1. Introduction
Apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APEX1) is a multifunctional protein that acts as a transcriptional coactivator in apoptosis, proliferation, and differentiation. It is also an essential factor in the DNA base excision repair pathways response to DNA damage and a negative regulator of the Ras-related GTPase Rac1 activity to control intracellular reactive oxygen species [
1]. APE1 refers to a member of the DNA base excision repair pathway; Ref-1 refers to redox regulation on diverse redox-dependent mechanisms [
1]. APEX1 has been associated with cancer, cardiovascular diseases, and neurodegeneration and seems to play a role in the inflammatory process. One study demonstrated that APEX1 induces interleukin-8 production by gastric cells in
Helicobacter pylori infection [
2], and another reported that APEX1 is a key upstream regulator in TLR2-dependent keratinocyte inflammatory responses [
3].
APEX1 protein is ubiquitous, but altered levels have been found in a variety of cancers, such as germ cell tumors, rhabdomyosarcomas, breast carcinomas, hepatocellular carcinomas (HCCs), non-small cell lung carcinomas, ovarian serous carcinomas, gastric carcinomas, esophageal squamous cell carcinomas, and bladder carcinomas [
4,
5]. Although APEX1 subcellular expression is mainly nuclear, cytoplasmic localization of APEX1 has been associated with different functional processes depending on the condition [
1]. The extracellular occurrence of APEX1 in the serum or plasma, which could result from tumor cells or immune cells, has been evaluated in HCC, bladder cancer, and non-small-cell lung cancer [
6,
7,
8]. The blood is a good source of biomarkers for screening or follow-up. The kidneys and liver have significant blood flow, so the effectiveness of using a serologic biomarker to diagnose cancer in these organs may be particularly high.
We hypothesized that the expression levels of subcellular or extracellular serum APEX1 (s-APEX1) could be the basis for a diagnostic or therapeutic strategy. This study aimed to evaluate subcellular APEX1 expression in cancer cells and the extracellular APEX1 level in serum as a possible diagnostic or prognostic biomarker in patients with clear cell renal cell carcinoma (ccRCC), HCC, or cholangiocarcioma (CC), compared to the levels in nontumor groups, including a healthy control group and patients with ≥58 copies/mL of hepatitis B viral DNA (HBV DNA (+)).
2. Materials and Methods
2.1. Patients and Samples
The study considered a total of 505 samples from South Korean individuals with ccRCC, HCC, or CC (
Table 1) who had not received preoperative chemotherapy or radiation therapy. All 505 tissue or serum samples were provided by the National Biobank of Korea at Chungnam National University Hospital, a member of the Korean Biobank Network. The frozen serum samples from cancer patients were obtained before operation for surgical resection.
The formalin-fixed, paraffin-embedded (FFPE) tissue samples used in this study were surgically resected between 1999 and 2014 at Chungnam National University Hospital in Daejeon, South Korea. All medical records of the patients were reviewed by Kim, K-H, and Kim, J-M to obtain clinical data. The tumor, node, and metastasis (TNM) staging and histologic grading for ccRCC, HCC, and CC were determined at the time of surgical resection and were based on the 8th edition of the American Joint Committee on Cancer (AJCC) staging system [
9]. ccRCC and hepatobiliary cancer recurrence or metastasis was determined via histological findings. Disease-free survival (DFS) was determined as the time between the date of initial surgical resection and the date of the cancer’s recurrence or metastasis. Overall survival (OS) was defined as the time from the initial surgical resection until the date of death due to any cause. Without death, recurrence, or metastasis confirmation, OS or DFS time was stopped at the last known date that the patient was alive. All paired tissue samples from the HCC, CC, and ccRCC patients consisted of a tumor tissue section and a matched nontumor tissue section, at least 3.0 cm from the tumor mass. Tissue microarrays (TMAs) were constructed as previously described [
10].
Quantitation of HBV DNA was performed using the Cobas HBV assay for the Cobas 6800 system (Roche Diagnostics GmbH, Mannheim, Germany). The analytical measurement range for HBV DNA levels was 58 to 5,820,000 copies/mL (10 to 1,000,000,000 IU/mL), and samples that had 58 or more HBV DNA copies/mL were defined as positive for HBV DNA (HBV DNA (+)).
This study was approved by the Institutional Review Board of Chungnam National University Hospital (CNUH 2018-06-030-002). All frozen serum or tissue samples and clinical data were obtained from the National Biobank of Korea at Chungnam National University Hospital. All patients signed a written informed consent form for biobanking before data were included in the register. The requirement for informed consent for the retrospective comparison study was waived because the study was based on immunohistochemical analysis using FFPE tissue.
