Concentrations and Fractionation of Carbon, Iron, Sulfur, Nitrogen and Phosphorus in Mangrove Sediments Along an Intertidal Gradient (Semi-Arid Climate, New Caledonia)
Abstract
:1. Introduction
2. Material and methods
2.1. Study Site
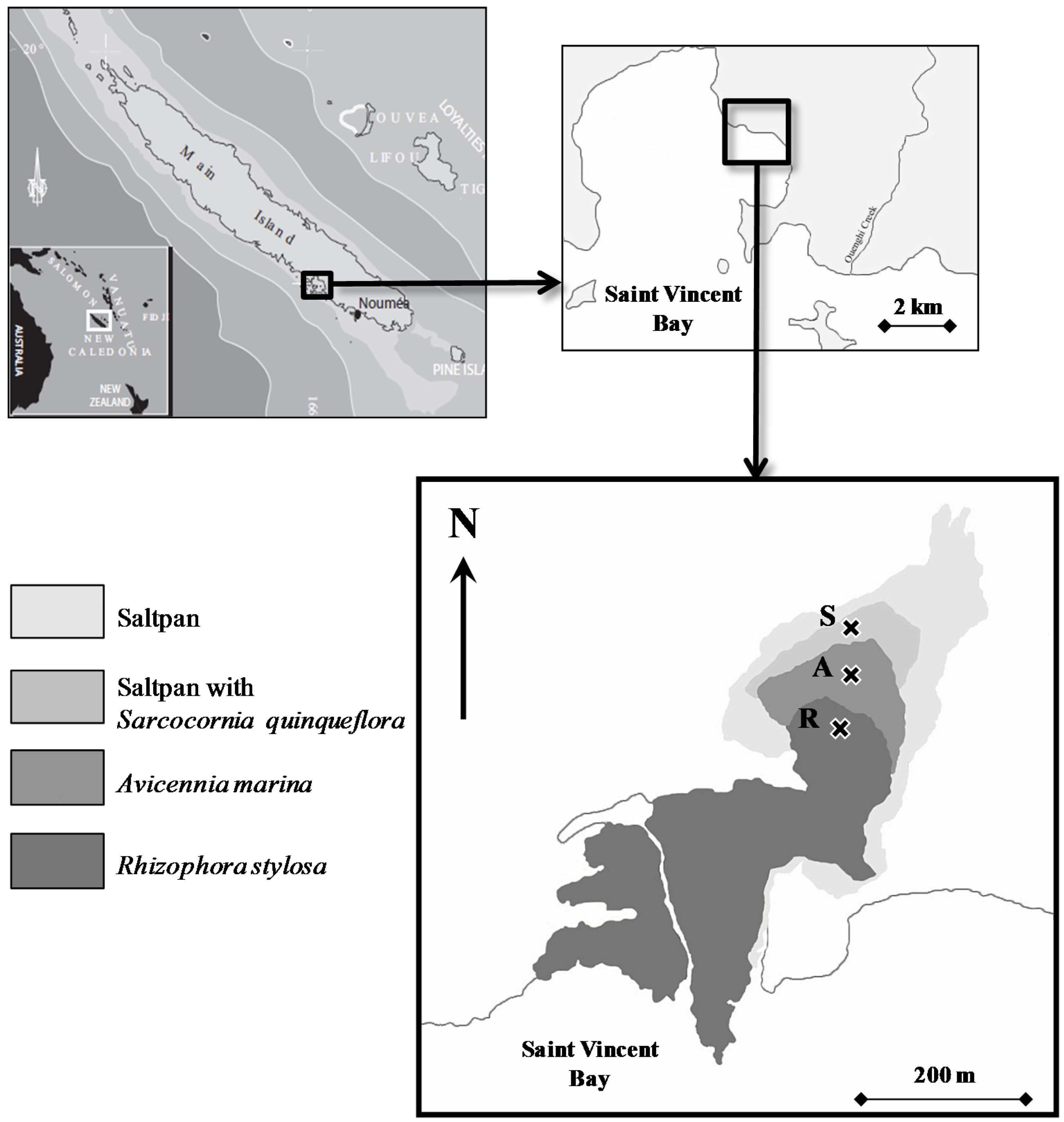
2.2. Sampling Collection
2.3. Analytical Methods
2.3.1. Salinity, pH, Redox Potential and ∑H2S Measurements
2.3.2. Pore-Water Analysis
2.3.3. Sediment Solid Phase Analysis
2.3.3.1. Total Organic Carbon, Total Sulfur
2.3.3.2. Stable Isotope Analysis
2.3.3.3. Particulate Iron and Phosphorus Extractions
3. Results and Discussion
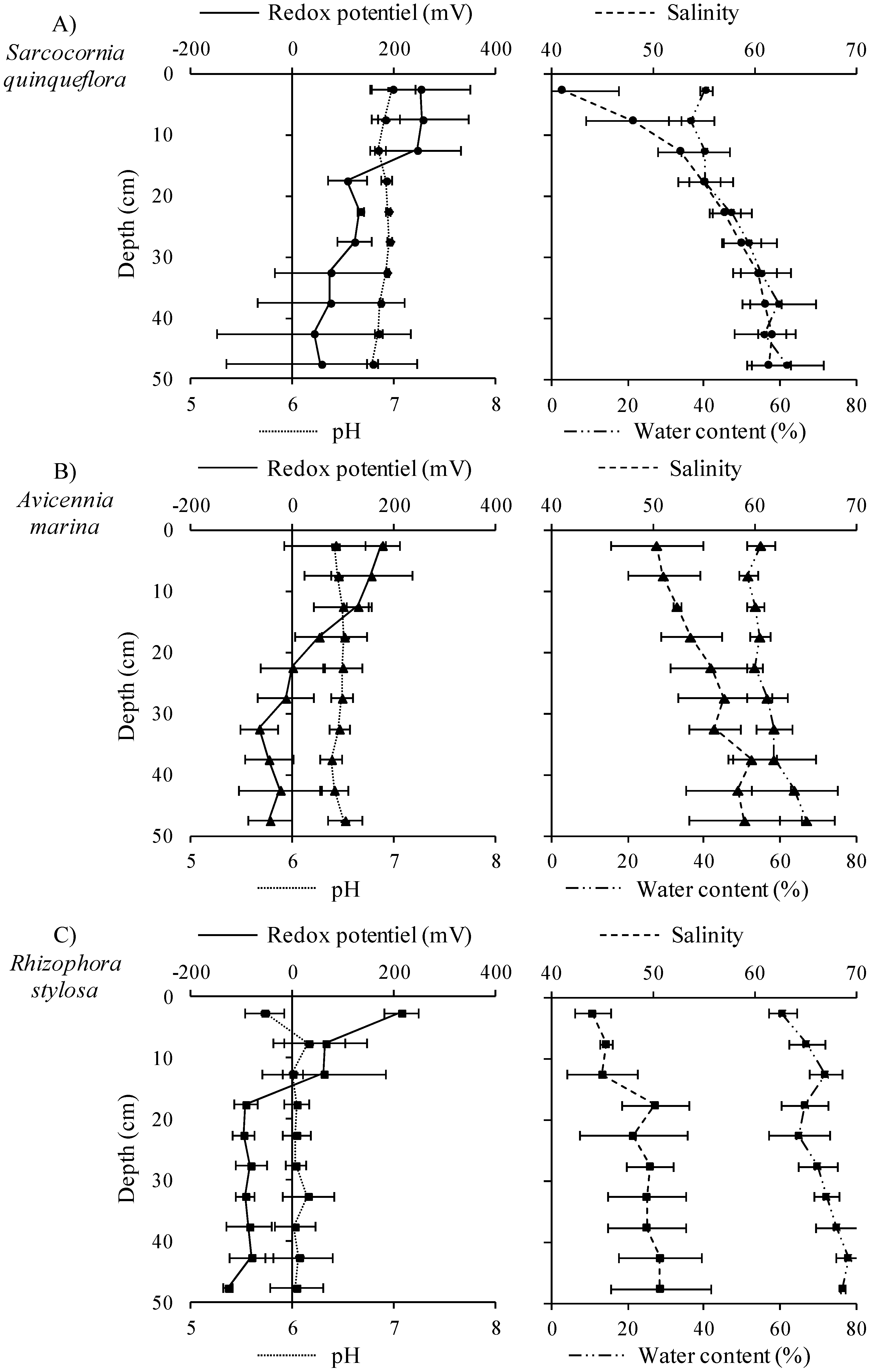
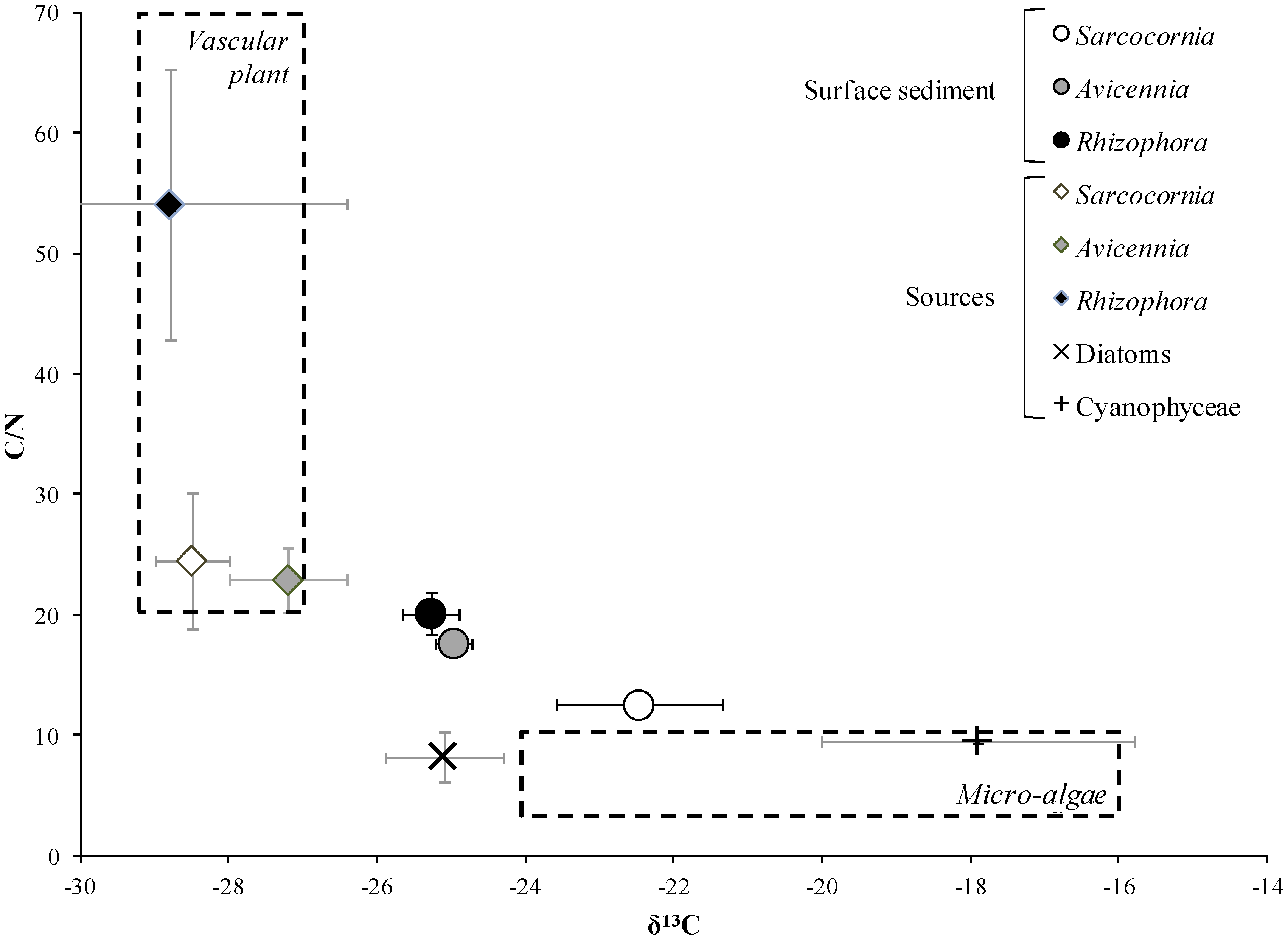
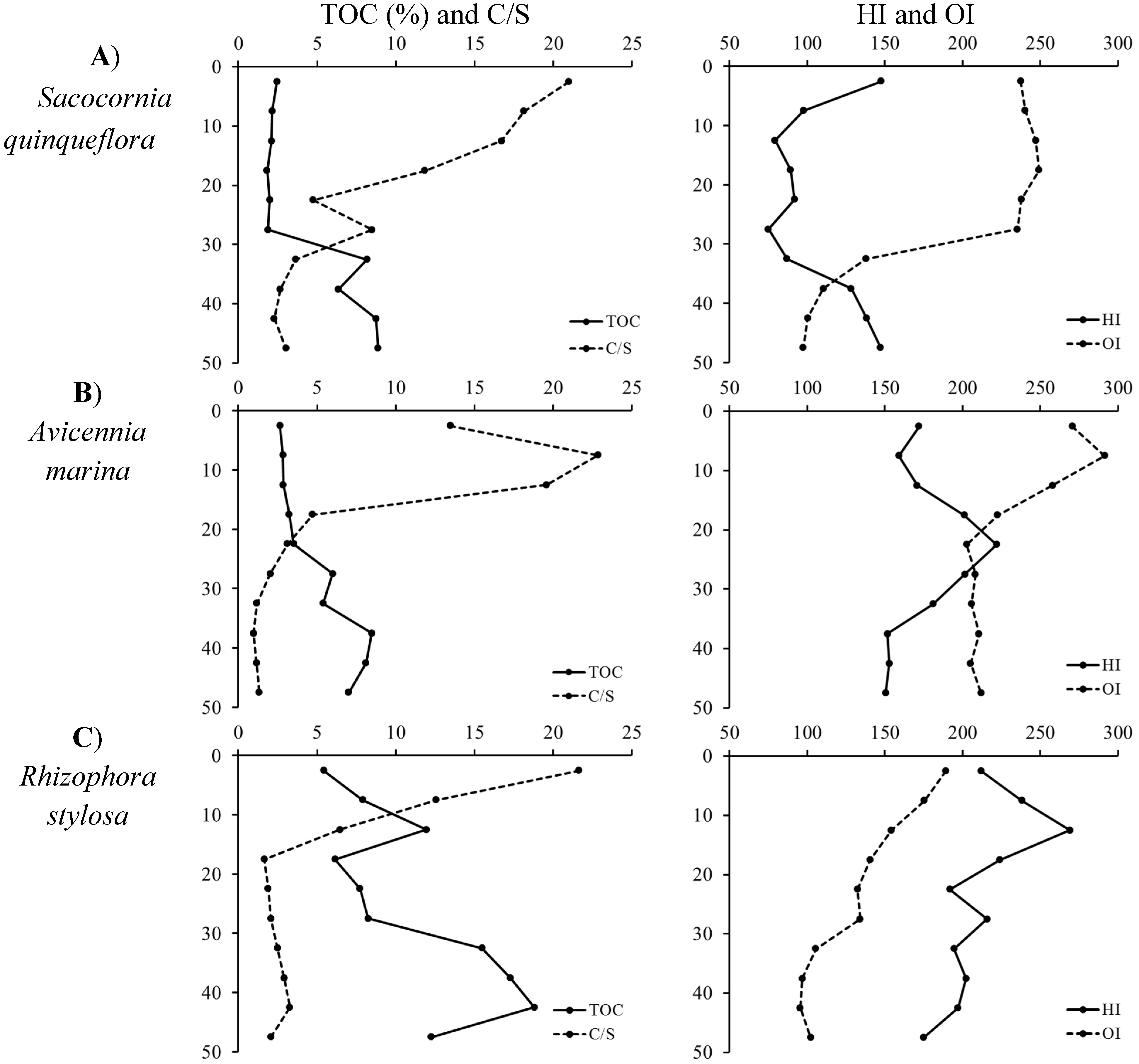
3.1. Influence of Mangrove Zonation on Sediment Physico-Chemical Properties and Organic Matter Content
| Depth (cm) | C/N | δ13C | δ15N | |||
|---|---|---|---|---|---|---|
| SD | SD | SD | ||||
| 0–5 | 12.4 | 1.5 | −23.3 | 1.1 | 2.8 | 0.8 |
| 5–10 | 14.7 | 1.1 | −24.1 | 0.2 | 2.3 | 0.1 |
| 15–20 | 16.4 | 2.1 | −23.9 | 0.2 | 2.0 | 0.5 |
| 30–35 | 29.9 | 8.8 | −25.2 | 1.1 | 1.8 | 0.4 |
| 40–45 | 41.7 | 12.5 | −26.1 | 0.6 | 1.7 | 0.2 |
3.2. Distribution and Speciation of Redox Sensitive Elements: Iron and Sulfur

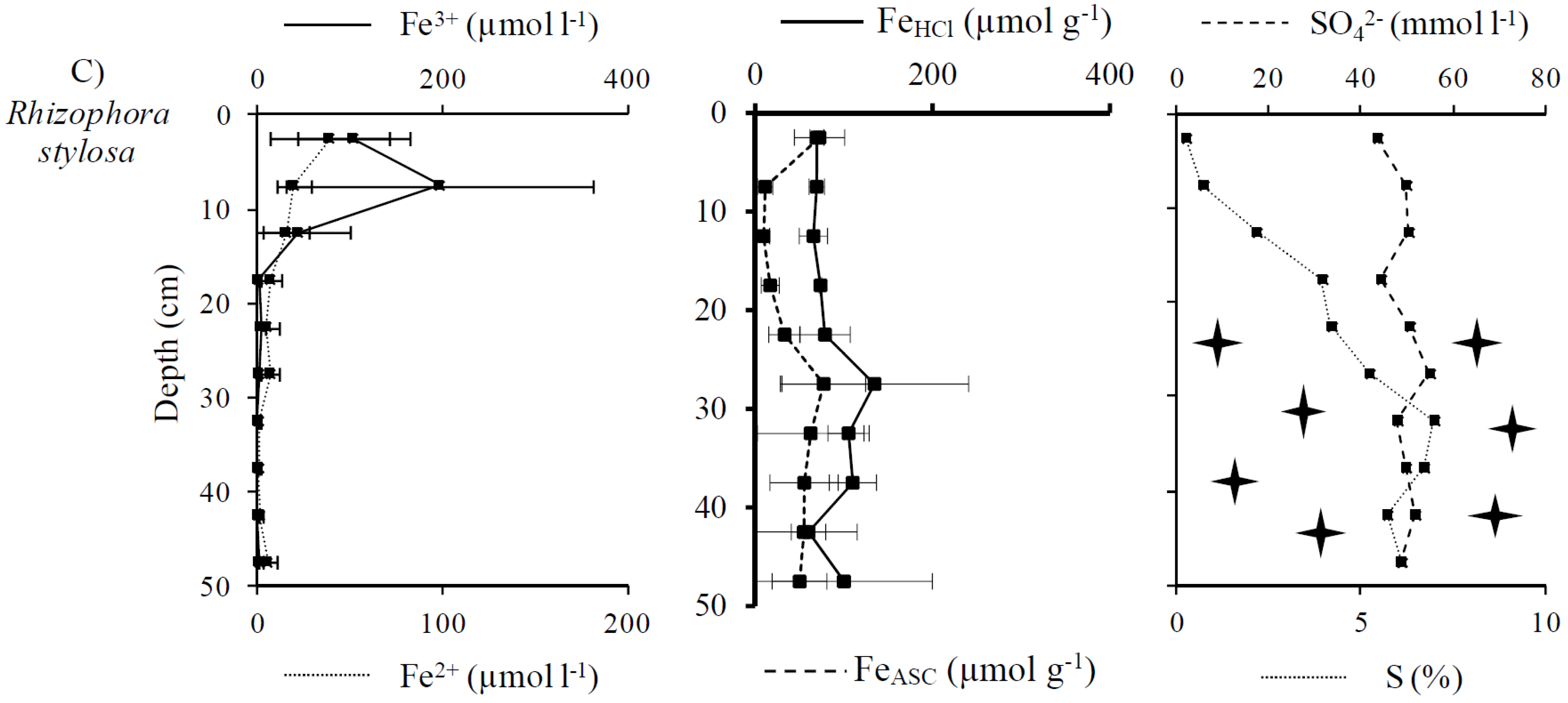
3.3. Distribution and Speciation of Limited Key Nutrients: Nitrogen and Phosphorus
3.3.1. Distribution and Speciation of Nitrogen Species along the Mangrove Zonation
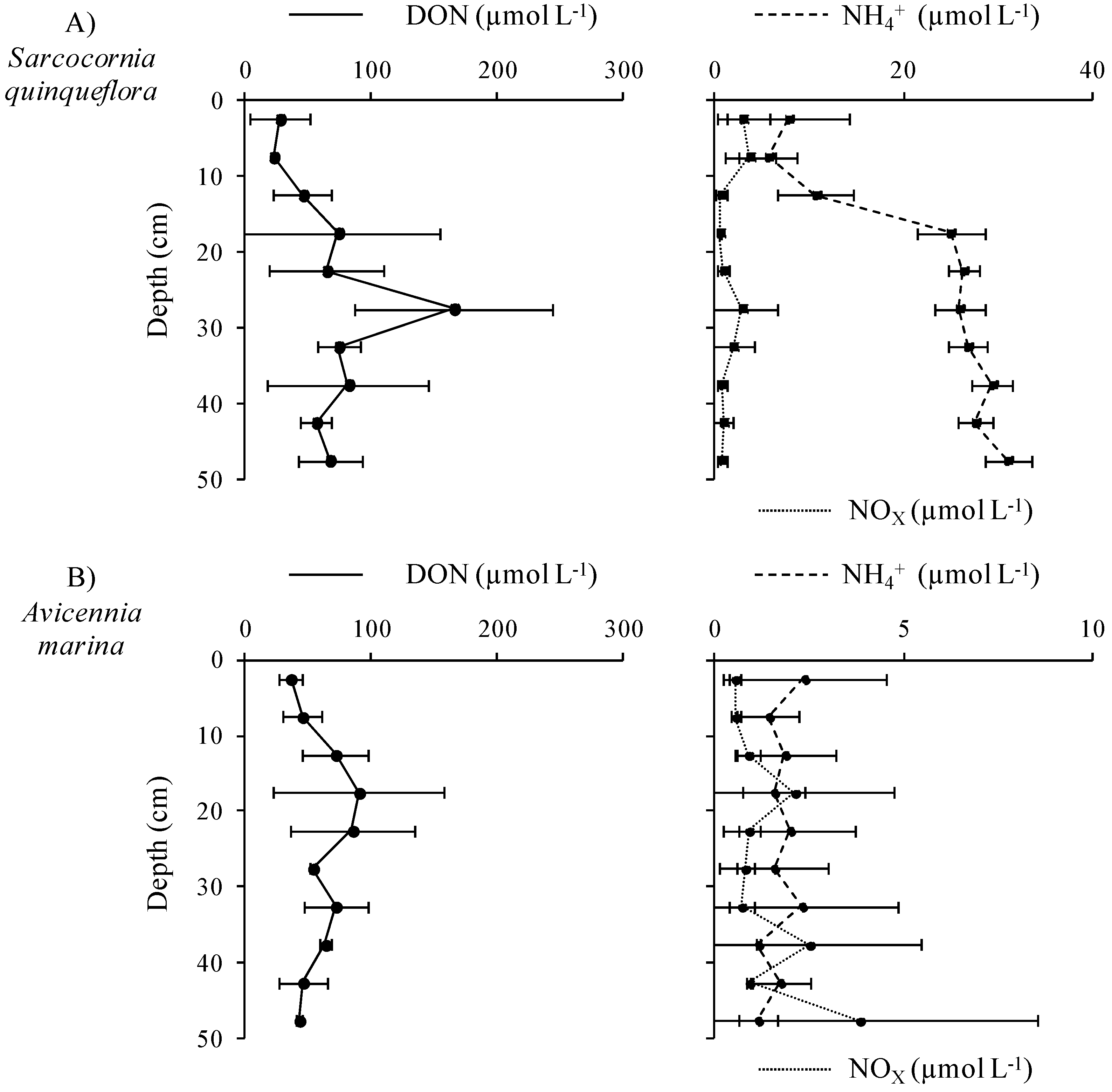
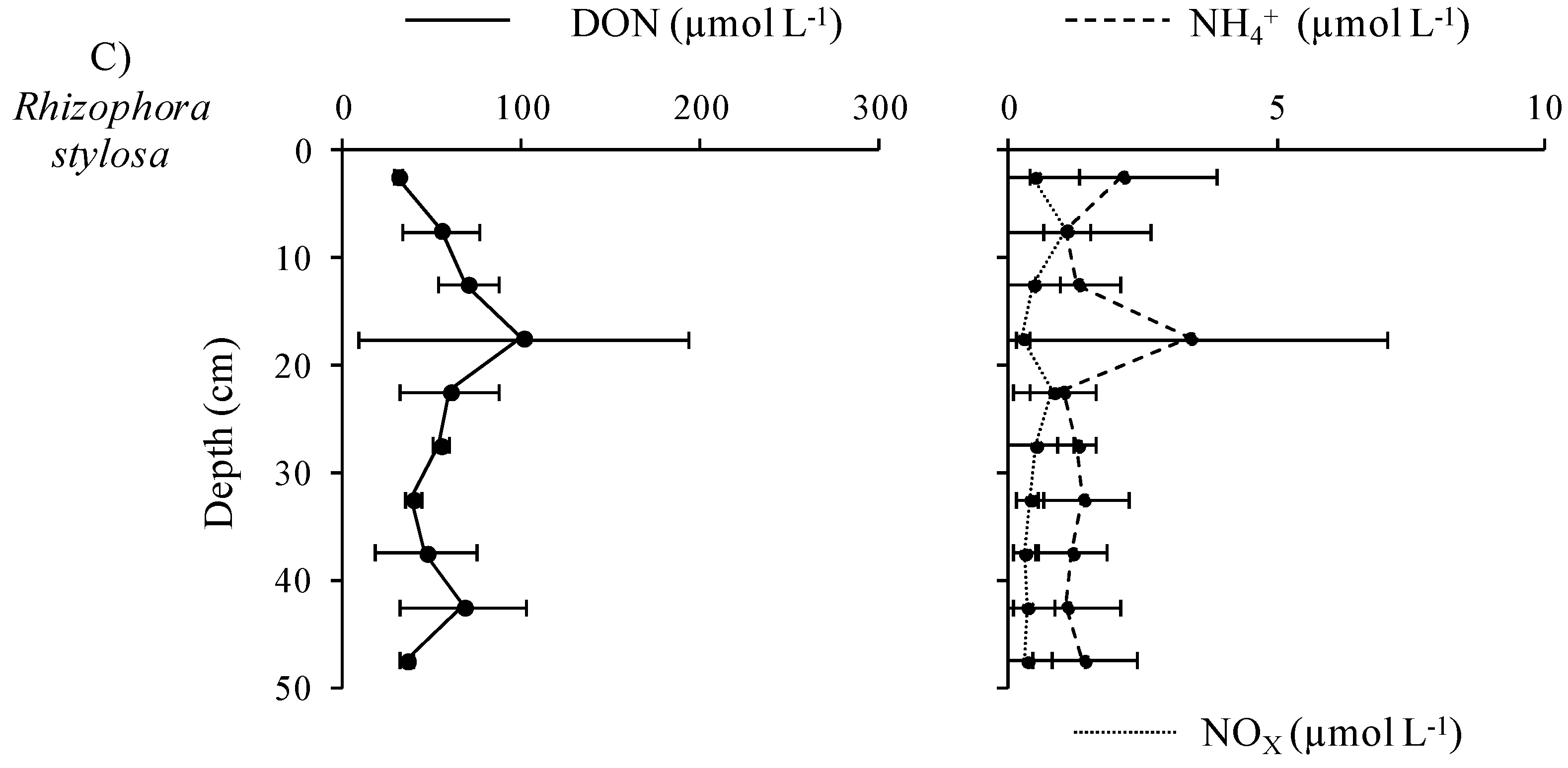
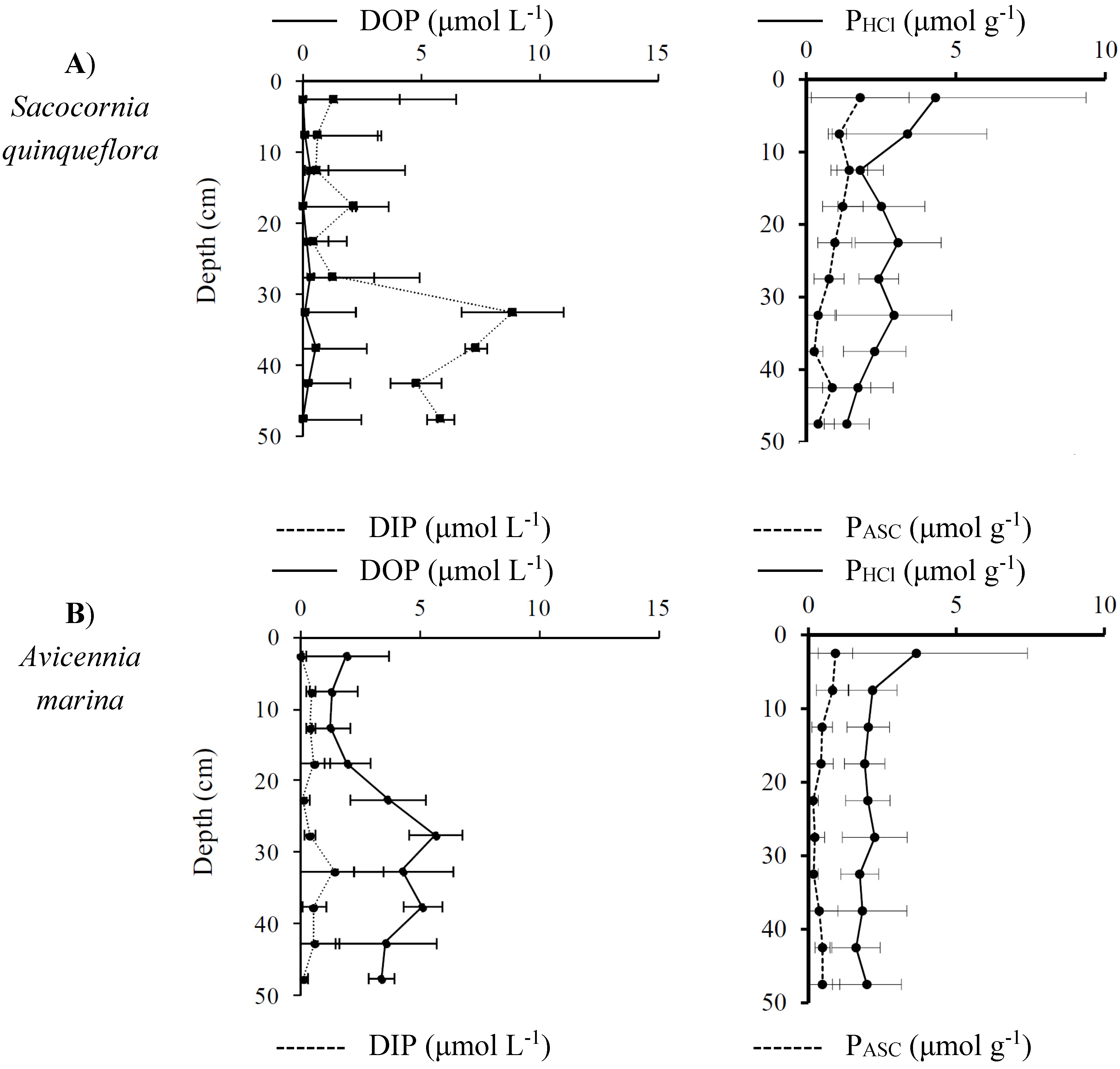

3.3.2. Distribution and Speciation of Phosphorus Species along the Mangrove Zonation
| Ratio | Fraction | Depth (cm) | Salt Flat | Avicennia Stand | Rhizophora Stand |
|---|---|---|---|---|---|
| Fe/P | Dissolved inorganic fraction | 0–15 | 4 | 94 | 44 |
| 15–50 | 9 | 20 | 19 | ||
| Particulate reactive fraction (Ascorbate) | 0–15 | 73 | 121 | 70 | |
