Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes
Abstract
:1. Introduction
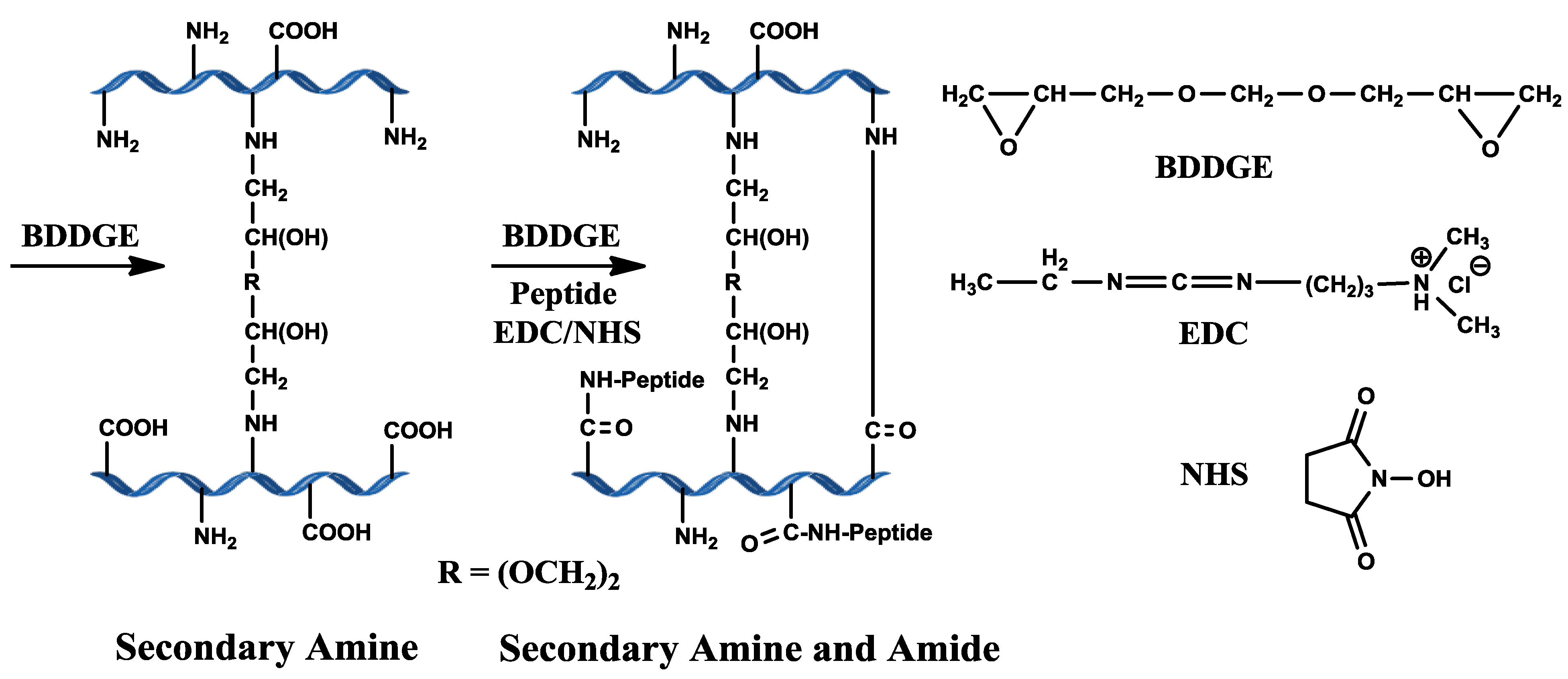
2. Results and Discussion
2.1. pH and BDDGE Cross-Linking
2.2. Properties of BDDGE-Collagen-Hydrogels
| Properties | Human cornea | Type 1 porcine collagen | ||
|---|---|---|---|---|
| Control EDC/NHS | BDDGE | BDDGE-EDC/NHS | ||
| pH | - | 5 | 11 | 5 |
| Optical properties | ||||
| Refractive index | 1.37–1.38 [24] | 1.35 | 1.35 | 1.35 |
| White light transmission (%) | >85 [25] | 82.1 (2.1%) | 86 (1.3%) | 86 (1.3%) |
| Backscatter (%) | 6.0-8.0 [25] | 2.8 (10.3%) | 1.9 (6%) | 0.4 (43%) |
| Mechanical properties | ||||
| Tensile strength (MPa) | 3.8 [26] | 0.19 (3%) | 0.21 (2.7%) | 0.44 (1.3%) * |
| Elongation at break (%) | - | 23.13 (2%) | 14.02 (0.7%) * | 147 (15.7%) * |
| Young’s modulus (MPa) | 3.0–13.0 [27] | 1.88 (6.4%) | 2.86 (1.6%) * | 2.69 (2.8%) * |
| Thermal stability | ||||
| Denaturation temperature (°C) | 65.1 [28] | 46.8 | 52.9 | 53.6 |
| Water content (%) | 80 [29] | 91 | 92 | 92 |
2.2.1. Physical and Optical Properties
2.2.2. Mechanical Properties of BDDGE-Collagen Hydrogels
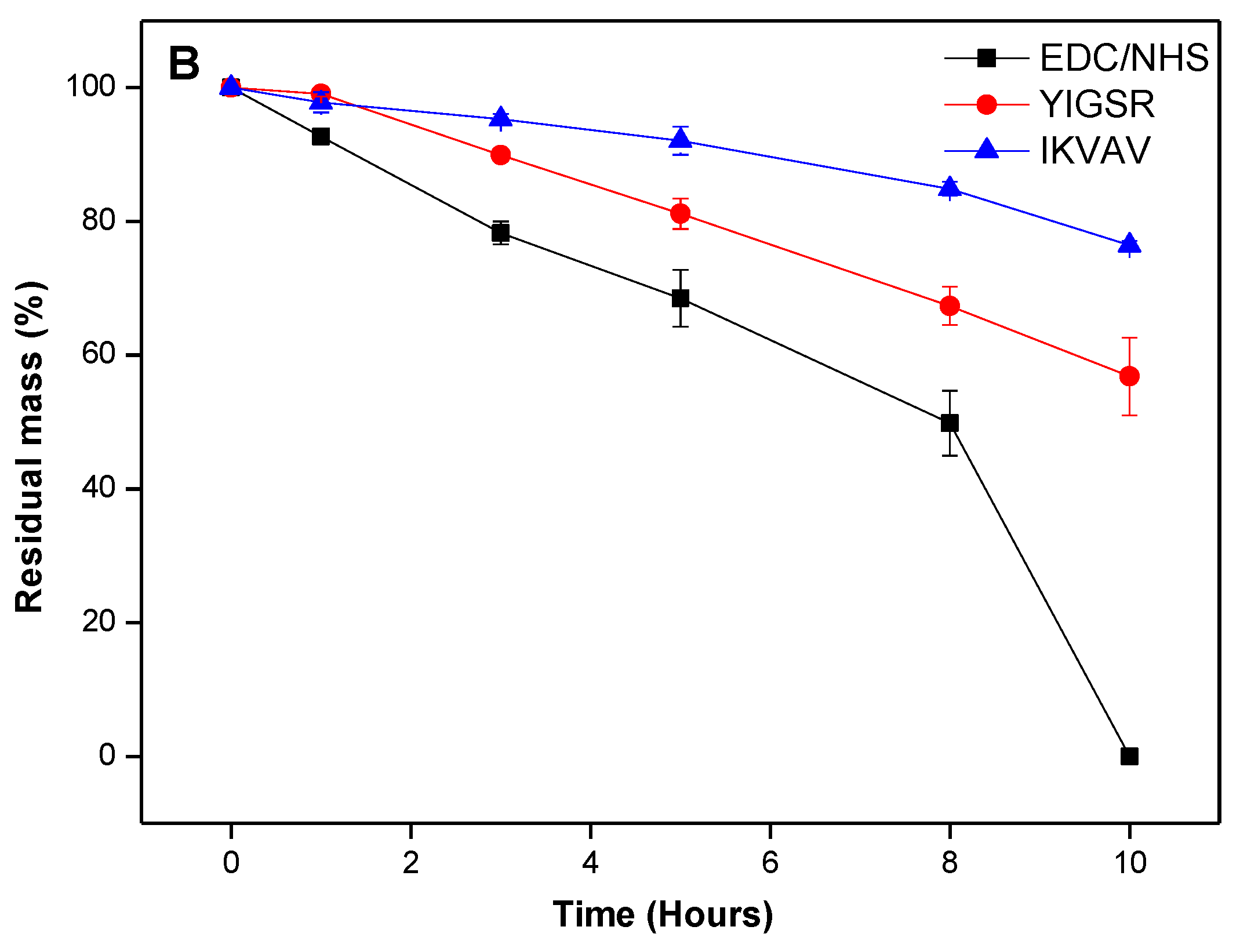
2.3. In Vitro Biological Stability

2.4. Femtosecond Laser-Assisted Cuts
2.5. Effects of addition of Laminin Peptides to BDDGE Cross-Linked Hydrogels
| Properties | Human cornea | Type 1 porcine collagen | ||||
|---|---|---|---|---|---|---|
| Control EDC/NHS | BDDGE | BDDGE-EDC/NHS | YIGSR | IKVAV | ||
| pH | - | 5 | 11 | 5 | 5 | 5 |
| Optical properties | ||||||
| White light transmission (%) | >85 [25] | 85.67 (0.8%) | 81.07 (0.7%) | 84.17 (0.8%) | 84.03 (1.3%) | 82.10 (0.8%) |
| Mechanical properties | ||||||