2.2. Frozen Serum APEX1 Measurement by Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was conducted as previously described using an APEX1 enzyme-linked immunosorbent assay kit (Cat#MR-APE064-HS. MediRedox Inc., Daejeon, Korea), including the mouse monoclonal anti-human APEX1 antibody (Cat#LS-C392589, LifeSpan BioSciences, WA, USA) and the rabbit polyclonal anti-human APEX1 antibody (MediRedox, Daejeon, Korea) [
11].
2.3. Tissue APEX1 Measurement by Immunohistochemical Staining Analysis
Immunohistochemical staining of the FFPE tissue section was conducted as previously described [
10] using a primary rabbit polyclonal antibody against human APEX1 (product # MR-PAAPE, diluted 1:50, incubation at 31 °C for 32 min; MediRedox, Daejeon, South Korea). We analyzed the nuclear and cytoplasmic APEX1 expression separately. Immunohistochemical staining was scored using the method described by Allred et al. [
12]. Specifically, semiquantitative scoring from 0 to 8 was used to evaluate the cytoplasmic and nuclear expression of stained cancer cells or nontumor hepatocytes, bile duct epithelial cells, and proximal convoluted tubular epithelial cells. The results were examined separately and scored by Kim, K-H, and Yeo, M-K, who were blinded to patients’ clinicopathological details. Discrepancies in the scores were discussed to obtain a consensus.
2.4. APEX1 mRNA Measurement by Reverse Transcriptase Digital Droplet PCR (RT-ddPCR)
Twenty paired frozen samples of HCC and nontumor liver tissue from 20 patients with HCC were used. All nontumor tissue samples were at least 3.0 cm from the tumor mass. The tissues were dissociated with a gentle MACS dissociator with a human tumor dissociation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). To remove as many nonparenchymal cells as possible, the supernatant was removed after centrifugation, and a precipitated cell suspension was used. The following primers were developed: the APEX1 forward primer sequence 5′-GGC-AAG-CTT-GAG-TCA-GGACC-3′ and the reverse primer sequence 5′-TTT-ACC-GCG-TTG-CTC-GC-3′ (input PCR template: NM_001244249.1). The QX200™ Droplet Digital™ PCR System (Bio-Rad, Pleasanton, CA, USA) was used for RT-ddPCR analysis of APEX1. RT-ddPCR was conducted as previously described [
13].
2.5. Statistics
The relationships between APEX1 expression and the clinicopathological parameters were evaluated using the Mann–Whitney U test. Differences in APEX1 mRNA expression between the paired HCC tissue and nontumor liver tissue sections were assessed using the Wilcoxon signed-rank test. The Cox proportional hazards model was applied for univariate and multivariate survival analyses. Differences in the s-APEX1 level between the control, HCC, CC, ccRCC, and HBV DNA (+) groups were assessed using the Kruskal–Wallis test. Receiver operating characteristic (ROC) curve analysis was applied to evaluate the utility of s-APEX1 levels in determining HCC, CC, or ccRCC. Optimal criteria for sensitivity and specificity were indicated by Youden’s index using MedCalc version 19.0.5 (MEDCALC, Ostend, Belgium). Associations between protein variables were assessed using the Spearman correlations. The statistical significance was set to p < 0.05 (SPSS v.24; SPSS, Inc., Chicago, IL, USA).
