Ghrelin Derangements in Idiopathic Dilated Cardiomyopathy: Impact of Myocardial Disease Duration and Left Ventricular Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.1.1. Patients
2.1.2. Histological Analyses
2.2. Biomarkers Assessment and Histological Analyses
2.2.1. Samples Processing
2.2.2. Measurement of Plasma Biomarkers Levels
2.3. Tissue Sampling and Immunohistochemistry Stains
2.4. Statistical Analysis
3. Results
3.1. Study Population: Comparison Controls vs. Patients with DCM
3.2. Ghrelin in Patients with DCM
3.3. Ghrelin and DCM Duration
3.4. Ghrelin and LVEF
3.5. Correlations Analyses
3.6. Predictors of Ghrelin Levels
3.7. Outcome Analyses
3.8. Histological Analysis
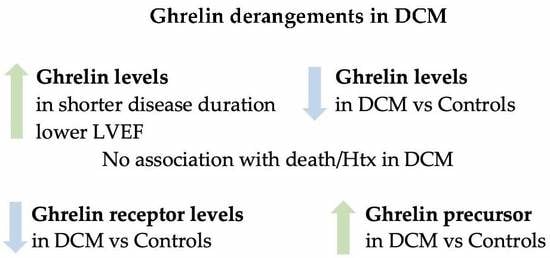
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [PubMed]
- Aleksova, A.; Sabbadini, G.; Merlo, M.; Pinamonti, B.; Barbati, G.; Zecchin, M.; Bussani, R.; Silvestri, F.; Iorio, A.M.; Stolfo, D.; et al. Natural history of dilated cardiomyopathy: From asymptomatic left ventricular dysfunction to heart failure—A subgroup analysis from the Trieste Cardiomyopathy Registry. J. Cardiovasc. Med. 2009, 10, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Pivetta, A.; Pinamonti, B.; Stolfo, D.; Zecchin, M.; Barbati, G.; Di Lenarda, A.; Sinagra, G. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: Changing mortality over the last 30 years. Eur. J. Heart Fail. 2014, 16, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.E.; Olsen, E.G.; Gatei, D. Dilated cardiomyopathy and myocarditis in Kenya: An endomyocardial biopsy study. Int. J. Cardiol. 1993, 41, 157–163. [Google Scholar] [CrossRef]
- Khatib, M.N.; Shankar, A.; Kirubakaran, R.; Agho, K.; Simkhada, P.; Gaidhane, S.; Saxena, D.; Bhaskaran, U.; Gode, D.; Gaidhane, A.; et al. Effect of ghrelin on mortality and cardiovascular outcomes in experimental rat and mice models of heart failure: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0126697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Baldanzi, G.; Filigheddu, N.; Cutrupi, S.; Catapano, F.; Bonissoni, S.; Fubini, A.; Malan, D.; Baj, G.; Granata, R.; Broglio, F.; et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002, 159, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Liu, J.; Liu, K.; Du, B.; Shi, K.; Ding, M.; Li, B.; Yang, P. Ghrelin suppresses cardiac fibrosis of post-myocardial infarction heart failure rats by adjusting the activin A-follistatin imbalance. Peptides 2018, 99, 27–35. [Google Scholar] [CrossRef]
- Penna, C.; Tullio, F.; Femmino, S.; Rocca, C.; Angelone, T.; Cerra, M.C.; Gallo, M.P.; Gesmundo, I.; Fanciulli, A.; Brizzi, M.F.; et al. Obestatin regulates cardiovascular function and promotes cardioprotection through the nitric oxide pathway. J Cell Mol. Med. 2017, 21, 3670–3678. [Google Scholar] [CrossRef]
- Angelino, E.; Reano, S.; Ferrara, M.; Agosti, E.; Graziani, A.; Filigheddu, N. Antifibrotic activity of acylated and unacylated ghrelin. Int. J. Endocrinol. 2015, 2015, 385682. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr. Opin. Pharm. 2003, 3, 146–151. [Google Scholar] [CrossRef]