| Tensile strength (MPa) | 3.8 [26] | 0.12 (14.4%) | 0.10 (17.3%) | 0.16 (7.2%) | 0.13 (4.4%) | 0.17 (44%) |
| Elongation at break (%) | - | 44.52 (24.4%) | 16.63 (6.2%) * | 120.48 (9.3%) * | 23.81 (5.9%) | 44.76 (2.6%) |
| Young’s modulus (MPa) | 3.0-13.0 [27] | 0.64 (39.6%) | 1.21 (15.7%) * | 0.21 (8.2%) * | 0.98 (1.7%) | 0.55 (46%) |
| Thermal properties | ||||||
| Denaturation temperature (°C) | 65.1 [28] | 49.1 | 65.9 | 49.5 | 47.2 | 55.0 & 64.4 |
2.6. In Vitro Biocompatibility and Performance
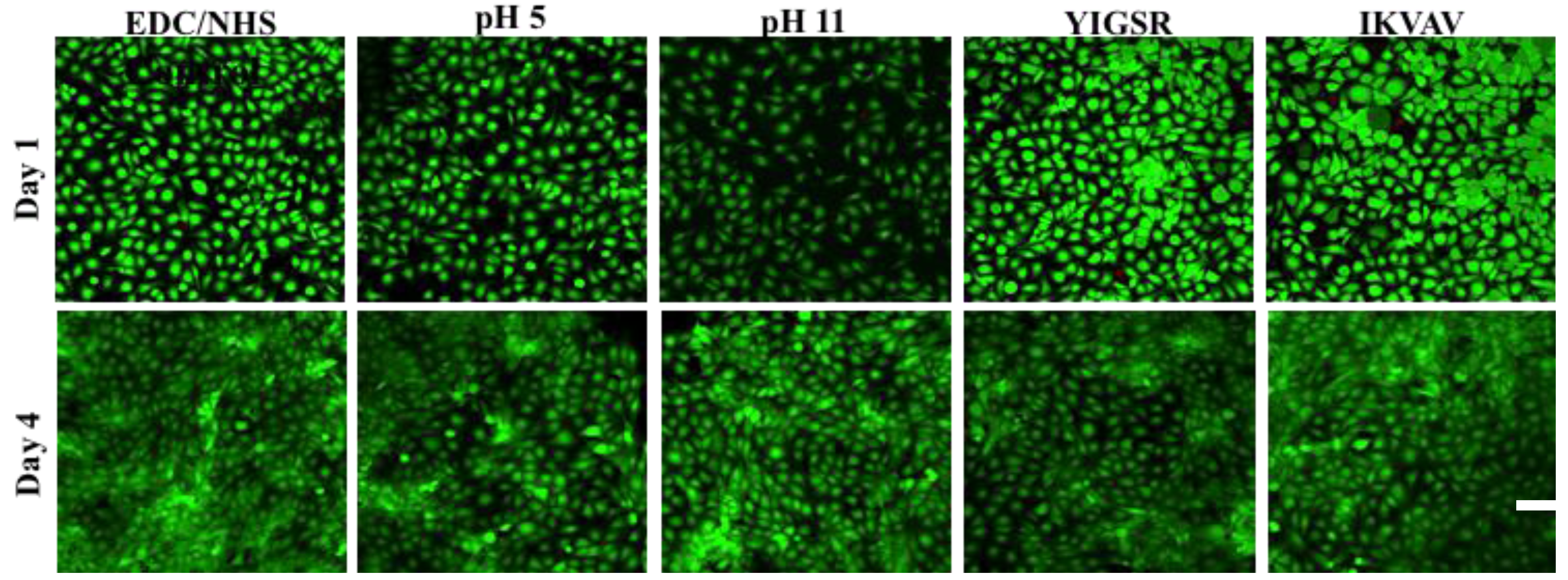
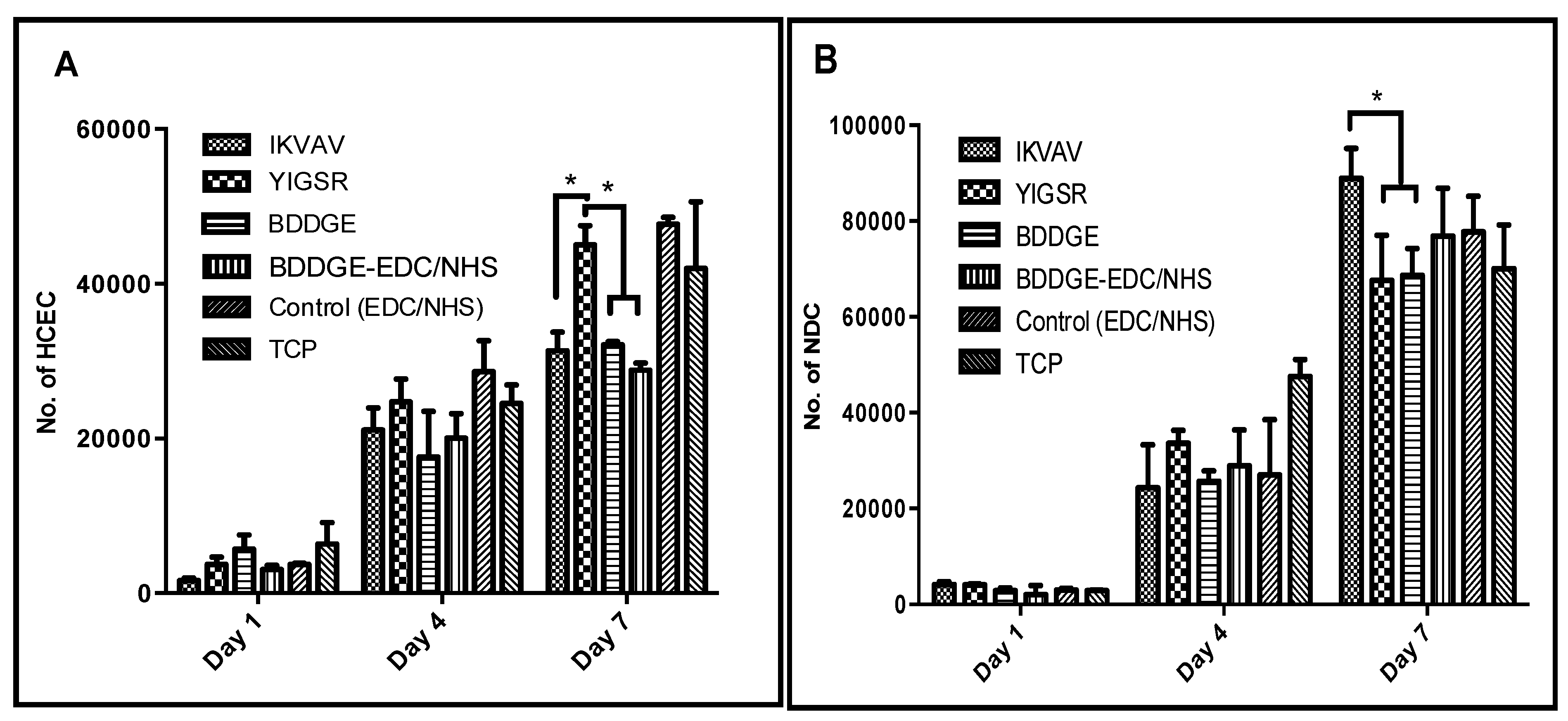
3. Experimental Section
3.1. Materials
3.2. Preparation of Collagen Cross-Linked Materials
3.3. Optical Property Measurements
3.4. Mechanical Property Measurements
3.5. Equilibrium Water Content Measurement
3.6. Thermal Properties: Differential Scanning Calorimetry (DSC)
3.7. In Vitro Degradation
3.8. Femtosecond Laser-Assisted Cuts of Epoxide and Carbodiimide Cross-linked Hydrogels: Feasibility
3.9. In Vitro Biocompatibility and Performance
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Heindl, L.M.; Riss, S.; Adler, W.; Bucher, F.; Hos, D.; Cursiefen, C. Split cornea transplantation: Relationship between storage time of split donor tissue and outcome. Ophthalmology 2013, in press. [Google Scholar]
- Du, Y.Q.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.X.; Kao, W.W.Y.; Funderburgh, J.L. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 2009, 27, 1635–1642. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Sinha Roy, A.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 13, 269–295. [Google Scholar] [CrossRef]
