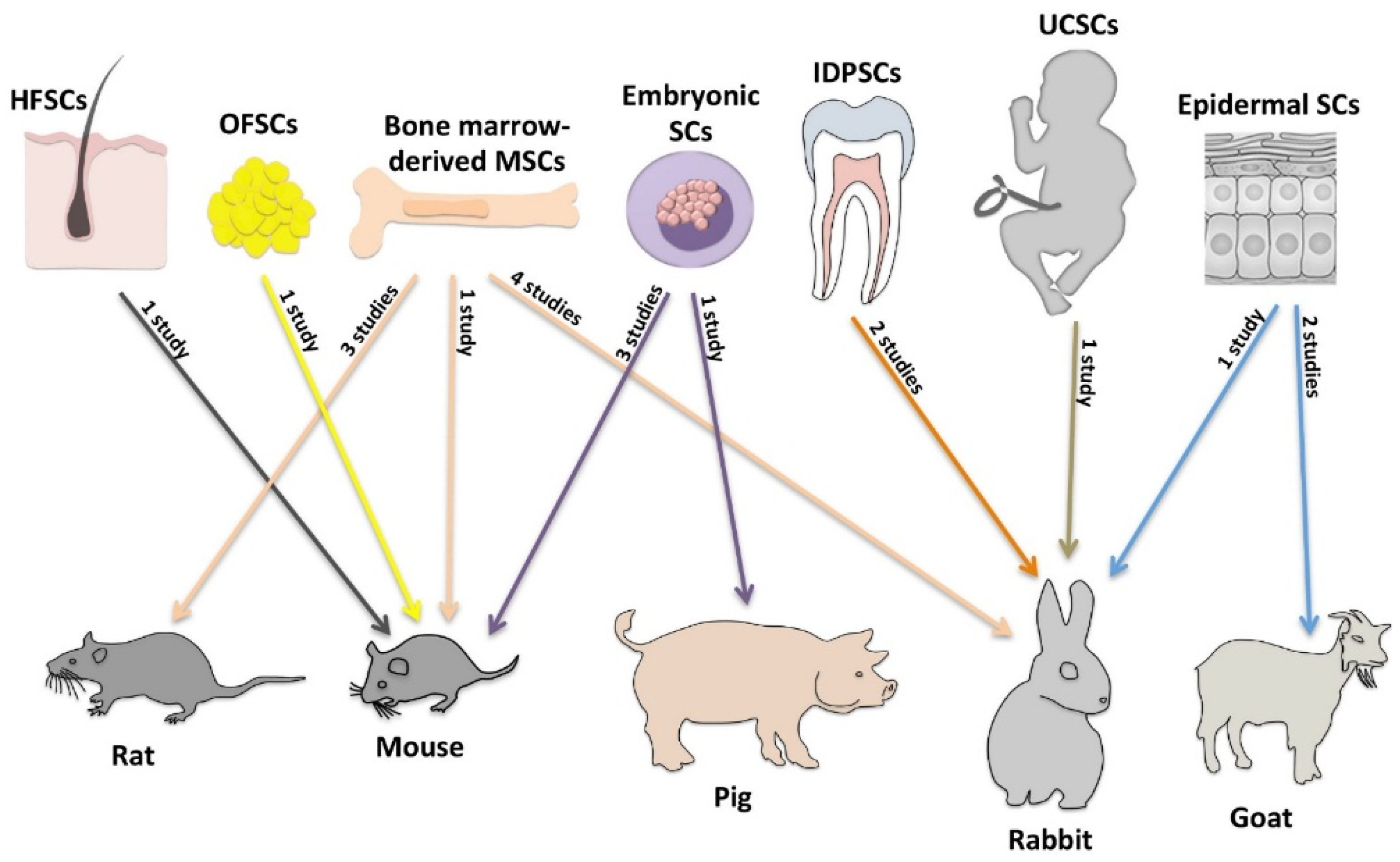
| Ma et al. 2006 [35] | Bone Marrow-Derived MSCs; Human | Cultured on AM carrier; Transplanted (n = 16); Control groups: 1) transplanted with fibroblast cells on AM (n = 8) and 2) transplanted with only AM (n = 7) | Rats; Disc paper saturated with 1 N NaOH onto cornea | 4 weeks | Slit lamp evaluation; Histology; IH | Reconstruction in 100% (16/16) of animals; Cornea completely transparent in 56.3% (9/16) of animals; Neovascularization detected within 2 mm and over 2 mm in 37.5% (6/16) and 12.5% (2/16) of animals, respectively; No complications |
| Ye et al. 2006 [39] | Bone Marrow-Derived MSCs; Rabbit | Cultured in α-MEM; IV injection; Four groups:
| Rabbits; Filter paper saturated with 1 N NaOH onto cornea | 1 month | Slit lamp evaluation; IH | Reconstruction in 100% (15/15) of animals in Group 2; Cornea more clear in group 2 compared with other groups; Neovascularization appeared on day 14 in Group 2; No complications |
| Gu et al. 2009 [33] | Bone Marrow-Derived MSCs; Rabbit | Cultured on fibrin carrier; Transplanted (n = 10); Control: eyes transplanted with only fibrin graft gel (n = 10) | Rabbits; Cornea treated with n-heptanol | 4 weeks | Slit lamp evaluation; Histology; FC; IF | Reconstruction in 100% (10/10) of animals; Iris partially clear in 30% (3/10) and completely obscure in 70% (7/10) of animals; Neovascularization detected over 3 mm from the limbus in 80% (8/10) of animals; No complications |
| Omoto et al. 2009 [36] | Bone Marrow-Derived MSCs; Human | Cultured in α-MEM; Carrier-free sheets transplanted; Control: no transplantation; Number of animals not reported | Rabbits; Cornea treated with n-heptanol | 4 weeks | Slit lamp evaluation; Histology; IH; RT-PCR | Reconstruction of corneal epithelium successful; Corneal clarity: no data; Neovascularization: no data; No complications |
| Jiang et al. 2010 [34] | Bone Marrow-Derived MSCs; Rat | Cultured on AM carrier; Three groups:
| Rats; Filter paper saturated with 1 N NaOH onto cornea | 10 weeks | Slit lamp evaluation; Histology; CLCM; SEM; FC; IF; IH | Reconstruction in 75% (9/12) of animals in group 3; Cornea completely transparent in 75% (9/12) of animals; Neovascularization limited within 2 mm of the limbus; No complications |
| Zajicova et al. 2010 [40] | Bone Marrow-Derived MSCs; Mouse | Cultured on nanofiber scaffold carrier; Co-transplantation of LSC and MSCs; Control: normal eyes; Number of animals not reported | Mice; Epithelial debridement with a needle | 2 weeks | Slit lamp evaluation; CLCM; FC; RT-PCR | Significantly inhibited local inflammatory reactions and supported healing process; Corneal clarity: no data; Neovascularization: no data; No complications |
| Reinshagen et al. 2011 [37] | Bone Marrow-Derived MSCs; Rabbit | Cultured in DMEM; Three groups:
| Rabbits; Cornea treated with n-heptanol | 6 months | Slit lamp evaluation; Histology; IH | Reconstruction in 100% (6/6) of animals in Group 1; Improved corneal clarity; Neovascularization of the entire cornea in all animals; No complications |
| Rohaina et al. 2014 [38] | Bone Marrow-Derived MSCs; Human | Cultured on AM carrier; Transplanted (n = 4); Control groups:
| Rats; Disc paper saturated with 1 N NaOH onto cornea | 8 weeks | Slit lamp evaluation; Histology; IH; OCT; RT-PCR | Reconstruction in 100% (4/4) of animals; Moderate corneal clarity; Minimal vascularization; No complications |
| Author, Year, (Reference) | Cell Source | Methods | LSCD Model | Follow-up Time | Evaluation | Results |
|---|---|---|---|---|---|---|
| Homma et al. 2004 [41] | Embryonic SCs; Mouse | Cultured on collagen IV-coated plates; Carrier-free sheets transplanted (n = 10); Control: no transplantation (n = 10) | Mice; Cornea treated with n-heptanol | 24 h | FC; Histology; RT-PCR; WB | Reconstruction in 100% (10/10) of animals; Corneal clarity: no data; Neovascularization: no data; No complications |
| Ueno et al. 2007 [44] | Embryonic SCs; Mouse | Cultured on gelatin-coated plates; Transfected with Pax6; Carrier-free sheets transplanted (n = 5); Control groups:
| Mice; Cornea treated with n-heptanol | 24 h | Histology; IF; RT-PCR | Reconstruction in 100% (5/5) of animals 12 h after transplantation; Corneal clarity: no data; Neovascularization: no data; No complications |
| Kumagai et al. 2010 [42] | Embryonic SCs; Monkey | Cultured on collagen IV-coated plates; Carrier-free sheets transplanted (n = 10); Control groups:
| Mice; Cornea treated with n-heptanol | 6 h | CLCM; IF; RT-PCR | Transplanted cells adhered to the corneal stroma and formed multiple cell layers in 100% (10/10) of animals; Corneal clarity: no data; Neovascularization: no data; No complications |
| Notara et al. 2013 [43] | Embryonic SCs; Mouse | Cultured on collagen IV-coated plates; Carrier-free sheets transplanted; Control: no transplantation; Number of animals not reported | Pigs; Epithelial debridement with a blade | 5 weeks | Histology; IH; RT-PCR; WB | Reconstruction after 1 week; Corneal clarity: no data; Neovascularization: no data; Mild immune reaction |
| Author, Year, (Reference) | Cell Source | Methods | LSCD Model | Follow-up Time | Evaluation | Results |
|---|---|---|---|---|---|---|
| Yang et al. 2007 [47] | Epidermal SCs; Goat | Cultured on AM carrier; Transplanted (n = 7); Control groups:
| Goats; Excision of the cornea and limbus | 24 months | IH; SEM; TEM | Reconstruction in 100% (7/7) of animals; Two or three quadrants of clear cornea in 71.4% (5/7) of animals at follow-up time to 24 months; Minimal neovascularization; Perforation through the pupil during operation in one eye |
| Yang et al. 2008 [46] | Epidermal SCs; Goat | Cultured on AM carrier; Transplanted (n = 10); Control groups:
| Goats; Excision of the cornea and limbus; Burned with 1 N NaOH | 30 months | Digital camera; Histology; IH | Reconstruction in 100% (10/10) of animals; Three or four quadrants of clear cornea in 80% (8/100) of animals at follow-up time to 30 months; Minimal neovascularization; No complications |
| Ouyang et al. 2014 [45] | Epidermal SCs; Human | Cultured on fibrin carrier; Transduction of Pax6 converted these cells into LSC-like cells; Transplanted and covered with AM (n = 5); Control: transplanted with only AM (n = 4) | Rabbits; Excision of the cornea and limbus | 3 months | CLCM; IF; Microarrays; Quantitative PCR; RNA-sequencing; WB | Reconstruction in 100% (5/5) of animals; Transparent cornea in 100% (5/5) of animals for over 3 months; Minimal neovascularization; No complications |
| Author, Year, (Reference) | Cell Source | Methods | LSCD Model | Follow-up Time | Evaluation | Results |
|---|---|---|---|---|---|---|
| Monteiro et al. 2009 [50] | IDPSCs; Human | Cultured on AM carrier; Transplanted (n = 5); Control: transplanted with only AM (n = 5) | Rabbits; Chemical burn of the cornea | 3 months | Slit lamp evaluation; CLCM; IF; RT-PCR | Reconstruction in 100% (5/5) of animals; Gradual improvement in corneal transparency in 100% (5/5) of animals during follow-up time of 3 months; Neovascularization: no data; No complications |
| Gomes et al. 2010 [49] | IDPSCs; Human | Cultured on AM carrier; MCB (n = 5), SCB (n = 4); Transplanted and covered with AM; Control: transplanted with only AM (n = 6) | Rabbits; Filter paper saturated with 0.5 M NaOH for 25 s (MCB), and for 45 s (SCB) | 3 months | Slit lamp evaluation; EM; Histology; IH | Reconstruction in 100% (5/5) of animals; Less organized and loose corneal epithelium in 75% (3/4) of SCB animals; Improved corneal clarity in 100% (5/5) of MCB animals; Superficial neovascularization in one animal No complications |
| Meyer-Blazejewska et al. 2011 [48] | HFSCs; Mouse | Cultured on fibrin carrier; Transplanted (n = 31); Control: no transplantation (n = 31) | Mice; Cornea and limbus removed | 5 weeks | Slit lamp evaluation; Histology; IF | Reconstruction in 87.5% (7/8) of animals after two weeks Improved corneal clarity; Neovascularization in 12.5% (1/8) of animals; No complications |
| Reza et al. 2011 [52] | UCSCs; Human | Cultured on AM carrier; Three groups:
| Rabbits; Cornea and limbus removed | 4 weeks | Slit lamp evaluation; Histology; IC; IH; RT-PCR | Reconstruction in 66.7% (4/6) of animals; Corneal clarity: no data; Severe neovascularization in one eye; Mild superficial inflammation in one other |
| Lin et al. 2013 [51] | OFSCs; Human | Cultured in MesenPro medium; Topical application of cells (n = 9), Intra-limbal injection of cells (n = 3); Control: Topical application of PBS (n = 6), Injection of PBS (n = 3), no treatment (n = 3) | Mice; Filter paper saturated with 0.5 N NaOH onto cornea | 1 week | Digital camera; Histology; IH; IF; WB | Reconstruction of corneal epithelium after 1 week; Improved corneal clarity; No neovascularization; No complications |
| Methods | Materials | References |
|---|---|---|
| Transplantation | Carrier-free cell sheets | [36,41,42,43,44] |
| Transplantation | Amniotic membrane | [34,38,46,47,49,50,52,53] |
| Intravenous injection | – | [39] |
| Transplantation | Fibrin scaffold | [33,45,48] |