4. Discussion
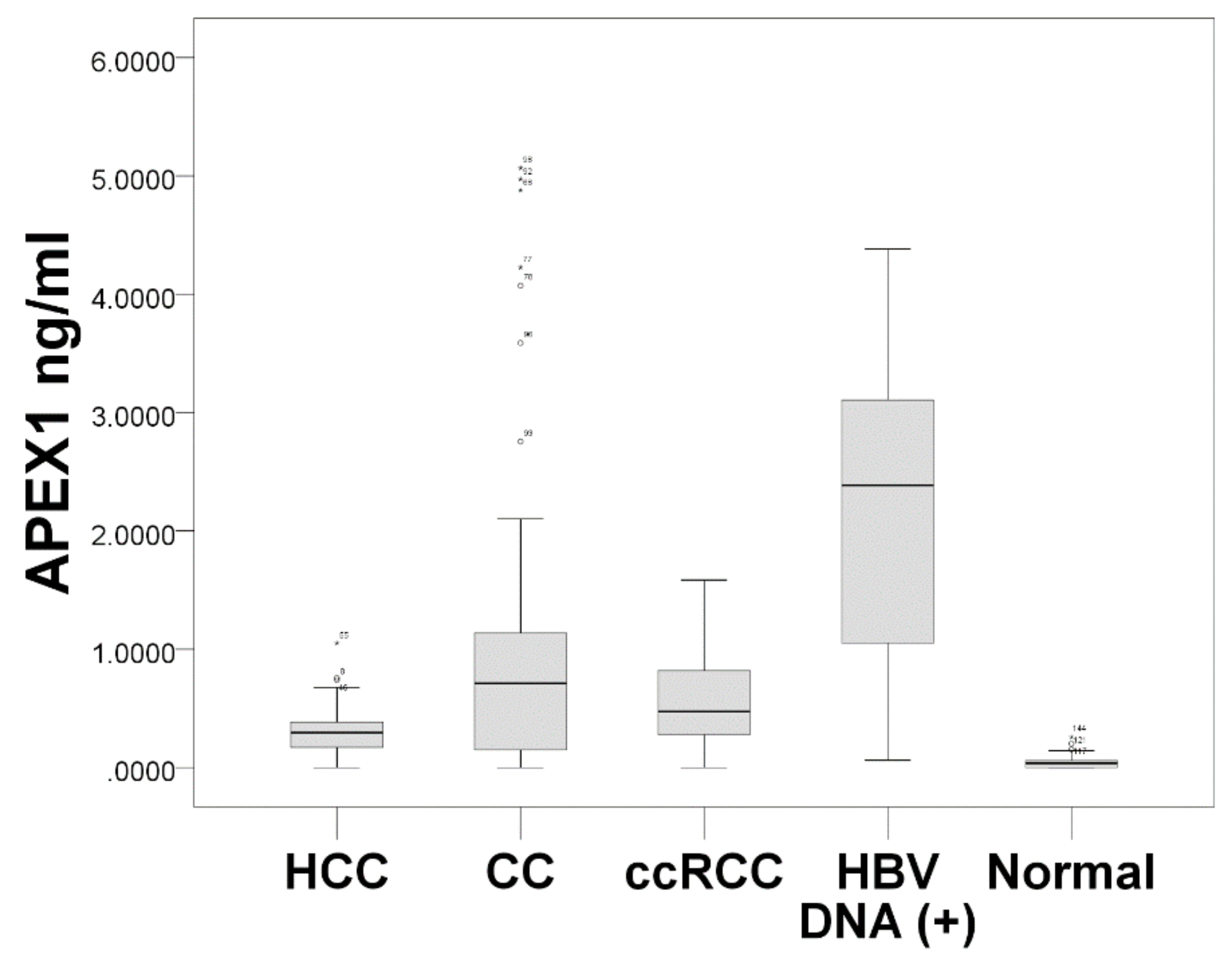
In this study, we evaluated the subcellular and extracellular expressions of APEX1 in ccRCC, HCC, and CC. We demonstrated that the s-APEX1 level was significantly higher in patients with the three cancers than in the healthy control group, while the serum level of individuals in the HBV DNA (+) group was higher than those with three cancers. In addition, increased cytoplasmic expression of APEX1 in HCC and intrahepatic CC cells was found to be associated with a shorter DFS period.
The s-APEX1 level was markedly increased in patients with HBV DNA (+), primary ccRCC, HCC, or CC, and the s-APEX1 levels exhibited positive correlations with AST, ALT, GGT, CRP, and HBV DNA (+). These findings suggest active extracellular secretion of APEX1 from cancer cells or activated stromal or inflammatory cells. Little is known about the mechanism or influence of extracellular secretion of APEX1 in the three cancers and under inflammatory conditions. In agreement with our study, a recent study showed markedly higher s-APEX1 levels in HCC patients in comparison to non-tumor cirrhosis and healthy control groups [
6]. Moreover, s-APEX1 levels were found to be significantly higher in individuals with HCC-related viral infection (either viral hepatitis B or C infection) compared to those with other non-viral etiologies, but the highest s-APEX1 level was seen in patients with multiple etiologies including viral, metabolic, or alcohol-related conditions [
6]. This demonstrates that APEX1 promotes IL-6 and IL-8 expression in JHH6 cells, suggesting that it influences the tumor microenvironment [
6]. In our study, the highest s-APEX1 level was observed in the non-tumor HBV DNA (+) group. The HBV DNA (+) state is generally associated with an active immune reaction or inflammation, while liver cirrhosis or the surgical preparation period were relatively stable in terms of immune or inflammatory reactions because of end stage fibrotic liver or the stable conditions required for surgery. Our results exhibit that, in all study groups, the s-APEX1 level has a positive correlation with AST, ALT, GGT, and CRP. Considering the role of APEX1 in triggering an inflammation or immune reaction, we speculate that elevated levels of s-APEX1 may reflect an inflammation/immune reaction. Therefore, an active inflammation/immune state and the three cancers need to be separated when the s-APEX1 level is considered to be a diagnostic tumor marker of the three cancers. Our data showed that s-APEX1 has a statistically significant ability to differentiate the three cancers from the healthy control group and the HBV DNA (+) group. Therefore, we suggest that s-APEX1 expression could be a suitable diagnostic tumor marker for the three cancers.