- Yang, C.L.; Hillas, P.J.; Baez, J.A.; Nokelainen, M.; Balan, J.; Tang, J.; Spiro, R.; Polarek, J.W. The application of recombinant human collagen in tissue engineering. Biodrugs 2004, 18, 103–119. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug. Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Merrett, K.; Jackson, W.B.; Munger, R.; Liu, Y.W.; Polarek, J.W.; Soderqvist, M.; Griffith, M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2010, 2. [Google Scholar] [CrossRef]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef]
- Nam, K.; Murakoshi, A.; Kimura, T.; Fujisato, T.; Kitamura, S.; Kishida, A. Study on the physical properties of tissue-engineered blood vessels made by chemical cross-linking and polymer-tissue cross-linking. J. Artif. Organs. 2009, 12, 47–54. [Google Scholar] [CrossRef]
- Zeeman, R. Cross-linking of Collagen-Based Materials. Ph.D. Dissertation, Universiteit Twente, Enschede, Netherlands, 1998. [Google Scholar]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.A.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials 1999, 20, 921–931. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.A.; Hendriks, M.; Cahalan, P.T.; Feijen, J. Crosslinking and modification of dermal sheep collagen using 1,4-butanediol diglycidyl ether. J. Biomed. Mater. Res. 1999, 46, 424–433. [Google Scholar] [CrossRef]
- Zeeman, R.; Dijkstra, P.J.; van Wachem, P.B.; van Luyn, M.J.A.; Hendriks, M.; Cahalan, P.T.; Feijen, J. The kinetics of 1,4-butanediol diglycidyl ether crosslinking of dermal sheep collagen. J. Biomed. Mater. Res. 2000, 51, 541–548. [Google Scholar] [CrossRef]