- Du, C.K.; Zhan, D.Y.; Morimoto, S.; Akiyama, T.; Schwenke, D.O.; Hosoda, H.; Kangawa, K.; Shirai, M. Survival benefit of ghrelin in the heart failure due to dilated cardiomyopathy. Pharm. Res. Perspect. 2014, 2, e00064. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Merlo, M.; Barbati, G.; Di Lenarda, A.; Brun, F.; Pinamonti, B.; Gregori, D.; Mestroni, L.; Sinagra, G. Prognostic impact of familial screening in dilated cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, J.S. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching package for R. J. Stat. Softw. 2011, 42, 52. [Google Scholar] [CrossRef]
- Merlo, M.; Stolfo, D.; Anzini, M.; Negri, F.; Pinamonti, B.; Barbati, G.; Ramani, F.; Lenarda, A.D.; Sinagra, G. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: Does real healing exist? J. Am. Heart Assoc. 2015, 4, e001504. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Aleksova, A.; Armellini, I.; Cattin, M.R.; Zanetti, M.; Carriere, C.; Giacca, M.; Dore, F.; Guarnieri, G.; Sinagra, G. Adipokines, ghrelin and obesity-associated insulin resistance in nondiabetic patients with acute coronary syndrome. Obesity 2012, 20, 2348–2353. [Google Scholar] [CrossRef]
- Muccioli, G.; Broglio, F.; Valetto, M.R.; Ghe, C.; Catapano, F.; Graziani, A.; Papotti, M.; Bisi, G.; Deghenghi, R.; Ghigo, E. Growth hormone-releasing peptides and the cardiovascular system. Ann. Endocrinol. 2000, 61, 27–31. [Google Scholar]
- Alloatti, G.; Arnoletti, E.; Bassino, E.; Penna, C.; Perrelli, M.G.; Ghe, C.; Muccioli, G. Obestatin affords cardioprotection to the ischemic-reperfused isolated rat heart and inhibits apoptosis in cultures of similarly stressed cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H470–H481. [Google Scholar] [CrossRef]
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S.; et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Pineiro, R.; Blanco, M.; Gallego, R.; Dieguez, C.; Gualillo, O.; Gonzalez-Juanatey, J.R.; Lago, F. Growth hormone releasing peptide (ghrelin) is synthesized and secreted by cardiomyocytes. Cardiovasc. Res. 2004, 62, 481–488. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, X.W.; Zhang, A.Y.; Lv, J.C.; Zhang, J.G.; Zhao, C.H. Prognostic value of plasma ghrelin in predicting the outcome of patients with chronic heart failure. Arch. Med. Res. 2014, 45, 263–269. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Vila, G.; Grimm, G.; Resl, M.; Heinisch, B.; Einwallner, E.; Esterbauer, H.; Dieplinger, B.; Mueller, T.; Luger, A.; Clodi, M. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes 2012, 61, 2592–2596. [Google Scholar] [CrossRef]
- Muller, T.D.; Perez-Tilve, D.; Tong, J.; Pfluger, P.T.; Tschop, M.H. Ghrelin and its potential in the treatment of eating/wasting disorders and cachexia. J. Cachexia Sarcopenia Muscle 2010, 1, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Lund, L.H.; Williams, J.J.; Freda, P.; LaManca, J.J.; LeJemtel, T.H.; Mancini, D.M. Ghrelin resistance occurs in severe heart failure and resolves after heart transplantation. Eur. J. Heart Fail. 2009, 11, 789–794. [Google Scholar] [CrossRef]
- Nagaya, N.; Kojima, M.; Uematsu, M.; Yamagishi, M.; Hosoda, H.; Oya, H.; Hayashi, Y.; Kangawa, K. Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1483–R1487. [Google Scholar] [CrossRef] [Green Version]
- Nagaya, N.; Miyatake, K.; Uematsu, M.; Oya, H.; Shimizu, W.; Hosoda, H.; Kojima, M.; Nakanishi, N.; Mori, H.; Kangawa, K. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J. Clin. Endocrinol. Metab. 2001, 86, 5854–5859. [Google Scholar] [CrossRef]
- Mao, Y.; Tokudome, T.; Kishimoto, I. Ghrelin as a treatment for cardiovascular diseases. Hypertension 2014, 64, 450–454. [Google Scholar] [CrossRef]