| 15–50 | 79 | 147 | 191 | ||
| Particulate amorphous fraction (HCl) | 0–15 | 78 | 79 | 50 | |
| 15–50 | 71 | 77 | 70 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Duke, N.C.; Meynecke, J.-O.; Dittmann, S.; Ellison, A.M.; Anger, K.; Berger, U.; Cannicci, S.; Diele, K.; Ewel, K.C.; Field, C.D.; et al. A world without mangroves? Science 2007, 317, 41–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, E.B. Valuing ecosystem services as productive inputs. Econ. Policy 2007, 22, 177–229. [Google Scholar] [CrossRef]
- Walters, B.B. Mangrove forests and environmental security. In Forests in the Balance: Linking Tradition and Technology, XXII IUFRO World Congress; Innes, J.I., Edwards, I.K., Wilford, D.J., Eds.; International Foresty Review: Brisbane, Australia, 2006; Volume 7, p. 290. [Google Scholar]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangrove among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Alongi, D.M.; Clough, B.F.; Robertson, A.I. Nutrient-use efficiency in arid-zone forests of the mangroves rhizophora stylosa and avicennia marina. Aquat. Bot. 2005, 82, 121–131. [Google Scholar] [CrossRef]
- Alongi, D.M.; Tirendi, F.; Clough, B.F. Below-ground decomposition of organic matter in forests of the mangrove rhizophora stylosa and avicennia marina along the arid coast of western australia. Aquat. Bot. 2000, 68, 97–122. [Google Scholar] [CrossRef]
- Marchand, C.; Baltzer, F.; Lallier-Vergès, E.; Albéric, P. Pore-water chemistry in mangrove sediments: Relationship with species composition and developmental stages (French Guiana). Mar. Geol. 2004, 208, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, T.O.; Otero, X.L.; Vidal-Torrado, P.; Macías, F. Effects of bioturbation by root and crab activity on iron and sulfur biogeochemistry in mangrove substrate. Geoderma 2007, 142, 36–46. [Google Scholar] [CrossRef]