- La Gatta, A.; Schiraldi, C.; Papa, A.; D’Agostino, A.; Cammarota, M.; de Rosa, A.; de Rosa, M. Hyaluronan scaffolds via diglycidyl ether crosslinking: Toward improvements in composition and performance. Carbohyd. Polym. 2013, 96, 536–544. [Google Scholar] [CrossRef]
- Nicoletti, A.; Fiorini, M.; Paolillo, J.; Dolcini, L.; Sandri, M.; Pressato, D. Effects of different crosslinking conditions on the chemical-physical properties of a novel bio-inspired composite scaffold stabilised with 1,4-butanediol diglycidyl ether (BDDGE). J. Mater. Sci.-Mater. M. 2013, 24, 17–35. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Cannon, F.B.; Laurie, G.W.; Hassell, J.R.; Aumailley, M.; Terranova, V.P.; Martin, G.R.; Duboisdalcq, M. Biological activities of laminin. J. Cell. Biochem. 1985, 27, 317–325. [Google Scholar] [CrossRef]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin—A glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar]
- Mecham, R.P. Laminin receptors. Annu. Rev. Cell. Biol. 1991, 7, 71–91. [Google Scholar] [CrossRef]
- Li, F.; Griffith, M.; Li, Z.; Tanodekaew, S.; Sheardown, H.; Hakim, M.; Carlsson, D.J. Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials 2005, 26, 3093–3104. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yeh, M.-H.; Lai, H.-M. Preparation of a Biodegradable Thermal-Sensitive Gel System. U.S. Patent 20040013733, 30 December 2002. [Google Scholar]
- Kamal, A.; Ramu, R.; Azhar, M.A.; Khanna, G.B.R. Copper(II) tetrafluoroborate-catalyzed ring-opening of epoxides by amines. Tetrahedron Lett. 2005, 46, 2675–2677. [Google Scholar] [CrossRef]
- André, I.; Linse, S.; Mulder, F.A.A. Residue-specific pKa determination of lysine and arginine side chains by indirect 15N and 13C NMR spectroscopy: Application to apo calmodulin. J. Am. Chem. Soc. 2007, 129, 15805–15813. [Google Scholar] [CrossRef]