- Imazio, M.; Bobbio, M.; Broglio, F.; Benso, A.; Podio, V.; Valetto, M.R.; Bisi, G.; Ghigo, E.; Trevi, G.P. GH-independent cardiotropic activities of hexarelin in patients with severe left ventricular dysfunction due to dilated and ischemic cardiomyopathy. Eur. J. Heart Fail. 2002, 4, 185–191. [Google Scholar] [CrossRef]
- Broglio, F.; Benso, A.; Valetto, M.R.; Gottero, C.; Quaranta, L.; Podio, V.; Arvat, E.; Bobbio, M.; Bisi, G.; Ghigo, E. Growth hormone-independent cardiotropic activities of growth hormone-releasing peptides in normal subjects, in patients with growth hormone deficiency, and in patients with idiopathic or ischemic dilated cardiomyopathy. Endocrine 2001, 14, 105–108. [Google Scholar] [CrossRef]
- Sullivan, R.; McGirr, R.; Hu, S.; Tan, A.; Wu, D.; Charron, C.; Lalonde, T.; Arany, E.; Chakrabarti, S.; Luyt, L.; et al. Changes in the Cardiac GHSR1a-Ghrelin System Correlate With Myocardial Dysfunction in Diabetic Cardiomyopathy in Mice. J. Endocr. Soc. 2018, 2, 178–189. [Google Scholar] [CrossRef]
- Beiras-Fernandez, A.; Kreth, S.; Weis, F.; Ledderose, C.; Pottinger, T.; Dieguez, C.; Beiras, A.; Reichart, B. Altered myocardial expression of ghrelin and its receptor (GHSR-1a) in patients with severe heart failure. Peptides 2010, 31, 2222–2228. [Google Scholar] [CrossRef]
- Sullivan, R.; Randhawa, V.K.; Stokes, A.; Wu, D.; Lalonde, T.; Kiaii, B.; Luyt, L.; Wisenberg, G.; Dhanvantari, S. Dynamics of the Ghrelin/Growth Hormone Secretagogue Receptor System in the Human Heart Before and after Cardiac Transplantation. J. Endocr. Soc. 2019, 3, 748–762. [Google Scholar] [CrossRef]
- Marleau, S.; Mulumba, M.; Lamontagne, D.; Ong, H. Cardiac and peripheral actions of growth hormone and its releasing peptides: Relevance for the treatment of cardiomyopathies. Cardiovasc. Res. 2006, 69, 26–35. [Google Scholar] [CrossRef]
- Laguens, R.; Alvarez, P.; Vigliano, C.; Cabeza Meckert, P.; Favaloro, L.; Diez, M.; Favaloro, R. Coronary microcirculation remodeling in patients with idiopathic dilated cardiomyopathy. Cardiology 2011, 119, 191–196. [Google Scholar] [CrossRef]
- Radu, R.I.; Bold, A.; Pop, O.T.; Malaescu, D.G.; Gheorghisor, I.; Mogoanta, L. Histological and immunohistochemical changes of the myocardium in dilated cardiomyopathy. Rom. J. Morphol. Embryol. 2012, 53, 269–275. [Google Scholar]
- Shiina, Y.; Murakami, T.; Matsumoto, N.; Okamura, D.; Takahashi, Y.; Nishihata, Y.; Komiyama, N.; Niwa, K. Body composition, appetite-related hormones, adipocytokines, and heart failure in adult patients with congenital heart disease: A preliminary study. Congenit. Heart Dis. 2018, 13, 79–84. [Google Scholar] [CrossRef]

| Healthy n = 200 | DCM n = 266 | p | |
|---|---|---|---|
| Age (years) | 52.4 (11.7) | 54.5 (14) | 0.074 |
| Male gender (%) | 80.8 | 77.7 | 0.4 |
| BMI (kg/m2) | 26.8 (5.15) | 26.7 (4.6) | 0.7 |
| Systolic blood pressure (mmHg) | 141.6 (16.6) | 119.9 (15.9) | <0.001 |
| Diastolic blood pressure (mmHg) | 84.8 (10.8) | 76 (12) | <0.001 |
| Total ghrelin (pg/mL) | 741 (481–1205.7) | 461 (293.7–798.7) | <0.001 |
| Acylated ghrelin (pg/mL) | 51.8 (35.5–127.1) | 21.7 (15.9–29.4) | <0.001 |
| Unacylated ghrelin (pg/mL) | 608.5 (443.3–1016.7) | 435.4 (263.8–769.3) | <0.001 |
| Acylated/unacylated ghrelin | 0.09 (0.059–0.186) | 0.05 (0.027–0.089) | <0.001 |