| Transplantation | Nanofiber scaffold | [40] |
| Injection under amniotic membrane | – | [37] |
| Topical application/Intra-limbal injection | – | [51] |
4. Substrates for Corneal Reconstruction
| Biological/Biosynthetic | Synthetic |
|---|---|
| Amniotic membrane [57] | Contact lenses [58] |
| Chemically cross-linked hyaluronic acid-based hydrogels [59] | Mebiol Gel (thermo-reversible polymer gel) [53] |
| Chitosan matrix/silver matrix/gold matrix [60] | Nanofiber scaffolds [40] |
| Collagen IV-coated plates [61] | Petrolatum gauze [24] |
| Collagen membranes [62] | Plastic [25] |
| Corneal stroma [63] | Poly(lactide-co-glycolide) electrospun scaffolds [64] |
| Fibrin [65] | Poly-ε-caprolactone electrospun scaffolds [66] |
| Human keratoplasty lenticules [67] | |
| Laminin-coated compressed collagen gel [68] | |
| Matrigel (reconstituted basement membrane extract) [69] | |
| Plastic compressed collagen [70] | |
| Recombinant human cross-linked collagen scaffold [71] | |
| Silk fibroin [72] | |
| Silk fibroin mixed with polyethylene glycol [72] |
| Carriers/Methods | Transparency | Mechanical Strength | Elasticity | Advantages | Disadvantages |
|---|---|---|---|---|---|
| AM | + | ++ | +++ | Stimulates cell growth, anti-inflammation, anti-angiogenesis, proper elasticity | Limited transparency, variable quality, risk of disease transmission, limited mechanical strength, poor standardization |
| Carrier-free method | N/A | N/A | N/A | Rapid adhesion, does not require preparation and standardization of membranes, does not require sutures | Possibility for detachment from the ocular surface in the early period after surgery |
| Fibrin gel | ++ | +++ | +++ | Proper transparency, good bioadsorbence, easy manipulation, good mechanical strength, elasticity, degradable | Possibility for immune response, risk for disease transmission |
| Nanofiber | ++ | ++++ | ++ | Good transparency, high mechanical strength, highly flexible, proper biocompatibility, easy to use, controlled shape and pore size, low cost | Limited elasticity, high cost |
5. Cultured Bone Marrow-Derived Mesenchymal Stem Cells
6. Cultured Embryonic Stem Cells
7. Cultured Epidermal Stem Cells
8. Cultured Hair Follicle-Derived Stem Cells
9. Cultured Immature Dental Pulp Stem Cells
10. Cultured Umbilical Cord Stem Cells
11. Cultured Orbital Fat-Derived Stem Cells
12. Challenges and Future Perspectives
13. Conclusions
| Types of Stem Cells | Success | Complications (Number of Animals) | Ease of Access | Number of Animals (Number of Studies) | Autologous Source | Ethical Concerns |
|---|---|---|---|---|---|---|
| Bone Marrow-Derived MSCs | +++ | – | ++ | 63 (8) 1 | Yes | No |
| Embryonic SCs | + | Mild immune reaction * | + | 25 (4) 2 | No | Yes |
| Epidermal SCs | ++++ | Perforation (1) | ++++ | 22 (3) | Yes | No |
| HFSCs | +++ | – | ++++ | 31 (1) | Yes | No |
| IDPSCs | +++ | – | ++ | 14 (2) | Yes | No |
| OFSCs | ++ | – | ++ | 12 (1) | Yes | No |
| UCSCs | ++ | Mild superficial inflammation (1) | ++ | 6 (1) | No | No |
Acknowledgments
Conflicts of Interest
References
- Land, M.F.; Fernald, R.D. The evolution of eyes. Annu. Rev. Neurosci. 1992, 15, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Beuerman, R.W.; Pedroza, L. Ultrastructure of the human cornea. Microsc. Res. Tech. 1996, 33, 320–335. [Google Scholar] [CrossRef]
- Robertson, D.M.; Ladage, P.M.; Yamamoto, N.; Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Bcl-2 and bax regulation of corneal homeostasis in genetically altered mice. Eye Contact Lens 2006, 32, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Moller-Pedersen, T.; Li, H.F.; Petroll, W.M.; Cavanagh, H.D.; Jester, J.V. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 1998, 39, 487–501. [Google Scholar] [PubMed]
- Cavanagh, H.D.; Ladage, P.M.; Li, S.L.; Yamamoto, K.; Molai, M.; Ren, D.H.; Petroll, W.M.; Jester, J.V. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: A 13-month clinical trial. Ophthalmology 2002, 109, 1957–1969. [Google Scholar] [CrossRef]
- Sharma, A.; Coles, W.H. Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Invest. Ophthalmol. Vis. Sci. 1989, 30, 1962–1971. [Google Scholar] [PubMed]
- Utheim, T.P. Limbal epithelial cell therapy: Past, present, and future. Methods Mol. Biol. 2013, 1014, 3–43. [Google Scholar] [PubMed]