- McKee, K.L. Soil physicochemical patterns and mangrove species distribution—Reciprocal effects? J. Ecol. 1993, 81, 477–487. [Google Scholar] [CrossRef]
- Walsh, G.E. Mangroves: A review. In Ecology of Halophytes; Queen, R.J.R.H., Ed.; Academic Press: Waltham, MA, USA, 1974; pp. 51–174. [Google Scholar]
- Ellison, J.C. Impacts of sediment burial on mangroves. Mar. Pollut. Bull. 1999, 37, 420–426. [Google Scholar] [CrossRef]
- Clark, M.W.; McConchie, D.; Lewis, D.W.; Saenger, P. Redox stratification and heavy metal partitioning in avicennia-dominated mangrove sediments: A geochemical model. Chem. Geol. 1998, 149, 147–171. [Google Scholar] [CrossRef]
- Virly, S. Atlas des mangroves de nouvelle-calédonie. In Typologies et Biodiversité des Mangroves de Nouvelle-Calédonie; ZoNéCo: Nouméa, New Caledonia, 2006; p. 213. [Google Scholar]
- Marchand, C.; Allenbach, M.; Lallier-Vergès, E. Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (conception bay, New Caledonia). Geoderma 2011, 160, 444–456. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.; Fernandez, J.M.; Moreton, B.; Landi, L.; Lallier-Vergès, E.; Baltzer, F. The partitioning of transitional metals (Fe, Mn, Ni, Cr) in mangrove sediments downstream of a ferralitized ultramafic watershed (New Caledonia). Chem. Geol. 2012, 300–301, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Baltzer, F. Géodynamique de la sédimentation et diagenèse précoce en domaine ultrabasique—Nouvelle calédonie. Trav. Doc. ORSTOM 1982, 152, 283. [Google Scholar]
- Song, J.; Luo, Y.M.; Zhao, Q.G.; Christie, P. Novel use of soil moisture samplers for studies on anaerobic ammonium fluxes across lake sediment-water interfaces. Chemosphere 2003, 50, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F.; Albéric, P.; Cossa, D.; Baillif, P. Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Mar. Chem. 2006, 98, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Vismann, B. Sulphide exposure experiments: The sulphide electrode and a set-up automatically controlling sulphide, oxygen and pH. J. Exp. Mar. Biol. Ecol. 1996, 204, 131–140. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal.Chim. Acta 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, Ontario, Canada, 1972; Volume 197. [Google Scholar]
- Rodier, J. L’analyse de L’eau, Eaux Naturelles, Eaux Résiduaires, Eau de Mer; Dunod: Paris, France, 1976; p. 364. [Google Scholar]
- Holmes, R.M.; Aminot, A.; Kérouel, R.; Hooker, B.A.; Peterson, B.J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Bendschneider, K.; Robinson, R.J. A new spectrophotometric method for the determination of nitrite in sea water. J. Mar. Res. 1952, 11, 87–96. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Raimbault, P.; Pouvesle, W.; Diaz, F.; Garcia, N.; Sempéré, R. Wet-oxidation and automated colorimetry for simultaneous determination of organic carbon, nitrogen and phosphorus dissolved in seawater. Mar. Chem. 1999, 66, 161–169. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Disnar, J.R.; Kéravis, D. Organic carbon sources and transformations in mangrove sediments: A rock-eval pyrolysis approach. Org. Geochem. 2008, 39, 408–421. [Google Scholar] [CrossRef] [Green Version]
- Lafargue, E.; Marquis, F.; Pillot, D. Rock-eval 6 applications in hydrocarbon exploration, production and soil contamination studies. Rev. Inst. Fr. Pét. 1998, 53, 421–437. [Google Scholar]
- Deborde, J.; Anschutz, P.; Chaillou, G.; Etcheber, H.; Commarieu, M.V.; Lecroart, P.; Abril, G. The dynamics of phosphorus in turbid estuarine systems: Example of the gironde estuary (France). Limnol. Oceanogr. 2007, 52, 862–872. [Google Scholar] [CrossRef]
- Anschutz, P.; Zhong, S.; Sundby, B.; Mucci, A.; Gobeil, C. Burial efficiency of phosphorus and the geochemistry of iron in continental margin sediments. Limnol. Oceanogr. 1998, 43, 53–64. [Google Scholar] [CrossRef]