| Acylated/total ghrelin | 0.083 (0.056–0.16) | 0.05 (0.03–0.08) | <0.001 |
| Healthy Controls | DCM | ||||||
|---|---|---|---|---|---|---|---|
| Age | Male Gender | BMI | Age | Male Gender | BMI | ||
| Total ghrelin | ρ p | −0.355 <0.001 | −0.239 <0.001 | −0.570 <0.001 | 0.107 0.08 | −0.126 0.04 | 0.04 0.52 |
| Acylated ghrelin | ρ p | −0.288 <0.001 | −0.152 0.01 | −0.494 <0.001 | 0.108 0.08 | −0.142 0.02 | −0.037 0.55 |
| Unacylated ghrelin | ρ p | −0.371 <0.001 | −0.253 <0.001 | −0.551 <0.001 | 0.102 0.1 | −0.126 0.04 | 0.039 0.53 |
| All Patients n = 266 (100%) | DCM ≤ 12 Months n = 58 (21.8%) | 12 < DCM ≤ 60 Months n = 72 (27.1%) | DCM > 60 Months n = 136 (51.1%) | p | |
|---|---|---|---|---|---|
| Age (years) | 54.5 (14.5) | 49.5 (14.8) | 51.47 (15.7) | 57.8 (12.9) | 0.001 |
| Male gender (%) | 77.7 | 79.3 | 75.4 | 78.7 | 0.8 |
| BMI (kg/m2) | 26.7 (4.6) | 26.4 (4.7) | 26.5 (4.9) | 26.8 (4.4) | 0.13 |
| Systolic Blood Pressure (mmHg) | 119.9 (15.9) | 121.6 (16.6) | 115 (16) | 122 (15) | 0.13 |
| Diastolic Blood Pressure (mmHg) | 76 (12) | 77.5 (11) | 76 (15) | 76 (10) | 0.59 |
| Heart Rate (bpm) | 69.9 (14.2) | 73 (18) | 70 (13) | 69 (13) | 0.18 |
| NYHA class I-II (%) | 73.5 | 67.2 | 72.5 | 77 | 0.35 |
| Sinus rhythm (%) | 86 | 93.1 | 89.9 | 81.6 | 0.20 |
| LBBB (%) | 38.5 | 32.8 | 37.7 | 40.4 | 0.6 |
| CRF (%) | 20.5 | 14 | 19.4 | 23.5 | 0.33 |
| Anemia (%) | 16.7 | 12.3 | 19.1 | 17.6 | 0.56 |
| Sodium (mEq/L) | 139.1 (2.7) | 139 (2.8) | 138.6 (3.3) | 139 (2.3) | 0.27 |
| Left atrial diameter indexed (cm/m2) | 21.9 (5.4) | 22 (4.8) | 20.7 (5) | 22.5 (6) | 0.10 |
| Left atrial area (cm2) | 25.3 (8.1) | 14 (4) | 12 (3.5) | 13 (4) | 0.09 |
| LVEDDI (cm/m2) | 32.3 (28.7–36) | 33.7 (28.6–37.6) | 30.7 (27.9–34.6) | 33 (29–36.2) | 0.088 |
| LVESDI (cm/m2) | 26 (22–30) | 27.8 (21.8–30.8) | 24.5 (20.1–28.1) | 26.5 (22.6–30.8) | 0.015 |
| IVS (cm) | 1.01 (0.2) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 0.53 |
| LVEDVI (mL/m2) | 89 (66.6–112) | 95.9 (74–126) | 82 (65–103) | 87 (66–106) | 0.051 |
| LVESVI (mL/m2) | 60 (39–78) | 72 (47–87) | 50 (39–68) | 57 (36–75) | 0.031 |
| LVEF (%) | 34.8 (26.7–42.9) | 32 (25–40) | 36 (29–42) | 36 (27–45) | 0.07 |
| E/A ratio | 1.06 (0.75–1.6) | 1.2 (0.8–2.2) | 1.1 (0.8–1.5) | 1 9(0.7–1.5) | 0.24 |
| E/E’ ratio | 10.7 (8–14) | 11 (8.7–14) | 10 (8–13) | 11 (8–14) | 0.18 |
| WMSI | 1.96 (0.4) | 2 (0.5) | 1.9 (0.4) | 1.9 (0.5) | 0.52 |
| LV mass (g) | 261 (208–317) | 257 (219–329) | 254 (197–290) | 262 (210–316) | 0.4 |
| MR grade 2–4 (%) | 47.1 | 47.4 | 42.6 | 48.9 | 0.7 |
| Total ghrelin (pg/mL) | 461 (293.7–798.7) | 617 (322–983) | 461 (231–841) | 407 (292–693) | 0.033 |
| Acylated ghrelin (pg/mL) | 21.7 (15.9–29.4) | 20.4 (14.8–27.5) | 22.6 (17–32) | 21.7 (17–30) | 0.39 |
| Unacylated ghrelin (pg/mL) | 435.4 (263.8–769.3) | 583 (294.3–897.6) | 438.2 (199.8–831) | 384.3 (273.5–663.7) | 0.075 |
| Acylated/unacylated ghrelin | 0.05 (0.0269–0.088) | 0.0326 (0.02–0.065) | 0.046 (0.017–0.11) | 0.0568 (0.033–0.097) | 0.019 |
| Acylated /total ghrelin | 0.049 (0.0267–0.081) | 0.0316 (0.02–0.061) | 0.0489 (0.02–0.1) | 0.0542 (0.032–0.089) | 0.013 |
| BNP (pg/mL) | 156 (68.9–256.4) | 123.6 (44.5–229.9) | 145 (78.6–243.3) | 168.1 (83.4–307.2) | 0.056 |
| sST2 (ng/mL) | 31.1 (17.9–62.1) | 30.9 (17.9–63.5) | 34 (20.6–60.7) | 28.9 (16–62.9) | 0.39 |
| Galectin-3 (ng/mL) | 9.08 (7–13.3) | 9.15 (6.7–11.7) | 8.6 (6.3–11.7) | 9.3 (7.5–15.3) | 0.07 |