- Patel, S.; Marshall, J.; Fitzke, F.W. Refractive index of the human corneal epithelium and stroma. J. Refract. Surg. 1995, 11, 100–105. [Google Scholar]
- Liu, Y.; Griffith, M.; Watsky, M.A.; Forrester, J.V.; Kuffova, L.; Grant, D.; Merrett, K.; Carlsson, D.J. Properties of porcine and recombinant human collagen matrices for optically clear tissue engineering applications. Biomacromolecules 2006, 7, 1819–1828. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Yang, J.; Huang, K.; Lee, Z.H.; Lee, X.Y. A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 2001, 34, 533–537. [Google Scholar] [CrossRef]
- Crabb, R.A.B.; Chau, E.P.; Evans, M.C.; Barocas, V.H.; Hubel, A. Biomechanical and microstructural characteristics of a collagen film-based corneal stroma equivalent. Tissue Eng. 2006, 12, 1565–1575. [Google Scholar] [CrossRef]
- Merrett, K.; Fagerholm, P.; McLaughlin, C.R.; Dravida, S.; Lagali, N.; Shinozaki, N.; Watsky, M.A.; Munger, R.; Kato, Y.; Li, F.F.; et al. Tissue-engineered recombinant human collagen-based corneal substitutes for implantation: Performance of type I versus type III collagen. Invest. Ophth. Vis. Sci. 2008, 49, 3887–3894. [Google Scholar] [CrossRef]
- Tighe, B. Eye contact. Chem. Brit. 1992, 28, 241–244. [Google Scholar]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Liu, Y.W.; Gan, L.H.; Carlsson, D.J.; Fagerholm, P.; Lagali, N.; Watsky, M.A.; Munger, R.; Hodge, W.G.; Priest, D.; Griffith, M. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest. Ophth. Vis. Sci. 2006, 47, 1869–1875. [Google Scholar] [CrossRef]