- Kostka, J.E.; Luther, G.W., III. Partitioning and speciation of solid phase iron in saltmarsh sediments. Geochim. Cosmochim. Acta 1994, 58, 1701–1710. [Google Scholar] [CrossRef]
- Anschutz, P.; Chaillou, G.; Lecroart, P. Phosphorus diagenesis in sediment of the thau lagoon. Estuar. Coast. Shelf Sci. 2007, 72, 447–456. [Google Scholar] [CrossRef]
- Bouillon, S.; Dahdouh-Guebas, F.; Rao, A.V.V.S.; Koedam, N.; Dehairs, F. Sources of organic carbon in mangrove sediments: Variability and possible ecological implications. Hydrobiologia 2003, 495, 33–39. [Google Scholar] [CrossRef]
- Marchand, C.; Disnar, J.R.; Lallier-Vergès, E.; Lottier, N. Early diagenesis of carbohydrates and lignin in mangrove sediments subject to variable redox conditions (French Guiana). Geochim. Cosmochim. Acta 2005, 69, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Lallier-Vergès, E.; Marchand, C.; Disnar, J.R.; Lottier, N. Origin and diagenesis of lignin and carbohydrates in mangrove sediments of guadeloupe (French West Indies): Evidence for a two-step evolution of organic deposits. Chem. Geol. 2008, 255, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Botto, F.; Iribarne, O. Contrasting effects of two burrowing crabs (Chasmagnathus granulata and Uca uruguayensis) on sediment composition and transport in estuarine environments. Estuar. Coast. Shelf Sci. 2000, 51, 141–151. [Google Scholar] [CrossRef]
- Nielsen, T.; Andersen, F.Ø. Phosphorus dynamics during decomposition of mangrove (Rhizophora apiculata) leaves in sediments. J. Exp. Mar. Biol. Ecol. 2003, 293, 73–88. [Google Scholar] [CrossRef]
- Thibodeau, F.R.; Nickerson, N.H. Differential oxidation of mangrove substrate by Avicennia germinans and Rhizophora mangle. Am. J. Bot. 1986, 73, 512–516. [Google Scholar] [CrossRef]