| IL-1β (pg/mL) | 0.6 (0.08–1.5) | 0.47 (0.06–1.55) | 0.038 (0.07–1.2) | 0.8 (0.09–1.67) | 0.28 |
| IL-6 (pg/mL) | 1.5 (0.6–3.3) | 1.28 (0.47–2.5) | 1.6 (0.49–4.01) | 1.67 (0.83–3.72) | 0.048 |
| IL-10 (pg/mL) | 1.4 (0.5–5.3) | 1 (0.14–6.76) | 1.22 (0.52–5.47) | 1.83 (0.56–4.89) | 0.52 |
| TNF-α (pg/mL) | 11.1 (8.6–16) | 10.3 (8.4–15.6) | 10.3 (7.7–14.1) | 11.7 (9.7–17.9) | 0.06 |
| ACE-I/ARBs (%) | 92.1 | 93.1 | 89.9 | 92.6 | 0.74 |
| Beta-blockers (%) | 90.2 | 87.9 | 91.2 | 90.4 | 0.8 |
| Digitalis (%) | 24.9 | 8.6 | 10.1 | 39.7 | <0.001 |
| Antiplatelets (%) | 27.5 | 22.4 | 27.5 | 29.4 | 0.61 |
| Oral Anticoagulants (%) | 26 | 22.4 | 16 | 33.1 | 0.023 |
| Amiodarone (%) | 17.4 | 19 | 13 | 19.1 | 0.53 |
| Diuretics (%) | 67.9 | 69 | 61 | 71 | 0.31 |
| Statins (%) | 29.5 | 20.4 | 27.5 | 34.6 | 0.19 |
| Oral antidiabetics (%) | 7.7 | 4.1 | 5.9 | 10.3 | 0.34 |
| ICD (%) | 25.1 | 12.2 | 27.5 | 29.9 | 0.06 |
| All Patients n = 266 (100%) | LVEF < 40% n = 174 (66%) | LVEF 40–49% n = 61 (23%) | LVEF ≥ 50% n = 30 (11%) | p | |
|---|---|---|---|---|---|
| Age (years) | 54.5 (14.5) | 56.1 (13.7) | 53.9 (15.7) | 45.9 (14.2) | 0.001 |
| Male gender (%) | 77.7 | 79.3 | 72.1 | 80 | 0.48 |
| BMI (kg/m2) | 26.7 (4.6) | 26.9 (4.8) | 25.6 (3.9) | 26.9 (4) | 0.13 |
| Overweight (%) | 60 | 63.8 | 47.5 | 63.3 | 0.08 |
| Obese (%) | 21.1 | 22.4 | 14.8 | 26.7 | 0.3 |
| Systolic blood pressure (mmHg) | 119.9 (15.9) | 118.4 (15.8) | 122.7 (17.2) | 123.2 (12.9) | 0.1 |
| Diastolic blood pressure (mmHg) | 76 (12) | 74.9 (9.8) | 78 (17.9) | 78.2 (6.4) | 0.1 |
| Heart rate (bpm) | 69.9 (14.2) | 71.7 (15.5) | 66.3 (11.2) | 66.5 (9.6) | 0.02 |
| NYHA class I-II (%) | 73.5 | 63.2 | 90.0 | 100.00 | <0.001 |
| Sinus rhythm (%) | 86 | 81.6 | 91.8 | 100.0 | 0.012 |
| LBBB (%) | 38.5 | 43.7 | 37.7 | 10.0 | 0.002 |
| Diabetes mellitus (%) | 9.8 | 10.9 | 8.2 | 6.7 | 0.7 |
| COPD (%) | 5.3 | 6.9 | 3.3 | 0 | 0.22 |
| Smoking (%) | 21.5 | 24.7 | 13.1 | 20 | 0.16 |
| GFR (mL/min/1.73m2) | 77.3 (61.3–93.9) | 74.7 (60–91) | 80.9 (61–102) | 83.5 (71–102) | 0.024 |
| CRF (%) | 20.5 | 22.7 | 20.7 | 7.1 | 0.2 |
| Anemia (%) | 16.7 | 22.4 | 3.3 | 10.7 | 0.002 |
| Haemoglobin (g/dL) | 13.9 (1.5) | 13.7 (1.6) | 14.2 (1.3) | 14.2 (1.4) | 0.02 |
| Sodium (mEq/L) | 139.1 (2.7) | 138.7 (2.9) | 139.8 (1.9) | 139.9 (1.9) | 0.005 |
| Creatinine (mg/dL) | 1.01 (0.87–1.2) | 1.04 (0.9–1.3) | 0.97 (0.8–1.12) | 0.98 (0.85–1.04) | 0.017 |
| Left atrial diameter indexed (mm/m2) | 21.9 (5.4) | 23.1 (5.5) | 19.9 (4.7) | 19.3 (4.2) | <0.001 |
| Left atrial area (cm2) | 25.3 (8.1) | 27.8 (8.6) | 22.3 (6.4) | 21 (4.8) | <0.001 |
| LVEDDI (cm/m2) | 32.3 (28.7–36) | 34 (30–38) | 31 (28–34) | 29 (26–32) | <0.001 |
| LVESDI (cm/m2) | 26 (22–30) | 28 (24–32) | 24 (20–26) | 20 (18–22) | <0.001 |
| IVS (cm) | 1.01 (0.2) | 1 (0.18) | 1 (0.16) | 1.1 (0.17) | 0.2 |
| LVEDVI (mL/m2) | 89 (66.6–112) | 101 (85–121) | 66 (55–79) | 57 (47–62) | <0.001 |
| LVESVI (mL/m2) | 60 (39–78) | 71 (59–90) | 38 (30–44) | 25 (18–29) | <0.001 |
| LVEF (%) | 34.8 (26.7–42.9) | 29 (24–35) | 44 (41–46) | 56 (52–60) | <0.001 |
| E/A ratio | 1.06 (0.75–1.6) | 1.1 (0.75–1.8) | 1.02 (0.78–1.3) | 1.2 (0.84–1.46) | 0.6 |
| E/E’ ratio | 10.7 (8–14) | 11.9 (8.9–16.7) | 10 (8–13) | 8.4 (6.9–11) | < 0.001 |
| WMSI | 1.96 (0.4) | 2.2 (0.45) | 1.85 (0.3) | 1.3 (0.3) | < 0.001 |
| LV mass (g) | 261 (208–317) | 284 (242–342) | 234 (191–268) | 226 (183–282) | < 0.001 |
