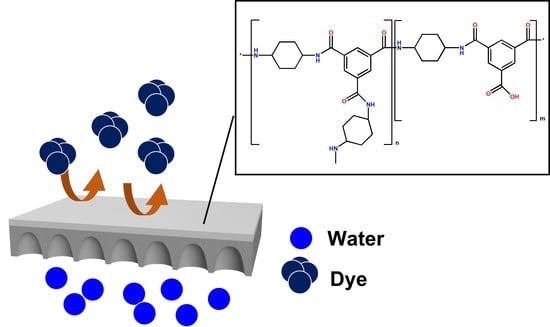
Nanofiltration Membranes Formed through Interfacial Polymerization Involving Cycloalkane Amine Monomer and Trimesoyl Chloride Showing Some Tolerance to Chlorine during Dye Desalination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Thin-Film Composite Membranes
2.3. Membrane Characterization
2.4. Filtration Test
2.5. Evaluation of Chlorine Resistance
3. Results and Discussion
3.1. Surface Chemical Property
3.2. Morphology and Roughness
3.3. Surface Hydrophilicity and Charged
3.4. Membrane Separation Performance and Chlorine Resistant Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lau, W.-J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Rawlings, G.D.; Samfield, M. Textile plant wastewater toxicity. Environ. Sci. Technol. 1979, 13, 160–164. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, A.M.; El-Hamshary, H.; Al-Enizi, A.M.; El-Newehy, M.H. Effective adsorption of Coomassie brilliant blue dye using poly(phenylene diamine)grafted electrospun carbon nanofibers as a novel adsorbent. Mater. Chem. Phys. 2019, 234, 133–145. [Google Scholar] [CrossRef]
- Ata, S.; Imran Din, M.; Rasool, A.; Qasim, I.; Ul Mohsin, I. Equilibrium, thermodynamics, and kinetic sorption studies for the removal of coomassie brilliant blue on wheat bran as a low-cost adsorbent. J. Anal. Methods Chem. 2012, 2012, 405980. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xing, H.; Yan, K.; Han, D.; Chen, J. Oyster shell derived hydroxyapatite microspheres as an effective adsorbent for remediation of Coomassie brilliant blue. Adv. Powder Technol. 2022, 33, 103425. [Google Scholar] [CrossRef]
- Katrahalli, U.; Kalanur, S.S.; Seetharamappa, J. Interaction of bioactive coomassie brilliant blue g with protein: Insights from spectroscopic methods. Sci. Pharm. 2010, 78, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Faria, R.X.; Ferreira, N.C.; Soares-Bezerra, R.J. Brilliant Blue Dyes in Daily Food: How Could Purinergic System Be Affected? Int. J. Food Sci. 2016, 2016, 7548498. [Google Scholar] [CrossRef] [PubMed]
- Cecille, L.; Toussaint, J.-C. (Eds.) Future Industrial Prospects of Membrane Processes; Elsevier Applied Science: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2021, 79, 2603–2631. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Vacchi, F.I.; Albuquerque, A.F.; Vendemiatti, J.A.; Morales, D.A.; Ormond, A.B.; Freeman, H.S.; Zocolo, G.J.; Zanoni, M.V.; Umbuzeiro, G. Chlorine disinfection of dye wastewater: Implications for a commercial azo dye mixture. Sci. Total Environ. 2013, 442, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, D.; Kim, J.G. Oxidation of various reactive dyes with in situ electro-generated active chlorine for textile dyeing industry wastewater treatment. J. Hazard. Mater. 2006, 136, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.K.; Joshi, S.V. Performance evaluation of thin film composite polyamide nanofiltration membrane with variation in monomer type. J. Membr. Sci. 2009, 342, 60–69. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Ji, Y.L.; Huang, S.H.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Incorporation of carboxylic monoamines into thin-film composite polyamide membranes to enhance nanofiltration performance. J. Membr. Sci. 2017, 539, 52–64. [Google Scholar] [CrossRef]