- Dublet, G.; Juillot, F.; Morin, G.; Fritsch, E.; Fandeur, D.; Ona-Nguema, G.; Brown, G.E., Jr. Ni speciation in a new caledonian lateritic regolith: A quantitative x-ray absorption spectroscopy investigation. Geochim. Cosmochim. Acta 2012, 95, 119–133. [Google Scholar] [CrossRef]
- Fernandez, J.-M.; Ouillon, S.; Chevillon, C.; Douillet, P.; Fichez, R.; Gendre, R.L. A combined modelling and geochemical study of the fate of terrigenous inputs from mixed natural and mining sources in a coral reef lagoon (New Caledonia). Mar. Pollut. Bull. 2006, 52, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Noël, V.; Marchand, C.; Juillot, F.; Ona-Nguema, G.; Viollier, E.; Marakovic, G.; Olivi, L.; Delbes, L.; Gelebart, F.; Morin, G. Exafs analysis of iron cycling in mangrove sediments downstream a lateritized ultramafic watershed (Vavouto Bay, New Caledonia). Geochim. Cosmochim. Acta 2014, 136, 211–228. [Google Scholar] [CrossRef]
- Canfield, D.E.; Raiswell, R.; Bottrell, S.H. The reactivity of sedimentary iron minerals toward sulfide. Am. J. Sci. 1992, 292, 659–683. [Google Scholar] [CrossRef]
- Holmer, M.; Kristensen, E.; Banta, G.; Hansen, K.; Jensen, M.H.; Bussawarit, N. Biogeochemical cycling of sulfur and iron in sediments of a south-east asian mangrove, phuket island, thailand. Bioelectrochemistry 1994, 26, 145–161. [Google Scholar]
- Bouillon, S.; Borges, A.V.; Castañeda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 2008, 22, GB2013. [Google Scholar] [CrossRef]
- Alongi, D.M. The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J. Mar. Res. 1996, 54, 123–148. [Google Scholar] [CrossRef]
- Feller, I.C.; Lovelock, C.; Mckee, K.L. Nutrient addition differentially affects ecological processes of avicennia germinans in nitrogen versus phosphorus limited mangrove ecosystems. Ecosystems 2007, 10, 347–359. [Google Scholar] [CrossRef]
