Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
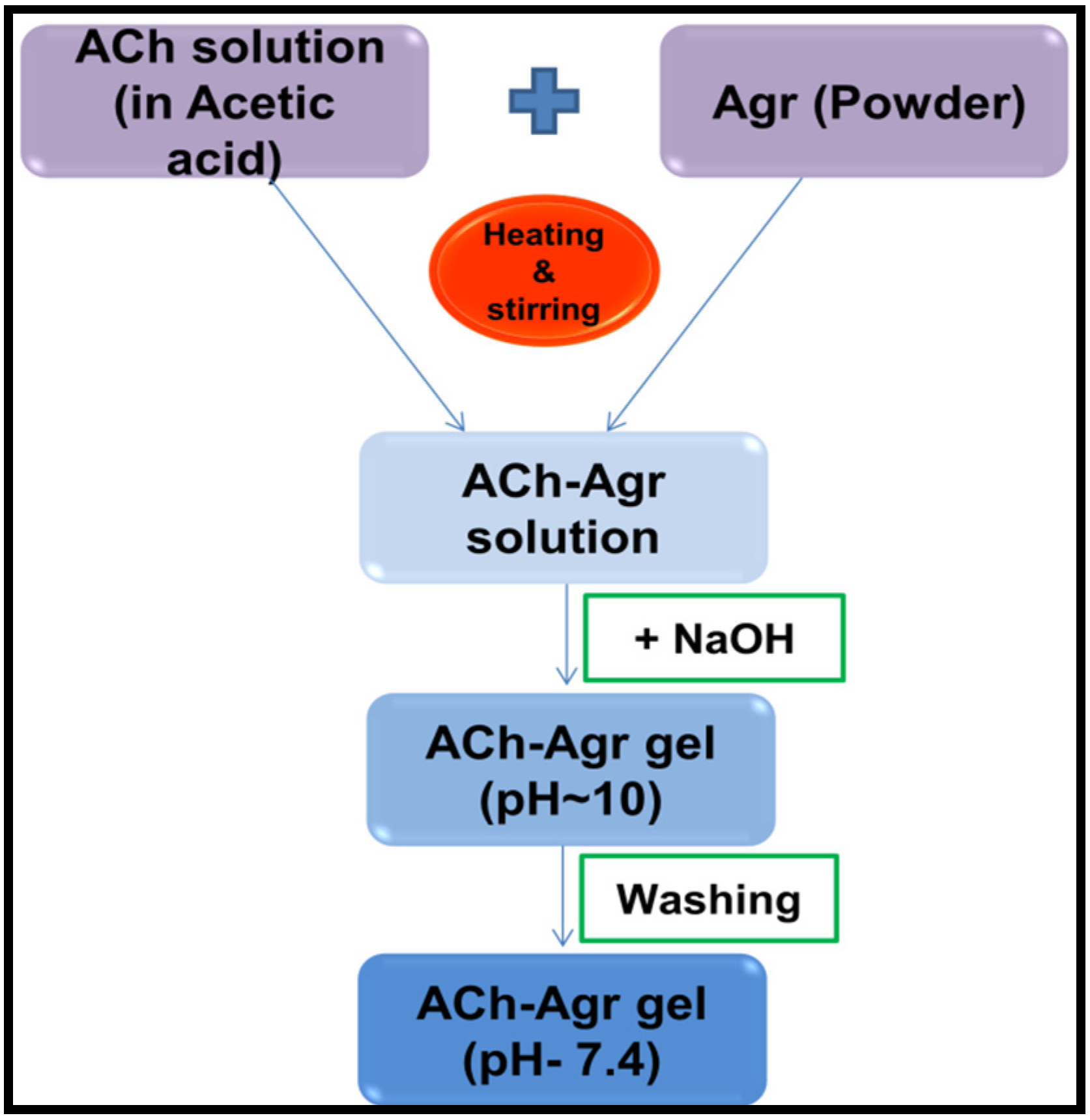
2.1. Preparation of ACh-Agr Composite Gel
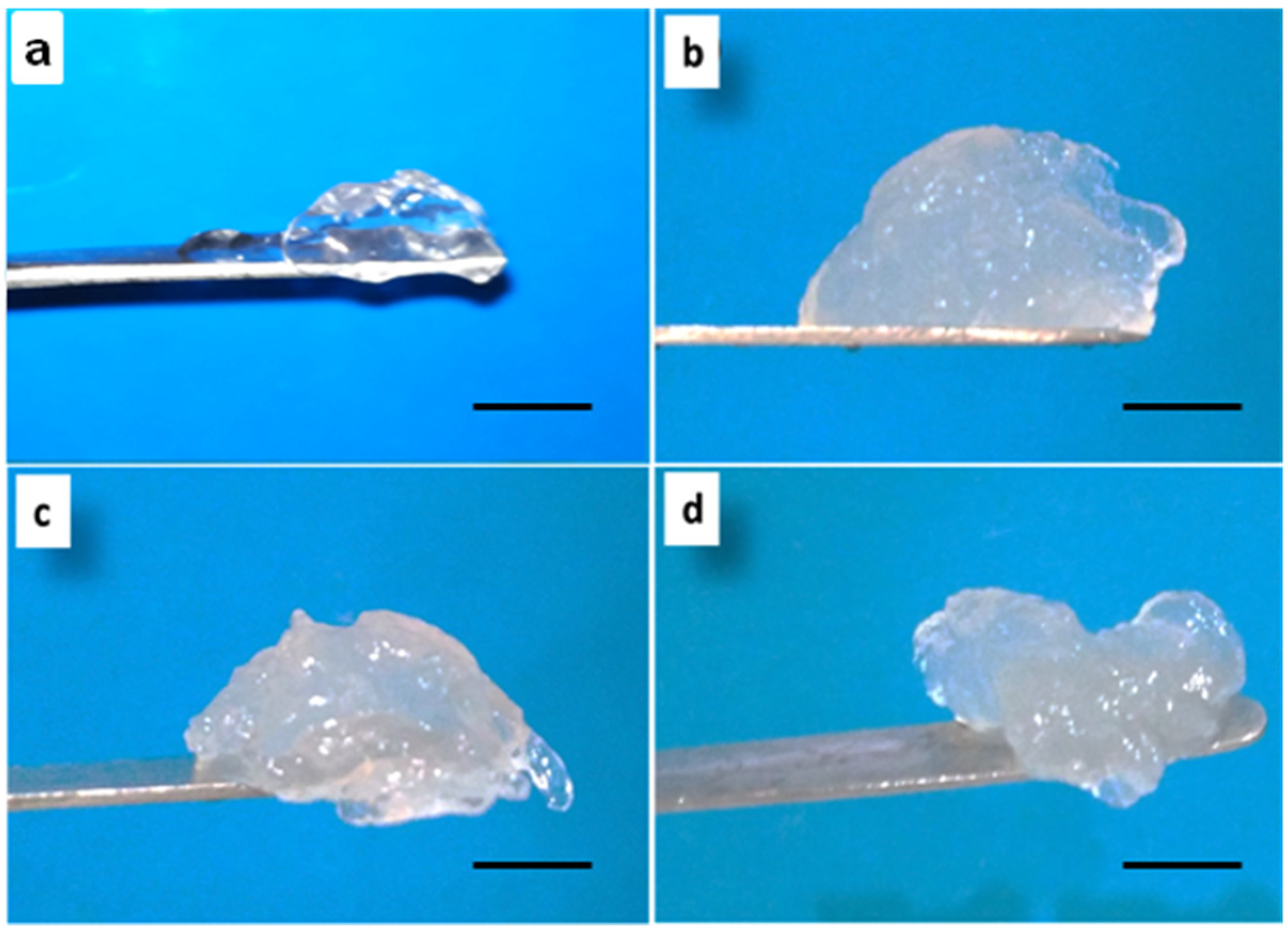
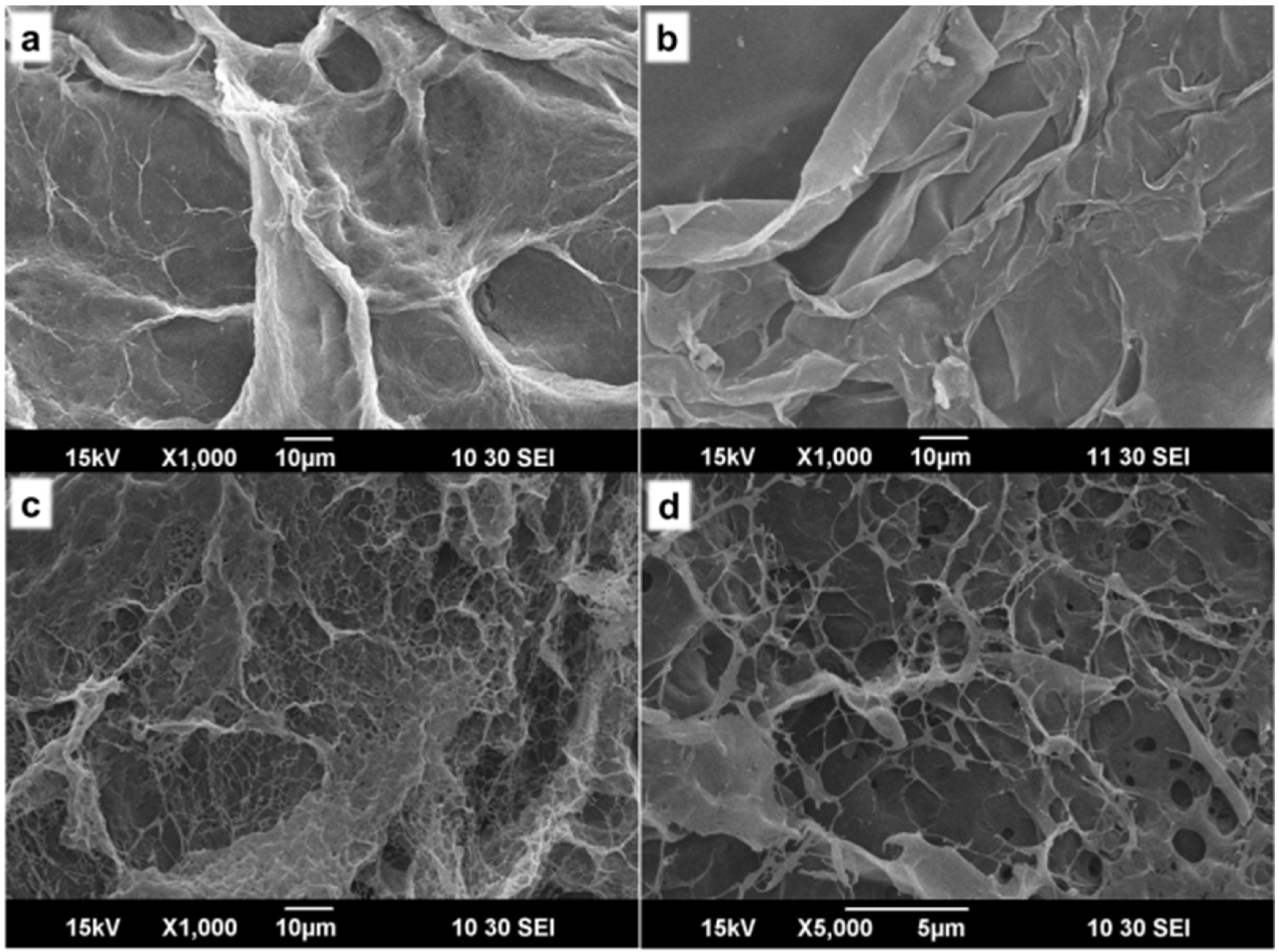
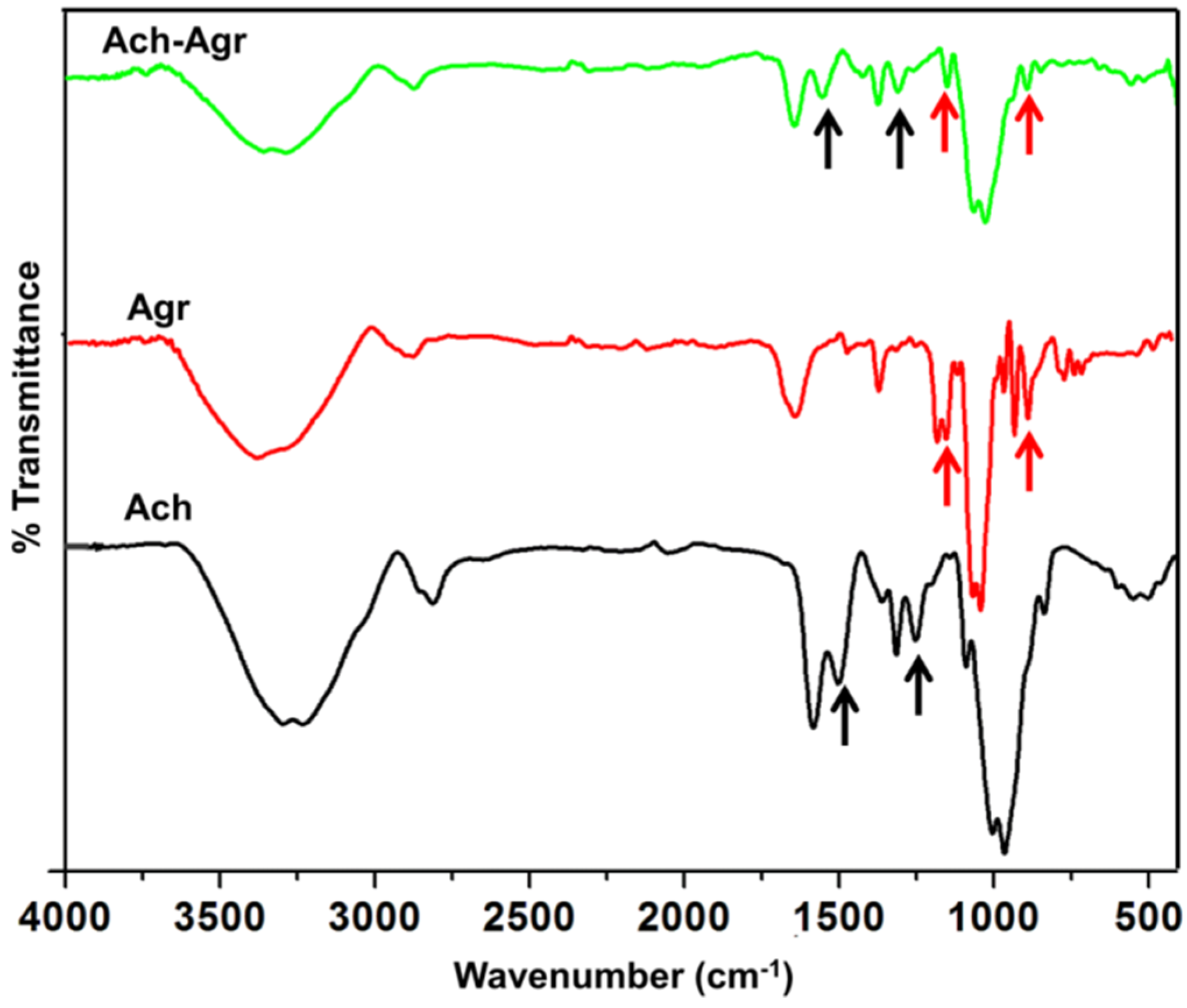
2.2. Characterization of ACh-Agr Gel
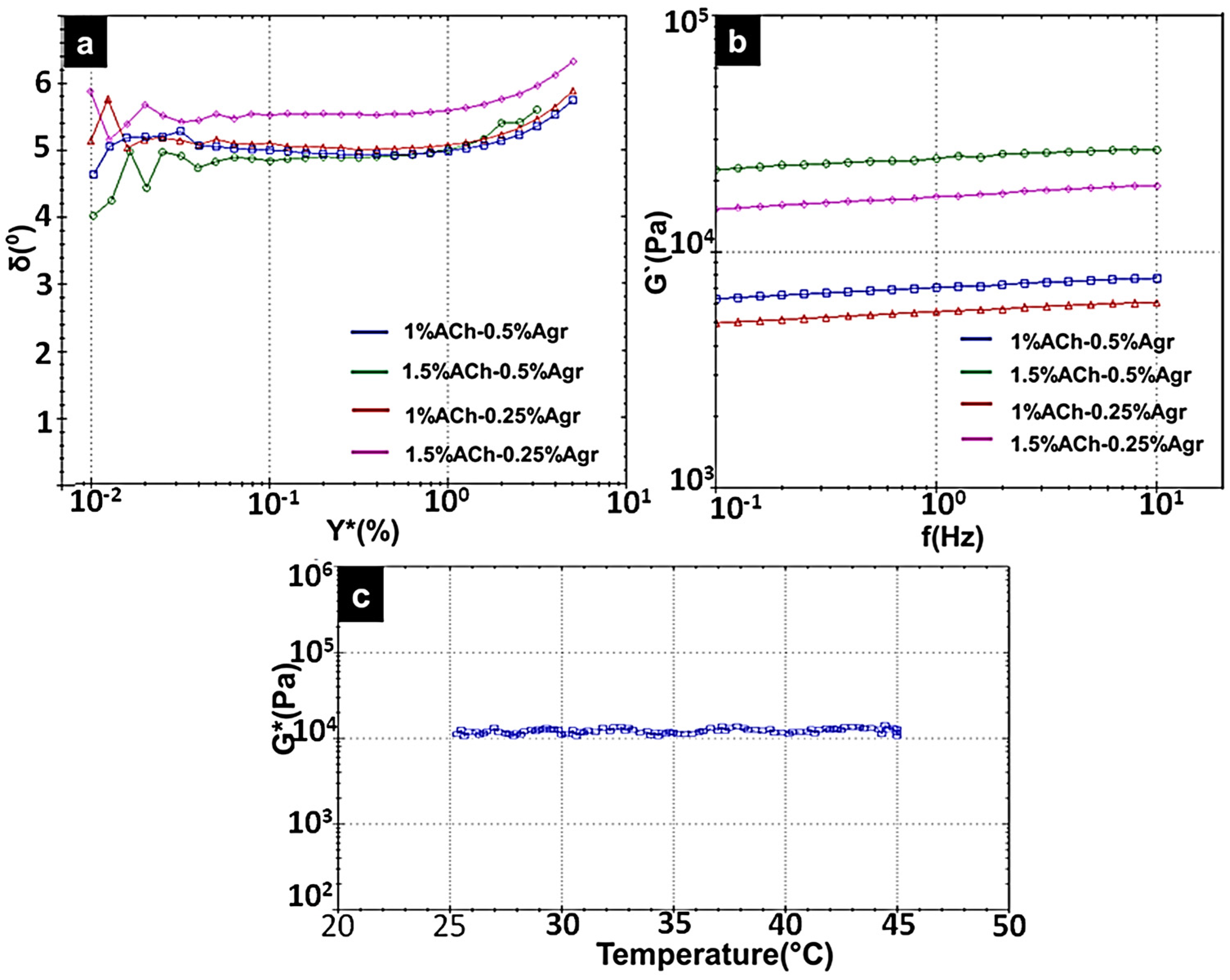
2.3. Rheological Analysis of Composite Gels

| Amorphous Chitin (%) | Agarose (%) | G′ (kPa) | Sigma Prime (Pa) | Complex Shear Strain (%) | n | k (kPa) | Corr. Coeff |
|---|---|---|---|---|---|---|---|
| 1 | 0.25 | 5.6 | 0.981 × 103 | 40.27 | 0.04488 | 5.11 | 0.9985 |
| 1 | 0.5 | 7.1 | 1.3 × 103 | 42.33 | 0.04433 | 6.48 | 0.9983 |