- Zheng, D.; Hua, D.; Yao, A.; Hong, Y.; Cha, X.; Yang, X.; Japip, S.; Zhan, G. Fabrication of thin-film composite membranes for organic solvent nanofiltration by mixed monomeric polymerization on ionic liquid/water interfaces. J. Membr. Sci. 2021, 636, 119551. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, J.; Chen, C.; Wan, Y. Highly permeable acid-resistant nanofiltration membrane based on a novel sulfonamide aqueous monomer for efficient acidic wastewater treatment. Chem. Eng. J. 2021, 425, 131791. [Google Scholar] [CrossRef]
- Wu, X.; Yang, L.; Meng, F.; Shao, W.; Liu, X.; Li, M. ZIF-8-incorporated thin-film nanocomposite (TFN) nanofiltration membranes: Importance of particle deposition methods on structure and performance. J. Membr. Sci. 2021, 632, 119356. [Google Scholar] [CrossRef]
- Xu, C.; Chen, W.; Gao, H.; Xie, X.; Chen, Y. Cellulose nanocrystal/silver (CNC/Ag) thin-film nanocomposite nanofiltration membranes with multifunctional properties. Environ. Sci. Nano 2020, 7, 803–816. [Google Scholar] [CrossRef]
- Gu, Z.; Yu, S.; Zhu, J.; Li, P.; Gao, X.; Zhang, R. Incorporation of lysine-modified UiO-66 for the construction of thin-film nanocomposite nanofiltration membrane with enhanced water flux and salt selectivity. Desalination 2020, 493, 114661. [Google Scholar] [CrossRef]
- Wu, J.; Xia, M.; Li, Z.; Shen, L.; Li, R.; Zhang, M.; Jiao, Y.; Xu, Y.; Lin, H. Facile preparation of polyvinylidene fluoride substrate supported thin film composite polyamide nanofiltration: Effect of substrate pore size. J. Membr. Sci. 2021, 638, 119699. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, X.; Luo, X.; Liu, Y.; Xu, D.; Tang, X.; Gan, Z.; Yang, L.; Li, G.; Liang, H. Ultrathin Thin-Film Composite Polyamide Membranes Constructed on Hydrophilic Poly(vinyl alcohol) Decorated Support Toward Enhanced Nanofiltration Performance. Environ. Sci. Technol. 2020, 54, 6365–6374. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.; Li, X.; Wang, Z.; Tang, C.Y. Metal–Organic Framework Nanosheets for Thin-Film Composite Membranes with Enhanced Permeability and Selectivity. ACS Appl. Nano Mater. 2020, 3, 9238–9248. [Google Scholar] [CrossRef]
- Yao, Q.; Li, S.; Zhang, R.; Han, L.; Su, B. High-throughput thin-film composite membrane via interfacial polymerization using monomers of ultra-low concentration on tannic acid—Copper interlayer for organic solvent nanofiltration. Sep. Purif. Technol. 2021, 258, 118027. [Google Scholar] [CrossRef]
- Freger, V.; Gilron, J.; Belfer, S. TFC polyamide membranes modified by grafting of hydrophilic polymers: An FT-IR/AFM/TEM study. J. Membr. Sci. 2002, 209, 283–292. [Google Scholar] [CrossRef]
- Zhan, Z.-M.; Tang, Y.-J.; Zhu, K.-K.; Xue, S.-M.; Ji, C.-H.; Tang, C.Y.; Xu, Z.-L. Coupling heat curing and surface modification for the fabrication of high permselectivity polyamide nanofiltration membranes. J. Membr. Sci. 2021, 623, 119073. [Google Scholar] [CrossRef]