- Nishida, T. Fundamentals, diagnosis and management. In Cornea, 2nd ed.; Krachmer, J.H., Mannis, M.J., Holland, E., Eds.; Elsevier: Philadelphia, PA, USA, 2005; pp. 3–26. [Google Scholar]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D. Perpetuation of stem cells in the eye. Eye 1994, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Schermer, A.; Galvin, S.; Sun, T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986, 103, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Rama, P.; Mavilio, F.; De Luca, M. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J. Pathol. 2009, 217, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Holland, E.J. Cornea: Fundamentals, diagnosis and management. In Classification and Staging of Ocular Surface Disease, 2nd ed.; Krachmer, J.H., Mannis, M.J., Holland, E., Eds.; Elsevier: Philadelphia, PA, USA, 2005; pp. 1785–1797. [Google Scholar]
- Vemuganti, G.K.; Sangwan, V.S. Interview: Affordability at the cutting edge: Stem cell therapy for ocular surface reconstruction. Regen. Med. 2010, 5, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Schwab, I.R.; Isseroff, R.R. Bioengineered corneas—The promise and the challenge. N. Engl. J. Med. 2000, 343, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Puangsricharern, V.; Tseng, S.C. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 1995, 102, 1476–1485. [Google Scholar] [CrossRef]
- Espana, E.M.; Grueterich, M.; Romano, A.C.; Touhami, A.; Tseng, S.C. Idiopathic limbal stem cell deficiency. Ophthalmology 2002, 109, 2004–2010. [Google Scholar] [CrossRef]
- Dua, H.S.; Joseph, A.; Shanmuganathan, V.A.; Jones, R.E. Stem cell differentiation and the effects of deficiency. Eye 2003, 17, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Ferrari, S.; Fasolo, A.; Bohm, E.; Ponzin, D.; Barbaro, V. Techniques for culture and assessment of limbal stem cell grafts. Ocul. Surf. 2010, 8, 146–153. [Google Scholar] [CrossRef]
- Shortt, A.J.; Secker, G.A.; Rajan, M.S.; Meligonis, G.; Dart, J.K.; Tuft, S.J.; Daniels, J.T. Ex vivo expansion and transplantation of limbal epithelial stem cells. Ophthalmology 2008, 115, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Rauz, S.; Saw, V.P. Serum eye drops, amniotic membrane and limbal epithelial stem cells—tools in the treatment of ocular surface disease. Cell Tissue Bank. 2010, 11, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, K.R.; Tseng, S.C. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989, 96, 709–722. [Google Scholar] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Daya, S.M.; Watson, A.; Sharpe, J.R.; Giledi, O.; Rowe, A.; Martin, R.; James, S.E. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology 2005, 112, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PloS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Sareen, D.; Saghizadeh, M.; Ornelas, L.; Winkler, M.A.; Narwani, K.; Sahabian, A.; Funari, V.A.; Tang, J.; Spurka, L.; Punj, V.; et al. Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Transl. Med. 2014, 3, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, D.; Kummerle-Deschner, J.; Dannecker, G.E. Side-effects of long-term immunosuppression versus morbidity in autologous stem cell rescue: Striking the balance. Rheumatology 1999, 38, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Utheim, T.P. Concise review: Transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency-current status and future perspectives. Stem Cells 2015, 33, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Bosch, M.; Zamora, K.; Coroneo, M.T.; Wakefield, D.; Watson, S.L. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation 2009, 87, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Park, I.K.; Kim, J.C. Technique for autologous nasal mucosa transplantation in severe ocular surface disease. Eur. J. Ophthalmol. 2011, 21, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chun, Y.S.; Lee, S.H.; Mun, S.K.; Jung, H.S.; Lee, S.H.; Son, Y.; Kim, J.C. Ocular surface reconstruction with autologous nasal mucosa in cicatricial ocular surface disease. Am. J. Ophthalmol. 2010, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Molecular vision 2009, 15, 99–107. [Google Scholar] [PubMed]
- Jiang, T.S.; Cai, L.; Ji, W.Y.; Hui, Y.N.; Wang, Y.S.; Hu, D.; Zhu, J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol. Vis. 2010, 16, 1304–1316. [Google Scholar] [PubMed]