- Griffith, M.; Osborne, R.; Munger, R.; Xiong, X.J.; Doillon, C.J.; Laycock, N.L.C.; Hakim, M.; Song, Y.; Watsky, M.A. Functional human corneal equivalents constructed from cell lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef]
- Wood, J.N.; Bevan, S.J.; Coote, P.R.; Dunn, P.M.; Harmar, A.; Hogan, P.; Latchman, D.S.; Morrison, C.; Rougon, G.; Theveniau, M.; et al. Novel cell lines display properties of nociceptive sensory neurons. P. R. Soc. B Biol. Sci. 1990, 241, 187–194. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koh, L.B.; Islam, M.M.; Mitra, D.; Noel, C.W.; Merrett, K.; Odorcic, S.; Fagerholm, P.; Jackson, W.B.; Liedberg, B.; Phopase, J.; et al. Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes. J. Funct. Biomater. 2013, 4, 162-177. https://doi.org/10.3390/jfb4030162
Koh LB, Islam MM, Mitra D, Noel CW, Merrett K, Odorcic S, Fagerholm P, Jackson WB, Liedberg B, Phopase J, et al. Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes. Journal of Functional Biomaterials. 2013; 4(3):162-177. https://doi.org/10.3390/jfb4030162
Chicago/Turabian StyleKoh, Li Buay, Mohammad Mirazul Islam, Debbie Mitra, Christopher W. Noel, Kimberley Merrett, Silvia Odorcic, Per Fagerholm, William. Bruce Jackson, Bo Liedberg, Jaywant Phopase, and et al. 2013. "Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes" Journal of Functional Biomaterials 4, no. 3: 162-177. https://doi.org/10.3390/jfb4030162
APA StyleKoh, L. B., Islam, M. M., Mitra, D., Noel, C. W., Merrett, K., Odorcic, S., Fagerholm, P., Jackson, W. B., Liedberg, B., Phopase, J., & Griffith, M. (2013). Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes. Journal of Functional Biomaterials, 4(3), 162-177. https://doi.org/10.3390/jfb4030162