- Rho, H.; Im, S.J.; Alrehaili, O.; Lee, S.; Jang, A.; Perreault, F.; Westerhoff, P. Facile Surface Modification of Polyamide Membranes Using UV-Photooxidation Improves Permeability and Reduces Natural Organic Matter Fouling. Environ. Sci. Technol. 2021, 55, 6984–6994. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, K.; Guan, H.; Liu, M.; Yu, S.; Gao, C. Improved separation efficiency of polyamide-based composite nanofiltration membrane by surface modification using 3-aminopropyltriethoxysilane. Sep. Purif. Technol. 2021, 274, 119142. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Chen, S.T.; Patra, T.; Hsu, C.H.; Sengupta, A.; Hung, W.S.; Huang, S.H.; Qian, X.; Wickramasinghe, R.; Chang, Y.; et al. Zwitterionic forward osmosis membrane modified by fast second interfacial polymerization with enhanced antifouling and antimicrobial properties for produced water pretreatment. Desalination 2019, 469, 114090. [Google Scholar] [CrossRef]
- Feng, X.; Liu, D.; Ye, H.; Peng, D.; Wang, J.; Han, S.; Zhang, Y. High-flux polyamide membrane with improved chlorine resistance for efficient dye/salt separation based on a new N-rich amine monomer. Sep. Purif. Technol. 2021, 278, 119533. [Google Scholar] [CrossRef]
- Yu, S.; Liu, M.; Lü, Z.; Zhou, Y.; Gao, C. Aromatic-cycloaliphatic polyamide thin-film composite membrane with improved chlorine resistance prepared from m-phenylenediamine-4-methyl and cyclohexane-1,3,5-tricarbonyl chloride. J. Membr. Sci. 2009, 344, 155–164. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, B.; Liu, W.; Li, J.; Gao, C.; Pan, Q. Preparation and characterization of a novel nanofiltration membrane with chlorine-tolerant property and good separation performance. RSC Adv. 2018, 8, 36430–36440. [Google Scholar] [CrossRef]
- Verbeke, R.; Gómez, V.; Vankelecom, I.F.J. Chlorine-resistance of reverse osmosis (RO) polyamide membranes. Prog. Polym. Sci. 2017, 72, 1–15. [Google Scholar] [CrossRef]
- Valamohammadi, E.; Behdarvand, F.; Tofighy, M.A.; Mohammadi, T. Preparation of positively charged thin-film nanocomposite membranes based on the reaction between hydrolyzed polyacrylonitrile containing carbon nanomaterials and HPEI for water treatment application. Sep. Purif. Technol. 2020, 242, 116826. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, H.; Ji, S.; Liu, Z. Self-assembly of polyelectrolyte multilayer pervaporation membranes by a dynamic layer-by-layer technique on a hydrolyzed polyacrylonitrile ultrafiltration membrane. J. Membr. Sci. 2007, 292, 1–8. [Google Scholar] [CrossRef]
- An, Q.F.; Ang, M.B.M.Y.; Huang, Y.H.; Huang, S.H.; Chia, Y.H.; Lai, C.L.; Tsai, H.A.; Hun, W.S.; Hu, C.C.; Wu, Y.P.; et al. Microstructural characterization and evaluation of pervaporation performance of thin-film composite membranes fabricated through interfacial polymerization on hydrolyzed polyacrylonitrile substrate. J. Membr. Sci. 2019, 583, 31–39. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.H.; Li, Y.C.; Cahatol, A.T.C.; Tayo, L.L.; Hung, W.S.; Tsai, H.A.; Hu, C.C.; Lee, K.R.; Lai, J.Y. High-performance thin-film composite polyetheramide membranes for the dehydration of tetrahydrofuran. J. Membr. Sci. 2020, 611, 118373. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Veríssimo, S.; Peinemann, K.V.; Bordado, J. Influence of the diamine structure on the nanofiltration performance, surface morphology and surface charge of the composite polyamide membranes. J. Membr. Sci. 2006, 279, 266–275. [Google Scholar] [CrossRef]