- Ma, Y.; Xu, Y.; Xiao, Z.; Yang, W.; Zhang, C.; Song, E.; Du, Y.; Li, L. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 2006, 24, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.; Miyashita, H.; Shimmura, S.; Higa, K.; Kawakita, T.; Yoshida, S.; McGrogan, M.; Shimazaki, J.; Tsubota, K. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest. Ophthalmol. Vis. Sci. 2009, 50, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Reinshagen, H.; Auw-Haedrich, C.; Sorg, R.V.; Boehringer, D.; Eberwein, P.; Schwartzkopff, J.; Sundmacher, R.; Reinhard, T. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011, 89, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Rohaina, C.M.; Then, K.Y.; Ng, A.M.; Wan Abdul Halim, W.H.; Zahidin, A.Z.; Saim, A.; Idrus, R.B. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl. Res. 2014, 163, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yao, K.; Kim, J.C. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: Engraftment and involvement in wound healing. Eye 2006, 20, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Zajicova, A.; Pokorna, K.; Lencova, A.; Krulova, M.; Svobodova, E.; Kubinova, S.; Sykova, E.; Pradny, M.; Michalek, J.; Svobodova, J.; et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transpl. 2010, 19, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Homma, R.; Yoshikawa, H.; Takeno, M.; Kurokawa, M.S.; Masuda, C.; Takada, E.; Tsubota, K.; Ueno, S.; Suzuki, N. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, Y.; Kurokawa, M.S.; Ueno, H.; Kayama, M.; Tsubota, K.; Nakatsuji, N.; Kondo, Y.; Ueno, S.; Suzuki, N. Induction of corneal epithelium-like cells from cynomolgus monkey embryonic stem cells and their experimental transplantation to damaged cornea. Cornea 2010, 29, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Hernandez, D.; Mason, C.; Daniels, J.T. Characterization of the phenotype and functionality of corneal epithelial cells derived from mouse embryonic stem cells. Regen. Med. 2012, 7, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kurokawa, M.S.; Kayama, M.; Homma, R.; Kumagai, Y.; Masuda, C.; Takada, E.; Tsubota, K.; Ueno, S.; Suzuki, N. Experimental transplantation of corneal epithelium-like cells induced by PAX6 gene transfection of mouse embryonic stem cells. Cornea 2007, 26, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Xue, Y.; Lin, Y.; Zhang, X.; Xi, L.; Patel, S.; Cai, H.; Luo, J.; Zhang, M.; Zhang, M.; et al. WNT7a and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 2014, 511, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Moldovan, N.I.; Zhao, Q.; Mi, S.; Zhou, Z.; Chen, D.; Gao, Z.; Tong, D.; Dou, Z. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol. Vis. 2008, 14, 1064–1070. [Google Scholar] [PubMed]
- Yang, X.; Qu, L.; Wang, X.; Zhao, M.; Li, W.; Hua, J.; Shi, M.; Moldovan, N.; Wang, H.; Dou, Z. Plasticity of epidermal adult stem cells derived from adult goat ear skin. Mol. Reprod. Dev. 2007, 74, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Blazejewska, E.A.; Call, M.K.; Yamanaka, O.; Liu, H.; Schlotzer-Schrehardt, U.; Kruse, F.E.; Kao, W.W. From hair to cornea: Toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011, 29, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.; Geraldes Monteiro, B.; Melo, G.B.; Smith, R.L.; Cavenaghi Pereira da Silva, M.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.G.; Serafim, R.C.; Melo, G.B.; Silva, M.C.; Lizier, N.F.; Maranduba, C.M.; Smith, R.L.; Kerkis, A.; Cerruti, H.; Gomes, J.A.; et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009, 42, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Loi, M.X.; Lien, G.S.; Cheng, C.F.; Pao, H.Y.; Chang, Y.C.; Ji, A.T.; Ho, J.H. Topical administration of orbital fat-derived stem cells promotes corneal tissue regeneration. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Reza, H.M.; Ng, B.Y.; Gimeno, F.L.; Phan, T.T.; Ang, L.P. Umbilical cord lining stem cells as a novel and promising source for ocular surface regeneration. Stem Cell Rev. 2011, 7, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Sudha, B.; Madhavan, H.N.; Sitalakshmi, G.; Malathi, J.; Krishnakumar, S.; Mori, Y.; Yoshioka, H.; Abraham, S. Cultivation of human corneal limbal stem cells in mebiol gel—A thermo-reversible gelation polymer. Indian J. Med. Res. 2006, 124, 655–664. [Google Scholar] [PubMed]
- Feng, Y.; Borrelli, M.; Reichl, S.; Schrader, S.; Geerling, G. Review of alternative carrier materials for ocular surface reconstruction. Curr. Eye Res. 2014, 39, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Levis, H.; Daniels, J.T. New technologies in limbal epithelial stem cell transplantation. Curr. Opin. Biotechnol. 2009, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Ntoukas, T.; Hofmann-Rummelt, C.; Kruse, F.E.; Schlotzer-Schrehardt, U. Comparative analysis of the basement membrane composition of the human limbus epithelium and amniotic membrane epithelium. Cornea 2012, 31, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Meller, D.; Tseng, S.C. Conjunctival epithelial cell differentiation on amniotic membrane. Investig. Ophthalmol. Vis. Sci. 1999, 40, 878–886. [Google Scholar]