| MR grade 2–4 (%) | 47.1 | 58.2 | 31.1 | 16.7 | < 0.001 |
| Total ghrelin (pg/mL) | 461 (293.7–798.7) | 480.8 (306.2–855.2) | 429.7 (293–807) | 329.5 (223.2–557.6) | 0.05 |
| Acylated ghrelin (pg/mL) | 21.7 (15.9–29.4) | 22.2 (15.5–29.9) | 21.6 (16–29.1) | 20.8 (17.1–28.7) | 0.9 |
| Unacylated ghrelin (pg/mL) | 435.4 (263.8–769.3) | 452.1 (274.7–833.8) | 406.3 (230–744.8) | 305.9 (200–544.7) | 0.076 |
| Acylated/unacylated ghrelin | 0.05 (0.0269–0.088) | 0.0465 (0.024–0.085) | 0.0461 (0.025–0.95) | 0.075 (0.035–0.094) | 0.092 |
| Acylated /total ghrelin | 0.049 (0.0267–0.081) | 0.04 (0.024–0.08) | 0.048 (0.025–0.09) | 0.069 (0.034–0.086) | 0.11 |
| BNP (pg/mL) | 156 (68.9–256.4) | 155.5 (67.3–243.2) | 135.8 (79.4–341.6) | 201.8 (76.5–271.8) | 0.9 |
| sST2 (ng/mL) | 31.1 (17.9–62.1) | 31.6 (18.6–63.3) | 30.2 (16.9–61.8) | 30 (15.7–49.1) | 0.4 |
| Galectin-3 (ng/mL) | 9.08 (7–13.3) | 9 (7–12.9) | 10.8 (7.6–17.4) | 9.1 (6.5–10.6) | 0.07 |
| IL-1β (pg/mL) | 0.6 (0.08–1.5) | 0.5 (0.08–1.39) | 0.6 (0.08–1.7) | 1.1 (0.16–2.17) | 0.3 |
| IL-6 (pg/mL) | 1.5 (0.6–3.3) | 1.7 (0.68–3.9) | 1.51 (0.48–3.11) | 0.99 (0.61–1.49) | 0.03 |
| IL-10 (pg/mL) | 1.4 (0.5–5.3) | 1.35 (0.47–5.69) | 0.9 (0.53–3.68) | 3.19 (1.31–7.52) | 0.06 |
| TNF-α (pg/mL) | 11.1 (8.6–16) | 11.5 (9.01–17.2) | 10.3 (7.9–13.9) | 12.05 (7.4–21.9) | 0.11 |
| ACE-I/ARBs (%) | 92.1 | 94.8 | 91.8 | 76.7 | 0.003 |
| Beta-blockers (%) | 90.2 | 91.4 | 90.2 | 83.3 | 0.4 |
| Digitalis (%) | 24.9 | 23.6 | 24.6 | 33.3 | 0.5 |
| Antiplatelets (%) | 27.5 | 28.2 | 27.9 | 23.3 | 0.9 |
| Oral Anticoagulants (%) | 26 | 30.5 | 13.1 | 26.7 | 0.03 |
| Amiodarone (%) | 17.4 | 20.1 | 9.8 | 16.7 | 0.2 |
| Diuretics (%) | 67.9 | 75.9 | 52.5 | 53.3 | 0.001 |
| Statins (%) | 29.5 | 30.3 | 32.7 | 20 | 0.4 |
| Oral antidiabetics (%) | 7.7 | 9 | 5.5 | 6.7 | 0.7 |
| ICD (%) | 25.1 | 32 | 14.5 | 16.7 | 0.02 |
| Total Ghrelin | Acylated Ghrelin | Unacylated Ghrelin | ||
|---|---|---|---|---|
| Age | ρ p | 0.1 0.1 | 0.11 0.08 | 0.10 0.09 |
| Male gender | ρ p | −0.124 0.04 | −0.14 0.02 | −0.13 0.039 |
| BMI | ρ p | 0.033 0.6 | −0.03 0.6 | 0.04 0.56 |
| NYHA class | ρ p | 0.1 0.09 | 0.12 0.08 | 0.07 0.28 |
| LBBB | ρ p | 0.04 0.5 | 0.007 0.9 | 0.036 0.56 |
| Atrial fibrillation | ρ p | 0.037 0.5 | 0.023 0.7 | 0.04 0.5 |
| Diabetes mellitus | ρ p | −0.062 0.32 | −0.003 0.96 | −0.06 0.36 |
| Smoking | ρ p | 0.1 0.09 | 0.119 0.055 | 0.09 0.14 |
| GFR | ρ p | −0.1 0.11 | −0.119 0.057 | −0.1 0.1 |
| LVEDDI | ρ p | 0.01 0.9 | −0.02 0.7 | 0.01 0.86 |
| LVESDI | ρ p | 0.007 0.9 | −0.023 0.7 | −0.006 0.9 |
| LVEDVI | ρ p | 0.12 0.08 | −0.007 0.91 | 0.08 0.2 |
| LVESVI | ρ p | 0.1 0.08 | 0.01 0.87 | 0.08 0.19 |
| LVEF | ρ p | −0.09 0.13 | −0.05 0.42 | −0.09 0.16 |
| LV mass | ρ p | 0.04 0.57 | −0.16 0.019 | 0.02 0.7 |
| Death/transplant | ρ p | 0.12 0.05 | 0.045 0.5 | 0.09 0.12 |
| BNP | ρ p | 0.11 0.08 | 0.131 0.03 | 0.12 0.055 |
| sST2 | ρ p | 0.12 0.048 | 0.124 0.045 | 0.12 0.049 |
| Galectin-3 | ρ p | 0.042 0.5 | 0.075 0.23 | 0.025 0.7 |
| IL-1β | ρ p | −0.015 0.8 | 0.3 <0.001 | −0.004 0.95 |
| IL-6 | ρ p | 0.15 0.013 | −0.015 0.81 | 0.015 0.018 |
| IL-10 | ρ p | 0.096 0.12 | 0.013 0.41 | 0.11 0.07 |
| TNF-α | ρ p | 0.064 0.31 | 0.22 <0.001 | 0.05 0.38 |
| All Patients n = 266 (100%) | Death/Transpl n = 42 (16%) | Alive n = 224 (84%) | p | |
|---|---|---|---|---|