- Li, P.; Lan, H.; Chen, K.; Ma, X.; Wei, B.; Wang, M.; Li, P.; Hou, Y.; Jason Niu, Q. Novel high-flux positively charged aliphatic polyamide nanofiltration membrane for selective removal of heavy metals. Sep. Purif. Technol. 2022, 280, 119949. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, C.; Du, S.; Wang, T.; Luan, Z.; Wang, J.; Hou, D. Fabrication of asymmetric poly (m-phenylene isophthalamide) nanofiltration membrane for chromium(VI) removal. J. Environ. Sci. 2010, 22, 1335–1341. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Luo, Z.Y.; Marquez, J.A.D.; Tsai, H.A.; Huang, S.H.; Hung, W.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Merits of using cellulose triacetate as a substrate in producing thin-film composite nanofiltration polyamide membranes with ultra-high performance. J. Taiwan Inst. Chem. Eng. 2020, 112, 251–258. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Wei, X.; Wang, C.; Qu, M.; Schubert, D.W.; Zhang, C. An outstanding antichlorine and antibacterial membrane with quaternary ammonium salts of alkenes via in situ polymerization for textile wastewater treatment. Chem. Eng. J. 2020, 384, 123306. [Google Scholar] [CrossRef]
- Bengani-Lutz, P.; Zaf, R.D.; Culfaz-Emecen, P.Z.; Asatekin, A. Extremely fouling resistant zwitterionic copolymer membranes with ~1 nm pore size for treating municipal, oily and textile wastewater streams. J. Membr. Sci. 2017, 543, 184–194. [Google Scholar] [CrossRef]
- Liu, M.; Yu, C.; Dong, Z.; Jiang, P.; Lü, Z.; Yu, S.; Gao, C. Improved separation performance and durability of polyamide reverse osmosis membrane in tertiary treatment of textile effluent through grafting monomethoxy-poly(ethylene glycol) brushes. Sep. Purif. Technol. 2019, 209, 443–451. [Google Scholar] [CrossRef]
- Kwon, Y.; Leckie, J. Hypochlorite degradation of crosslinked polyamide membranes: II. Changes in hydrogen bonding behavior and performance. J. Membr. Sci. 2006, 282, 456–464. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Effects of chlorine exposure conditions on physiochemical properties and performance of a polyamide membrane—Mechanisms and implications. Environ. Sci. Technol. 2012, 46, 13184–13192. [Google Scholar] [CrossRef] [PubMed]







| C | O | N | N/O | |
|---|---|---|---|---|
| TFCCHD | 75.39 | 16.7 | 7.91 | 0.473653 |
| TFCEDA | 67.71 | 18.96 | 13.33 | 0.703059 |
| TFCPPD | 71.12 | 19.24 | 9.65 | 0.501559 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, M.B.M.Y.; Wu, Y.-L.; Chu, M.-Y.; Wu, P.-H.; Chiao, Y.-H.; Millare, J.C.; Huang, S.-H.; Tsai, H.-A.; Lee, K.-R. Nanofiltration Membranes Formed through Interfacial Polymerization Involving Cycloalkane Amine Monomer and Trimesoyl Chloride Showing Some Tolerance to Chlorine during Dye Desalination. Membranes 2022, 12, 333. https://doi.org/10.3390/membranes12030333
Ang MBMY, Wu Y-L, Chu M-Y, Wu P-H, Chiao Y-H, Millare JC, Huang S-H, Tsai H-A, Lee K-R. Nanofiltration Membranes Formed through Interfacial Polymerization Involving Cycloalkane Amine Monomer and Trimesoyl Chloride Showing Some Tolerance to Chlorine during Dye Desalination. Membranes. 2022; 12(3):333. https://doi.org/10.3390/membranes12030333
Chicago/Turabian StyleAng, Micah Belle Marie Yap, Yi-Ling Wu, Min-Yi Chu, Ping-Han Wu, Yu-Hsuan Chiao, Jeremiah C. Millare, Shu-Hsien Huang, Hui-An Tsai, and Kueir-Rarn Lee. 2022. "Nanofiltration Membranes Formed through Interfacial Polymerization Involving Cycloalkane Amine Monomer and Trimesoyl Chloride Showing Some Tolerance to Chlorine during Dye Desalination" Membranes 12, no. 3: 333. https://doi.org/10.3390/membranes12030333