APEX1 a variety of conditions ranging from physiologic to pathologic, acting as an essential protein in base excision repair pathways and as a transcriptional coactivator for ubiquitous or tissue-specific transcription factors on redox-dependent or redox-independent mechanisms. Some studies have suggested that the regulation of APEX1 functions is controlled by posttranslational modification and the heterogeneity of subcellular localization [
1,
14]. Different conditions change the APEX1 subcellular localization, for example, to the nucleus, mitochondria, or endoplasmic reticulum. APEX1 subcellular localization is prominently nuclear, whereas cytoplasmic APEX1 expression has also been shown in highly metabolic or proliferative non-neoplastic cells [
15,
16]. APEX1 modulates intracellular redox status in oxidative stress [
17]. A mitochondrial targeting sequence (MTS) of APEX1 resides within residues 289–318 in the C terminus, normally masked by the intact N-terminal structure [
18]. The APEX1 and Mia40 interaction in mitochondria highlights the role of maintaining mitochondrial DNA stability and cell survival [
19]. Photoirradiation of a lung cancer therapy induces the mitochondrial translocation of APEX1 to control mitochondrial transcription activity by mitochondrial transcription factor A as a redox regulation [
20]. HCC and ccRCC can have metabolic reprogramming to cover highly proliferative state [
21]. Recent studies have been reported an important mitochondrial regulatory role for redox regulation that mitochondrial NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 4-like 2 (NDUFA4L2) were overexpressed in ccRCC and HCC cells. The NDUFA4L2 overexpression by glycolysis in solid cancer cells is suggestive of one of metabolic adaptations in hypoxic state to reduce mitochondrial oxidative phosphorylation and control increased reactive oxygen species production [
22,
23].
In both colonic adenoma and adenocarcinoma, APEX1 expression has been shown to be pronounced in the cytoplasm, in contrast to the predominantly nuclear expression in normal colonic epithelial cells [
24]. Although the molecular basis of changing the subcellular localization is not clear, the cytoplasmic distribution of APEX1 has been reported to be associated with aggressiveness in some cancers including lung, ovarian, thyroid, breast, and liver cancers [
25,
26,
27]. In HEK293 cells derived from human embryonal kidneys, cytoplasmic APEX1 protein seems to be secreted extracellularly in response to treatment with Trichostatic A, an inhibitor of histone deacetylases [
28]. Therefore, the extracellular APEX1 level could be associated with special functions. According to the TCGA database, the correlation between mRNA expression level and ccRCC patient survival rate was lower in the low-expression ccRCC patients than in the high-expression patients (
https://www.proteinatlas.org/ENSG00000100823-APEX1/pathology/tissue/renal+cancer/KIRC). In our results, but, nuclear or cytoplasmic APEX1 protein expression was not statistically significant in survival analysis of ccRCC patients although there was a significant positive correlation between nuclear or cytoplasmic APEX1 expression and histologic nuclear grade in the ccRCC cases. Differences in patient survival outcome between the APEX1 mRNA and protein expression may be due to differences in APEX1 protein expression in ccRCC cells and total mRNA expression of ccRCC cells and various nontumor cells in tumor tissue sections.
Our evaluation of APEX1 expression and clinicopathological data revealed a correlation between cytoplasmic APEX1 expression in tumor cells and shorter DFS times in patients with HCC or intrahepatic CC. Cytoplasmic expression levels of APEX1 in HCC, CC, and ccRCC cells were higher than those in the matched non-neoplastic hepatocytes, bile duct epithelial cells, and proximal convoluted tubular epithelial cells. The s-APEX1 levels of the HCC, CC, and ccRCC groups were between the levels of the healthy control group and the HBV DNA (+) group and showed sufficient diagnostic accuracy to distinguish between the healthy control and the HBV DNA (+) group.