| 1.5 | 0.25 | 17.3 | 3.8 × 103 | 62.54 | 0.05155 | 15.5 | 0.9981 |
| 1.5 | 0.5 | 25 | 2.9 × 103 | 15.88 | 0.04389 | 22.9 | 0.9917 |

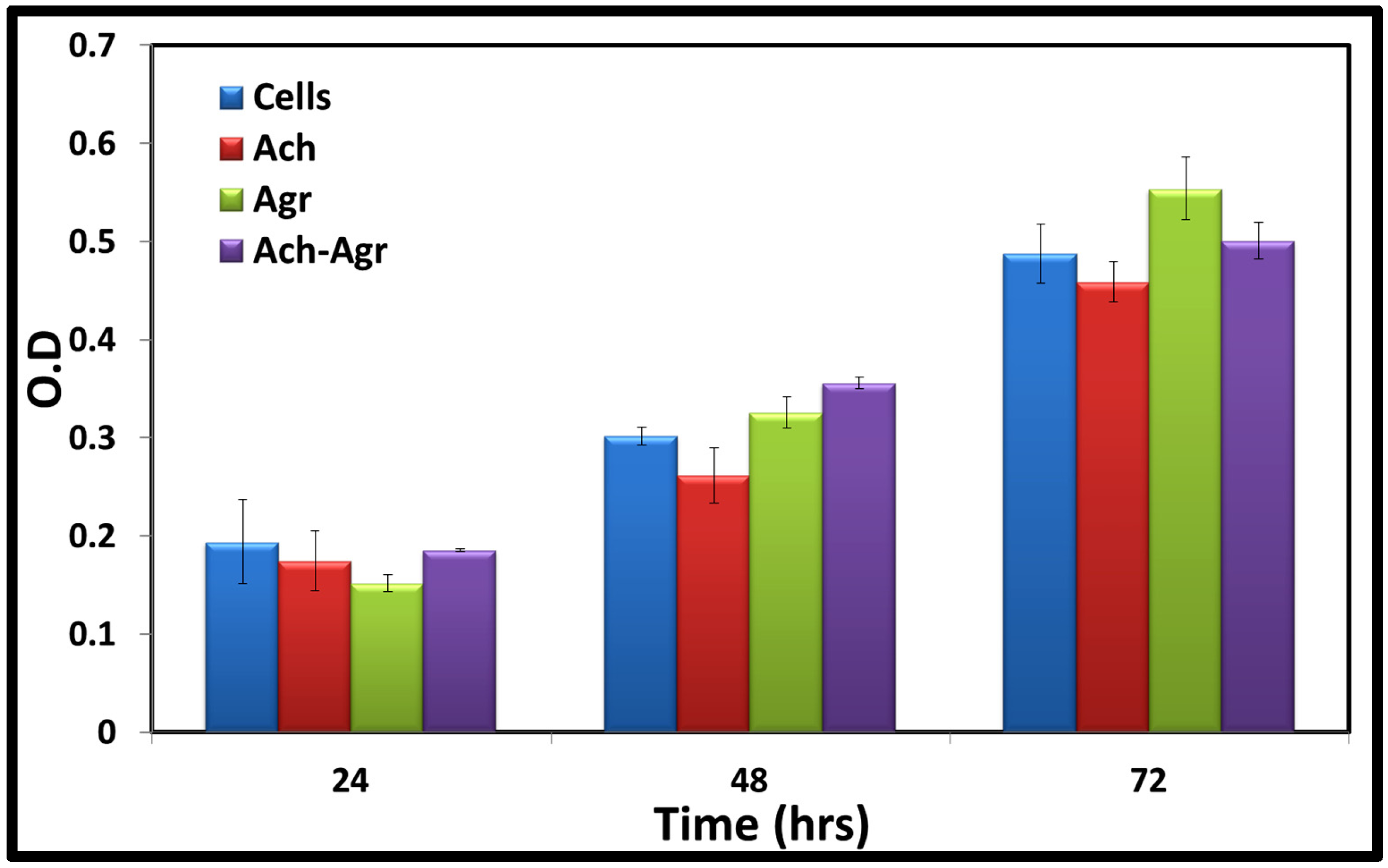
2.4. Cytocompatibility
3. Experimental Section
3.1. Materials
3.2. Preparation of ACh-Agr Composite Gel
3.3. Physicochemical Characterization of the Gel
3.4. Rheological Studies
3.4.1. Viscoelastic Studies
3.4.2. Temperature Stability
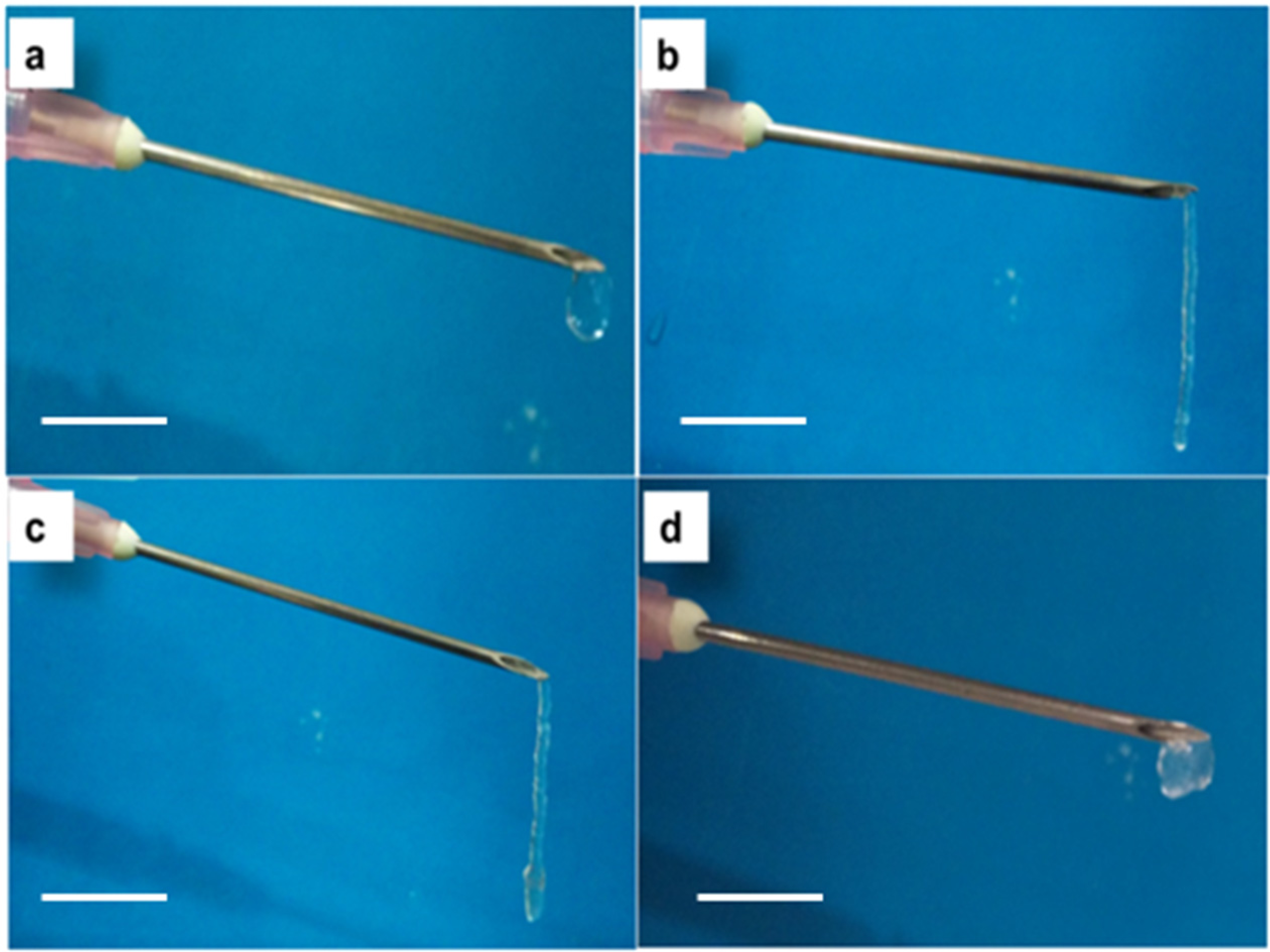
3.4.3. Injectability andInversion Study
3.5. Cytocompatibility
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jhon, M.S.; Andrade, J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Banerjee, R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef] [PubMed]