| Age (years) | 54.5 (14.5) | 55.1 (15.2) | 54.4 (14.4) | 0.8 |
| Male gender (%) | 77.7 | 92.9 | 74.9 | 0.01 |
| BMI (kg/m2) | 26.7 (4.6) | 26.9 (4.7) | 26.6 (4.5) | 0.75 |
| Overweight (%) | 60 | 66.7 | 58.7 | 0.2 |
| Obese (%) | 21.1 | 21.4 | 21.1 | 0.96 |
| Systolic blood pressure (mmHg) | 119.9 (15.9) | 117.4 (14.2) | 120.4 (16.2) | 0.3 |
| Diastolic blood pressure (mmHg) | 76 (12) | 74.9 (8.6) | 76.3 (12.5) | 0.5 |
| Heart rate (bpm) | 69.9 (14.2) | 72.9 (11.2) | 69.3 (14.6) | 0.2 |
| NYHA class I-II (%) | 73.5 | 57.1 | 76.6 | 0.009 |
| Sinus rhythm (%) | 86 | 69 | 89.2 | 0.002 |
| LBBB (%) | 38.5 | 47.6 | 36.8 | 0.18 |
| Diabetes mellitus (%) | 9.8 | 16.7 | 8.5 | 0.1 |
| COPD (%) | 5.3 | 11.9 | 4 | 0.052 |
| Smoking (%) | 21.5 | 28.6 | 20.2 | 0.2 |
| GFR (mL/min/1.73 m2) | 77.3 (61.3–93.9) | 68.9 (51.7–89.2) | 77.5 (64.1–94.7) | 0.045 |
| CRF (%) | 20.5 | 40.5 | 16.7 | 0.001 |
| Anemia (%) | 16.7 | 33.3 | 13.6 | 0.002 |
| Haemoglobin (g/dL) | 13.9 (1.5) | 13.8 (1.5) | 13.9 (1.6) | 0.7 |
| Sodium (mEq/L) | 139.1 (2.7) | 138.2 (2.9) | 139.2 (2.6) | 0.02 |
| Creatinine (mg/dL) | 1.01 (0.87–1.2) | 1.1 (0.97–1.4) | 1 (0.84–1.2) | 0.001 |
| Left atrial diameter indexed (mm/m2) | 21.9 (5.4) | 24.8 (5.6) | 21.4 (5.2) | 0.001 |
| Left atrial area (cm2) | 25.3 (8.1) | 28.9 (10) | 24.8 (7.7) | 0.02 |
| LVEDDI (cm/m2) | 32.3 (28.7–36) | 32.5 (27.9–37.6) | 32.2 (28.7–35.97) | 0.82 |
| LVESDI (cm/m2) | 26 (22–30) | 28 (24.38–33.8) | 25.34 (21.45–30) | 0.012 |
| IVS (cm) | 1.01 (0.2) | 1.02 (0.2) | 1 (0.17) | 0.7 |
| LVEDVI (mL/m2) | 89 (66.6–112) | 93.3 (78.9–113.1) | 88.1 (65.02–111.2) | 0.066 |
| LVESVI (mL/m2) | 60 (39–78) | 66.8 (49.9–78.3) | 56 (36.7–77.7) | 0.042 |
| LVEF (%) | 34.8 (26.7–42.9) | 29.7 (23.1–39.3) | 35.6 (27.5–44) | 0.012 |
| E/A ratio | 1.06 (0.75–1.6) | 1.48 (1–2.21) | 1.02 (0.75–1.5) | 0.02 |
| E/E’ ratio | 10.7 (8–14) | 13 (9.4–18.4) | 10.3 (8–13.5) | 0.04 |
| WMSI | 1.96 (0.4) | 2.14 (0.4) | 1.93 (0.4) | 0.03 |
| LV mass (g) | 261 (208–317) | 285.5 (215.8–332.3) | 256 (205.8–314.8) | 0.13 |
| MR grade 2–4 (%) | 47.1 | 60 | 44.8 | 0.07 |
| Total ghrelin (pg/mL) | 461 (293.7–798.7) | 517.4 (329.4–1059.6) | 430.3 (273–777) | 0.05 |
| Acylated ghrelin (pg/mL) | 21.7 (15.9–29.4) | 22.8 (18.2–29.9) | 21.6 (15.6–29.4) | 0.46 |
| Unacylated ghrelin (pg/mL) | 435.4 (263.8–769.3) | 462.5 (299.1–977.8) | 406.3 (244.7–735.6) | 0.12 |
| Acylated/unacylated ghrelin | 0.05 (0.0269–0.088) | 0.047 (0.031–0.075) | 0.053 (0.024–0.095) | 0.5 |
| Acylated /total ghrelin | 0.049 (0.0267–0.081) | 0.045 (0.03–0.07) | 0.05 (0.026–0.089) | 0.39 |
| BNP (pg/mL) | 156 (68.9–256.4) | 262 (130–437.6) | 135.4 (66.2–230.1) | <0.001 |
| sST2 (ng/mL) | 31.1 (17.9–62.1) | 45 (26.3–102.6) | 30.2 (17–57.6) | 0.009 |
| Galectin-3 (ng/mL) | 9.08 (7–13.3) | 10.5 (8.6–20.8) | 9 (6.9–12.2) | 0.01 |
| IL-1β (pg/mL) | 0.6 (0.08–1.5) | 0.9 (0.3–1.8) | 0.5 (0.1–1.4) | 0.08 |
| IL-6 (pg/mL) | 1.5 (0.6–3.3) | 3.28 (1.05–8.43) | 1.49 (0.62–2.96) | 0.002 |
| IL-10 (pg/mL) | 1.4 (0.5–5.3) | 2.86 (0.97–11.2) | 1.21 (0.5–4.14) | 0.012 |
| TNF-α (pg/mL) | 11.1 (8.6–16) | 12.3 (10.3–20.9) | 10.8 (8.3–15.3) | 0.028 |
| ACE-I/ARBs (%) | 92.1 | 95.2 | 91.5 | 0.4 |
| Beta-blockers (%) | 90.2 | 95.2 | 89.2 | 0.2 |
| Digitalis (%) | 24.9 | 38.1 | 22.4 | 0.03 |
| Antiplatelets (%) | 27.5 | 28.6 | 27.4 | 0.9 |
| Oral Anticoagulants (%) | 26 | 47.6 | 22 | 0.001 |
| Antiarrythmics (%) | 35.8 | 47.6 | 33.6 | 0.08 |