- Di Girolamo, N.; Chui, J.; Wakefield, D.; Coroneo, M.T. Cultured human ocular surface epithelium on therapeutic contact lenses. Br. J. Ophthalmol. 2007, 91, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Senior, R.A.; Pitarresi, G.; Palumbo, F.S.; Giammona, G.; Deshpande, P.; MacNeil, S. Biocompatible hydrogels based on hyaluronic acid cross-linked with a polyaspartamide derivative as delivery systems for epithelial limbal cells. Int. J. Pharm. 2011, 414, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sudha, B.; Jasty, S.; Krishnan, S.; Krishnakumar, S. Signal transduction pathway involved in the ex vivo expansion of limbal epithelial cells cultured on various substrates. Indian J. Med. Res. 2009, 129, 382–389. [Google Scholar] [PubMed]
- Li, D.Q.; Chen, Z.; Song, X.J.; de Paiva, C.S.; Kim, H.S.; Pflugfelder, S.C. Partial enrichment of a population of human limbal epithelial cells with putative stem cell properties based on collagen type IV adhesiveness. Exp. Eye Res. 2005, 80, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Kagami, H.; Kobayashi, C.; Ueda, M.; Terasaki, H. Effects of cryopreservation on histology and viability of cultured corneal epithelial cell sheets in rabbit. Cornea 2005, 24, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Friend, J.; Kinoshita, S.; Thoft, R.A.; Eliason, J.A. Corneal epithelial cell cultures on stromal carriers. Investig. Ophthalmol. Vis. Sci. 1982, 23, 41–49. [Google Scholar]
- Deshpande, P.; McKean, R.; Blackwood, K.A.; Senior, R.A.; Ogunbanjo, A.; Ryan, A.J.; MacNeil, S. Using poly(lactide-co-glycolide) electrospun scaffolds to deliver cultured epithelial cells to the cornea. Regen Med. 2010, 5, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Talbot, M.; Carrier, P.; Giasson, C.J.; Deschambeault, A.; Guerin, S.L.; Auger, F.A.; Bazin, R.; Germain, L. Autologous transplantation of rabbit limbal epithelia cultured on fibrin gels for ocular surface reconstruction. Mol. Vis. 2006, 12, 65–75. [Google Scholar] [PubMed]
- Sharma, S.; Mohanty, S.; Gupta, D.; Jassal, M.; Agrawal, A.K.; Tandon, R. Cellular response of limbal epithelial cells on electrospun poly-epsilon-caprolactone nanofibrous scaffolds for ocular surface bioengineering: A preliminary in vitro study. Mol. Vis. 2011, 17, 2898–2910. [Google Scholar] [PubMed]
- Barbaro, V.; Ferrari, S.; Fasolo, A.; Ponzin, D.; Di Iorio, E. Reconstruction of a human hemicornea through natural scaffolds compatible with the growth of corneal epithelial stem cells and stromal keratocytes. Mol. Vis. 2009, 15, 2084–2093. [Google Scholar] [PubMed]
- Mi, S.; Chen, B.; Wright, B.; Connon, C.J. Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Eng. Part A 2010, 16, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiankia, N.; Ebrahimi, M.; Hosseini, A.; Baharvand, H. Effects of different extracellular matrices and co-cultures on human limbal stem cell expansion in vitro. Cell Biol. Int. 2009, 33, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Levis, H.J.; Brown, R.A.; Daniels, J.T. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials 2010, 31, 7726–7737. [Google Scholar] [CrossRef] [PubMed]
- Dravida, S.; Gaddipati, S.; Griffith, M.; Merrett, K.; Lakshmi Madhira, S.; Sangwan, V.S.; Vemuganti, G.K. A biomimetic scaffold for culturing limbal stem cells: A promising alternative for clinical transplantation. J. Tissue Eng. Regen Med. 2008, 2, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Takeshima, N.; Moro, F.; Kawakita, T.; Kawashima, M.; Demura, M.; Shimazaki, J.; Asakura, T.; Tsubota, K.; Shimmura, S. Porous silk fibroin film ass a transparent carrier for cultivated corneal epithelial sheets. J. Biomater Sci. Polym. Ed. 2010, 22. [Google Scholar] [CrossRef]