- Van, V.S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Tamura, H.; Furuike, T.; Nair, S.V.; Jayakumar, R. Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr. Polym. 2011, 84, 820–824. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Nair, A.; Sabitha, M.; Chennazhi, K.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, characterization and in vitro cytocompatibility studies of chitin nanogels for biomedical applications. Carbohydr. Polym. 2012, 87, 943–949. [Google Scholar] [CrossRef]
- Yang, T.L. Chitin-based materials in tissue engineering: Applications in soft tissue and epithelial organ. Int. J. Mol. Sci. 2011, 12, 1936–1963. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Cho, Y.N.; Chung, S.H.; Yoo, G.; Ko, S.W. Water-soluble chitin as a wound healing accelerator. Biomaterials. 1999, 20, 2139–2145. [Google Scholar] [CrossRef]
- Shimojoh, M.; Sakuma, K.; Ohzeki, Y.; Matsumura, Y.; Kurita, K. Preparation and characteristics of reprecipitated chitin: A new morphological form easy to manipulate with versatile utility. Polym. Bull. 2011, 67, 1435–1441. [Google Scholar] [CrossRef]
- Smitha, K.; Anitha, A.; Furuike, T.; Tamura, H.; Nair, S.V.; Jayakumar, R. In vitro evaluation of paclitaxel loaded amorphous chitin nanoparticles for colon cancer drug delivery. Colloids Surf. B 2013, 104, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.M.; Wu, C.X.; Huang, J.Y.; Peng, X.H.; Chen, P.; Tang, S.Q. Synthesis and characterization of a degradable composite agarose/HA hydrogel. Carbohydr. Polym. 2012, 88, 1445–1452. [Google Scholar] [CrossRef]
- Lin, P.W.; Wu, C.C.; Chen, C.H.; Ho, H.O.; Chen, Y.C.; Sheu, M.T. Characterization of cortical neuron outgrowth in two-and three-dimensional culture systems. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.V.; Velasco, D.; Hernandez, R.; Mijangos, C.; Kumacheva, E. Chitosan/agarose hydrogels: Cooperative properties and microfluidic preparation. Carbohydr. Polym. 2014, 111, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Gilbert, R.J.; He, W. Simple agarose-chitosan gel composite system for enhanced neuronal growth in three dimensions. Biomacromolecules 2009, 10, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan-agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Arnott, S.; Fulmer, A.; Scott, W.; Dea, I.; Moorhouse, R.; Rees, D. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.; Lakshmanan, V.K.; Anilkumar, T.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.; Jayakumar, R.; Nair, S.V. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Interface Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Rehage, H.; Hoffmann, H. Viscoelastic surfactant solutions: Model systems for rheological research. Mol. Phys. 1991, 74, 933–973. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Cipriano, B.H. Gel formation: Phase diagrams using tabletop rheology and calorimetry. In Molecualr Gels Materials with Self-Accembled Fibrillar Networks; Weiss, R.G., Terech, P., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 241–252. [Google Scholar]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priya, M.V.; Kumar, R.A.; Sivashanmugam, A.; Nair, S.V.; Jayakumar, R. Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications. J. Funct. Biomater. 2015, 6, 849-862. https://doi.org/10.3390/jfb6030849
Priya MV, Kumar RA, Sivashanmugam A, Nair SV, Jayakumar R. Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications. Journal of Functional Biomaterials. 2015; 6(3):849-862. https://doi.org/10.3390/jfb6030849
Chicago/Turabian StylePriya, Murali Vishnu, Rajendran Arun Kumar, Amirthalingam Sivashanmugam, Shantikumar Vasudevan Nair, and Rangasamy Jayakumar. 2015. "Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications" Journal of Functional Biomaterials 6, no. 3: 849-862. https://doi.org/10.3390/jfb6030849
APA StylePriya, M. V., Kumar, R. A., Sivashanmugam, A., Nair, S. V., & Jayakumar, R. (2015). Injectable Amorphous Chitin-Agarose Composite Hydrogels for Biomedical Applications. Journal of Functional Biomaterials, 6(3), 849-862. https://doi.org/10.3390/jfb6030849