- Feller, I.C. Effects of nutient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecol. Monogr. 1995, 65, 477–505. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; McKee, K.L.; Engelbrecht, B.M.J.; MC., B. The effect of nutrient enrichment on growth, photosynthesis and hydraulic conductance of dwarf mangroves in Panama. Funct. Ecol. 2004, 18, 25–33. [Google Scholar] [CrossRef]
- Dangremond, E.M.; Feller, I.C. Functional traits and nutrient limitation in the rare mangrove pelliciera rhizophorae. Aquat. Bot. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Alongi, D.M.; Trott, L.A.; Wattayakorn, G.; Clough, B.F. Below ground nitrogen cycling in relation to net canopy production in mangrove forests of Southern Thailand. Mar. Biol. 2002, 140, 855–864. [Google Scholar] [CrossRef]
- Badr, E.-S.A.; Tappin, A.D.; Achterberg, E.P. Distributions and seasonal variability of dissolved organic nitrogen in two estuaries in sw england. Mar. Chem. 2008, 110, 153–164. [Google Scholar] [CrossRef]
- Molnar, N.; Welsh, D.T.; Marchand, C.; Deborde, J.; Meziane, T. Impacts of shrimp farm effluent on water quality, benthic metabolism and n-dynamics in a mangrove forest (New Caledonia). Estuar. Coast. Shelf Sci. 2013, 117, 12–21. [Google Scholar] [CrossRef]
- Canfield, D. Organic matter oxidation in marine sediments. In Interactions of C, N, P and S Biogeochemical Cycles and Global Change; Wollast, R., Mackenzie, F., Chou, L., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 1993; Volume 4, pp. 333–363. [Google Scholar]
- Kristensen, E.; Alongi, D.M. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron, and sulfur biogeochemistry in mangrove sediment. Limnol. Oceanogr. 2006, 51, 1557–1571. [Google Scholar] [CrossRef]
- Jordan, M.A.; Welsh, D.T.; Dunn, R.J.K.; Teasdale, P.R. Influence of trypaea australiensis population density on benthic metabolism and nitrogen dynamics in sandy estuarine sediment: A mesocosm simulation. J. Sea Res. 2009, 61, 144–152. [Google Scholar] [CrossRef]