- Redenti, S.; Tao, S.; Yang, J.; Gu, P.; Klassen, H.; Saigal, S.; Desai, T.; Young, M.J. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Infor. 2008, 1, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Holan, V.; Javorkova, E.; Trosan, P. The growth and delivery of mesenchymal and limbal stem cells using copolymer polyamide 6/12 nanofiber scaffolds. Methods Mol. Biol. 2013, 1014, 187–199. [Google Scholar] [PubMed]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Forni, M.F.; Loureiro, R.R.; Cristovam, P.C.; Bonatti, J.A.; Sogayar, M.C.; Gomes, J.A. Comparison between different biomaterial scaffolds for limbal-derived stem cells growth and enrichment. Curr. Eye Res. 2013, 38, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y.; et al. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Shimmura, S.; Kato, N.; Kawakita, T.; Miyashita, H.; Itabashi, Y.; Fukuda, K.; Shimazaki, J.; Tsubota, K. Proliferation and differentiation of transplantable rabbit epithelial sheets engineered with or without an amniotic membrane carrier. Invest. Ophthalmol. Vis. Sci. 2007, 48, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, C.; Yamagishi, M.; Yamahara, K.; Hagino, I.; Mori, H.; Sawa, Y.; Yagihara, T.; Kitamura, S.; Nagaya, N. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2008, 374, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.W.; Kim, S.S.; Lee, S.Y.; Lee, H.S.; Kim, H.S.; Lee, Y.D.; Suh-Kim, H. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp. Mol. Med. 2008, 40, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.B.; Ye, W.B.; Hu, Z.Z.; Yan, Y.; Wang, Y.; Takon, B.F.; Zhou, G.Q.; Zhou, Y.F. Intravenously administered BMSCs reduce neuronal apoptosis and promote neuronal proliferation through the release of VEGF after stroke in rats. Neurol. Res. 2010, 32, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, F.; Gu, Y.; Liu, J.; Gu, X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009, 1262, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; Koc, O.N.; Devine, S.M.; Curtin, P.; Maziarz, R.T.; Holland, H.K.; Shpall, E.J.; McCarthy, P.; Atkinson, K.; Cooper, B.W.; et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol. Blood Marrow Transpl. 2005, 11, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Jiao, C.; Zhao, S.; Li, X.; Ren, X.; Zhang, L.; Han, Z.C.; Zhang, X. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp. Eye Res. 2012, 102, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zhang, Y.Y.; Gu, H.W.; Guan, H.J. Effects of bone marrow mesenchymal stem cells on cell proliferation and growth factor expression of limbal epithelial cells in vitro. Ophthalmic Res. 2012, 48, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.N.; Edgar, A.J.; Samadikuchaksaraei, A.; Timson, C.M.; Romanska, H.M.; Polak, J.M.; Bishop, A.E. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002, 8, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, H.R.; Paul, S.; Haller, C.; Bluethmann, H.; Blum, C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature 2001, 414, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Stewart, R.; Yung, S.; Kolli, S.; Armstrong, L.; Stojkovic, M.; Figueiredo, F.; Lako, M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells 2007, 25, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Brzeszczynska, J.; Samuel, K.; Greenhough, S.; Ramaesh, K.; Dhillon, B.; Hay, D.C.; Ross, J.A. Differentiation and molecular profiling of human embryonic stem cell-derived corneal epithelial cells. Int. J. Mol. Med. 2014, 33, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guan, Y.; Qu, Z.; Zhang, J.; Liao, B.; Ma, B.; Qian, J.; Li, D.; Li, W.; Xu, G.T.; et al. WNT signaling determines tumorigenicity and function of ESC-derived retinal progenitors. J. Clin. Investg. 2013, 123, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Bickenbach, J.R. Somatic epidermal stem cells can produce multiple cell lineages during development. Stem Cells 2002, 20, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tsonis, P.A.; Fuentes, E.J. Focus on molecules: PAX-6, the eye master. Exp. Eye Res. 2006, 83, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Treisman, J.E. How to make an eye. Development 2004, 131, 3823–3827. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, C.A.; Whitehouse, J.; Reynolds, A.J.; Hole, N. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 2003, 12, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Lako, M.; Armstrong, L.; Cairns, P.M.; Harris, S.; Hole, N.; Jahoda, C.A. Hair follicle dermal cells repopulate the mouse haematopoietic system. J. Cell Sci. 2002, 115, 3967–3974. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.D.; Arnott, E.C.; Whitehouse, C.J.; Lawrence, C.M.; Reynolds, A.J.; Hole, N.; Jahoda, C.A. Plasticity of rodent and human hair follicle dermal cells: Implications for cell therapy and tissue engineering. J. Investig. Dermatol. Symp. Proc. 2005, 10, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G. Epithelial stem cells: A folliculocentric view. J. Investig. Dermatol. 2006, 126, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar] [CrossRef]