| Amiodarone (%) | 17.4 | 26.2 | 15.7 | 0.1 |
| Diuretics (%) | 67.9 | 83.3 | 65 | 0.02 |
| Statins (%) | 29.5 | 19.2 | 30.9 | 0.2 |
| Oral antidiabetics (%) | 7.7 | 15.4 | 6.6 | 0.2 |
| ICD (%) | 25.1 | 34.6 | 23.8 | 0.2 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| LnBNP | 1.51 (1.13–2.03) | 0.005 | 1.6 (1.22–2.23) | 0.001 |
| LVEF for 10% points increase | 0.96 (0.93–0.99) | 0.003 | 0.7 (0.51–0.98) | 0.04 |
| NYHA I-II vs. III-IV | 3.25 (1.75–6.02) | <0.001 | 2.47 (1.26–4.86) | 0.009 |
| LnsST2 | 1.47 (1.08–1.99) | 0.013 | - | - |
| Sodium | 0.88 (0.8–0.97) | 0.015 | - | - |
| LnGal-3 | 1.16 (0.75–1.8) | 0.5 | - | - |
| Ln-Total ghrelin | 1.3 (0.9–1.81) | 0.17 | - | - |
| MDRD | 0.99 (0.97–1.005) | 0.21 | - | - |
| LBBB | 1.5 (0.83–2.79) | 0.17 | - | - |
| DCM (n = 46) | Controls (n = 18) | Normal Values | p | |
| Age (Year) | 55.7 (41.9; 62.5) | 41.5 (31.5; 51.5) | - | <0.01 |
| Sex (M/F) | 38/8 | 8/10 | - | <0.01 |
| Duration of disease (Years) | 6.6 (3.1; 12.3) | - | - | |
| NYHA class (%) | ||||
| II | 9 | - | - | - |
| III | 67 | - | - | - |
| IV | 24 | - | - | - |
| Echocardiography * | ||||
| Left ventricular diameter (mm) | ||||
| Systolic | 66 (56; 72) | - | 21.6–34.8 | - |
| Diastolic | 74 (67; 83) | - | 37.8–52.2 | - |
| Left ventricular volumes (mL) | ||||
| End-diastolic | 183.5 (163.8; 262.3) | - | 46–106 | - |
| End-systolic | 132.5 (120.0; 168.8) | - | 14–42 | - |
| Left ventricular ejection fraction (%) | 23 (20; 27) | - | 54–74 | - |
| Hemodynamics† | ||||
| Pulmonary artery pressure (mmHg) | ||||
| Systolic | 46 (31.5; 51.5) | - | 15–25 | - |
| Diastolic | 24 (14.5; 29.5) | - | 8–12 | - |
| Mean | 31 (20.5; 37.5) | - | 10–20 | - |
| PCWP (mmHg) | 21 (15; 31) | - | 6–12 | - |
| CI (Lmin−1m−2) | 2.3 (1.8; 2.7) | - | 2.5–4.0 | - |
| Gross Anatomy ‡ | ||||
| Heart weight (g) | 465 (400; 561) | 340 (235; 412) | 196–516 | <0.01 |
| Transverse diameter (mm) | 130 (120; 140) | 102 (85; 122) | - | |
| Inner longitudinal diameter (mm) | 95 (85; 105) | 71 (6; 77) | - | <0.01 |
| Wall thickness (mm) | <0.01 | |||
| LV | 10 (8; 11.5) | 14 (12; 14) | - | 0.017 |
| RV | 3 (3; 5) | 3 (2; 5) | - | N.S. |
| Septum | 1 (1; 1.35) | - | N.S. | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksova, A.; Beltrami, A.P.; Bevilacqua, E.; Padoan, L.; Santon, D.; Biondi, F.; Barbati, G.; Stenner, E.; Gortan Cappellari, G.; Barazzoni, R.; et al. Ghrelin Derangements in Idiopathic Dilated Cardiomyopathy: Impact of Myocardial Disease Duration and Left Ventricular Ejection Fraction. J. Clin. Med. 2019, 8, 1152. https://doi.org/10.3390/jcm8081152
Aleksova A, Beltrami AP, Bevilacqua E, Padoan L, Santon D, Biondi F, Barbati G, Stenner E, Gortan Cappellari G, Barazzoni R, et al. Ghrelin Derangements in Idiopathic Dilated Cardiomyopathy: Impact of Myocardial Disease Duration and Left Ventricular Ejection Fraction. Journal of Clinical Medicine. 2019; 8(8):1152. https://doi.org/10.3390/jcm8081152
Chicago/Turabian StyleAleksova, Aneta, Antonio Paolo Beltrami, Elisa Bevilacqua, Laura Padoan, Daniela Santon, Federico Biondi, Giulia Barbati, Elisabetta Stenner, Gianluca Gortan Cappellari, Rocco Barazzoni, and et al. 2019. "Ghrelin Derangements in Idiopathic Dilated Cardiomyopathy: Impact of Myocardial Disease Duration and Left Ventricular Ejection Fraction" Journal of Clinical Medicine 8, no. 8: 1152. https://doi.org/10.3390/jcm8081152