- Clough, B.F. Primary productivity and growth of mangrove forests. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; American Geophysical Union: Washington, DC, USA, 1992; Volume Coastal and Estuarine Study No.41; pp. 225–250. [Google Scholar]
- Gleason, S.M.; Ewel, K.C.; Hue, N. Soil redox conditions and plant–soil relationships in a micronesian mangrove forest. Estuar. Coast. Shelf Sci. 2003, 56, 1065–1074. [Google Scholar] [CrossRef]
- Jensen, H.S.; Mortensen, P.B.; Andersen, F.Ø.; Rasmussen, E. Phosphorus cycling in coastal marine sediment, Aarhus Bay, Denmark. Mar. Ecol. Prog. Ser. 1995, 293, 49–58. [Google Scholar] [CrossRef]
- Cha, H.J.; Lee, C.B.; Kim, B.S.; Choi, M.S.; Ruttenberg, K.C. Early diagenetic redistribution and burial of phosphorus in the sediments of the southwestern East Sea (Japan Sea). Mar. Geol. 2005, 216, 127–143. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deborde, J.; Marchand, C.; Molnar, N.; Patrona, L.D.; Meziane, T. Concentrations and Fractionation of Carbon, Iron, Sulfur, Nitrogen and Phosphorus in Mangrove Sediments Along an Intertidal Gradient (Semi-Arid Climate, New Caledonia). J. Mar. Sci. Eng. 2015, 3, 52-72. https://doi.org/10.3390/jmse3010052
Deborde J, Marchand C, Molnar N, Patrona LD, Meziane T. Concentrations and Fractionation of Carbon, Iron, Sulfur, Nitrogen and Phosphorus in Mangrove Sediments Along an Intertidal Gradient (Semi-Arid Climate, New Caledonia). Journal of Marine Science and Engineering. 2015; 3(1):52-72. https://doi.org/10.3390/jmse3010052
Chicago/Turabian StyleDeborde, Jonathan, Cyril Marchand, Nathalie Molnar, Luc Della Patrona, and Tarik Meziane. 2015. "Concentrations and Fractionation of Carbon, Iron, Sulfur, Nitrogen and Phosphorus in Mangrove Sediments Along an Intertidal Gradient (Semi-Arid Climate, New Caledonia)" Journal of Marine Science and Engineering 3, no. 1: 52-72. https://doi.org/10.3390/jmse3010052