- Blazejewska, E.A.; Schlotzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009, 27, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Cao, X.; Zhang, Z.; Huang, J.; Chen, C.; Zhang, Z.; Jia, M.; Xiong, J.; Liang, Z.; Sun, S.; et al. Long-term efficacy and safety of human umbilical cord mesenchymal stromal cells in rotenone-induced hemiparkinsonian rats. Biol. Blood Marrow Transpl. 2010, 16, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.H.; Ma, W.H.; Tseng, T.C.; Chen, Y.F.; Chen, M.H.; Lee, O.K. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng. Part A 2011, 17, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Bien, M.Y.; Ku, C.C.; Chang, Y.C.; Pao, H.Y.; Yang, Y.L.; Hsiao, M.; Chen, C.L.; Ho, J.H. Systemic human orbital fat-derived stem/stromal cell transplantation ameliorates acute inflammation in lipopolysaccharide-induced acute lung injury. Crit Care Med. 2012, 40, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, W.; Ling, S.; Sheha, H.; Qiu, W.; Li, C.; Liu, Z. Amniotic membrane extraction solution for ocular chemical burns. Clin. Exp. Ophthalmol. 2009, 37, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Amirjamshidi, H.; Milani, B.Y.; Sagha, H.M.; Movahedan, A.; Shafiq, M.A.; Lavker, R.M.; Yue, B.Y.; Djalilian, A.R. Limbal fibroblast conditioned media: A non-invasive treatment for limbal stem cell deficiency. Mol. Vis. 2011, 17, 658–666. [Google Scholar] [PubMed]
- Geerling, G.; Maclennan, S.; Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C. Is autologous serum a tonic for the ailing corneal epithelium? Am. J. Ophthalmol. 2006, 142, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Utheim, T.P.; Raeder, S.; Utheim, O.A.; Cai, Y.; Roald, B.; Drolsum, L.; Lyberg, T.; Nicolaissen, B. A novel method for preserving cultured limbal epithelial cells. Br. J. Ophthalmol. 2007, 91, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Pasovic, L.; Utheim, T.P.; Maria, R.; Lyberg, T.; Messelt, E.B.; Aabel, P.; Chen, D.F.; Chen, X.; Eidet, J.R. Optimization of Storage Temperature for Cultured ARPE-19 Cells. J. Ophthalmol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Pasovic, L.; Eidet, J.R.; Lyberg, T.; Messelt, E.B.; Aabel, P.; Utheim, T.P. Antioxidants Improve the Viability of Stored Adult Retinal Pigment Epithelial-19 Cultures. Ophthalmol. Ther. 2014, 3, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Aabel, P.; Eidet, J.R.; Messelt, E.B.; Lyberg, T.; von Unge, M.; Utheim, T.P. Effect of storage temperature on cultured epidermal cell sheets stored in xenobiotic-free medium. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Jackson, C.; Eidet, J.R.; Messelt, E.B.; Corraya, R.M.; Lyberg, T.; Griffith, M.; Dartt, D.A.; Utheim, T.P. Effect of storage temperature on structure and function of cultured human oral keratinocytes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Eidet, J.R.; Utheim, O.A.; Raeder, S.; Dartt, D.A.; Lyberg, T.; Carreras, E.; Huynh, T.T.; Messelt, E.B.; Louch, W.E.; Roald, B.; et al. Effects of serum-free storage on morphology, phenotype, and viability of ex vivo cultured human conjunctival epithelium. Exp. Eye Res. 2012, 94, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Utheim, T.P.; Raeder, S.; Utheim, O.A.; de la Paz, M.; Roald, B.; Lyberg, T. Sterility control and long-term eye-bank storage of cultured human limbal epithelial cells for transplantation. Br. J. Ophthalmol. 2009, 93, 980–983. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.R.; Daniels, J.T. Concise review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells 2011, 29, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Osei-Bempong, C.; Dana, R.; Jurkunas, U. The culture and transplantation of human limbal stem cells. J. Cell Physiol. 2010, 225, 15–19. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehic, A.; Utheim, Ø.A.; Ommundsen, K.; Utheim, T.P. Pre-Clinical Cell-Based Therapy for Limbal Stem Cell Deficiency. J. Funct. Biomater. 2015, 6, 863-888. https://doi.org/10.3390/jfb6030863
Sehic A, Utheim ØA, Ommundsen K, Utheim TP. Pre-Clinical Cell-Based Therapy for Limbal Stem Cell Deficiency. Journal of Functional Biomaterials. 2015; 6(3):863-888. https://doi.org/10.3390/jfb6030863
Chicago/Turabian StyleSehic, Amer, Øygunn Aass Utheim, Kristoffer Ommundsen, and Tor Paaske Utheim. 2015. "Pre-Clinical Cell-Based Therapy for Limbal Stem Cell Deficiency" Journal of Functional Biomaterials 6, no. 3: 863-888. https://doi.org/10.3390/jfb6030863