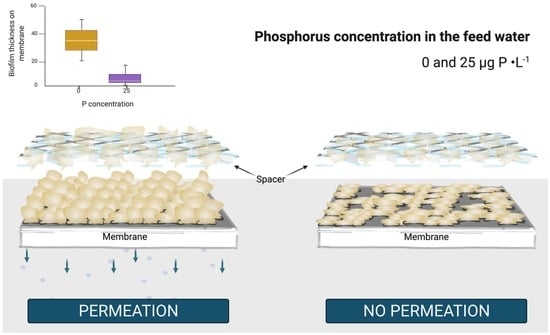
Permeation Increases Biofilm Development in Nanofiltration Membranes Operated with Varying Feed Water Phosphorous Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Water Composition
2.2. Phosphorus Concentration in the Feed Water
2.3. Experimental Setup and Operational Parameters
2.4. Biofilm in Situ Visualization in the MFS
2.5. Total Cell Count, Adenosine Triphosphate, and Extracellular Polymeric Substances Quantification
2.6. Total Phosphorus on the Membrane and Spacer Characterization
3. Results
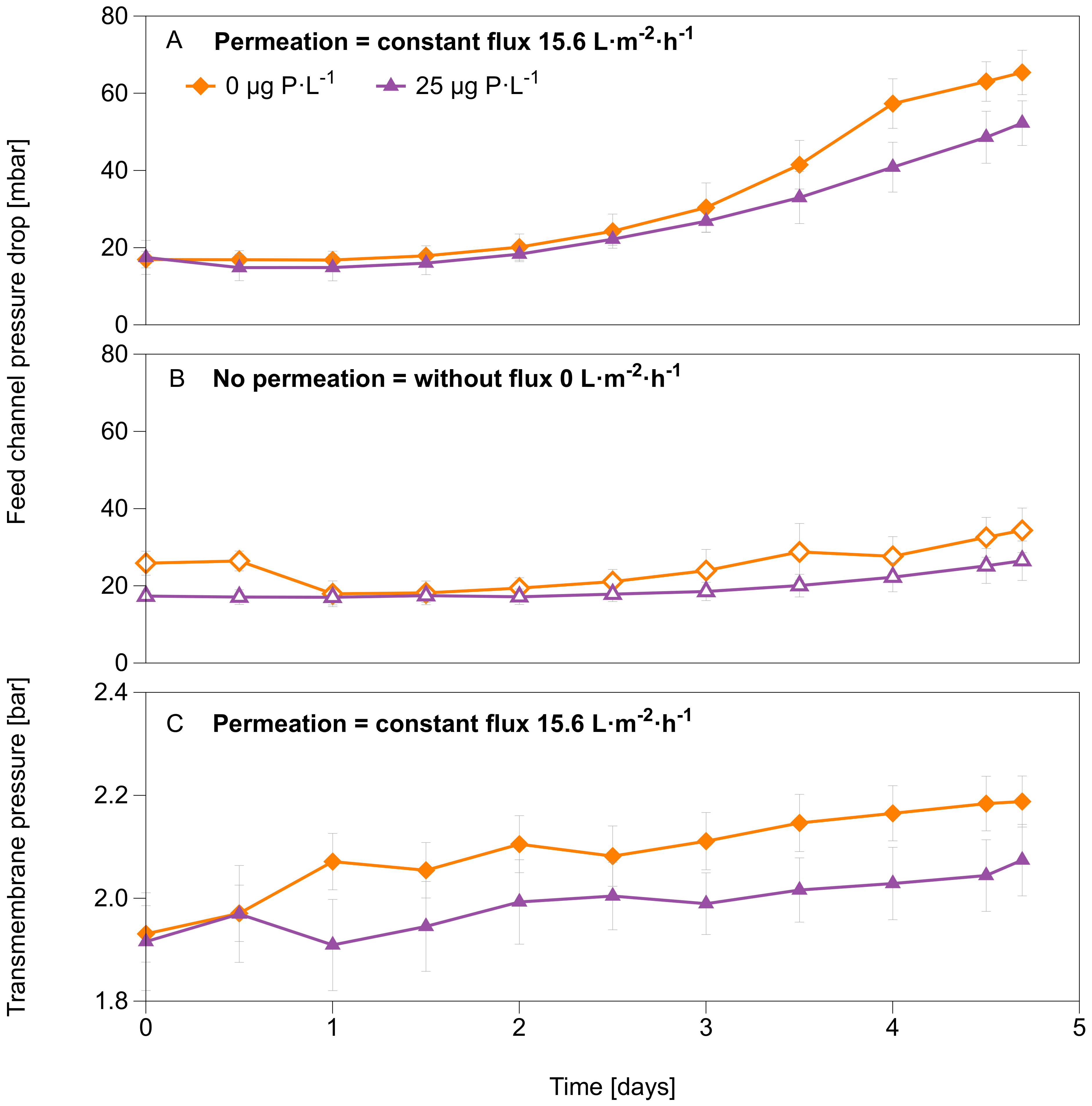
3.1. System Performance Parameters: Feed Channel Pressure Drop, Transmembrane Pressure, and Flux
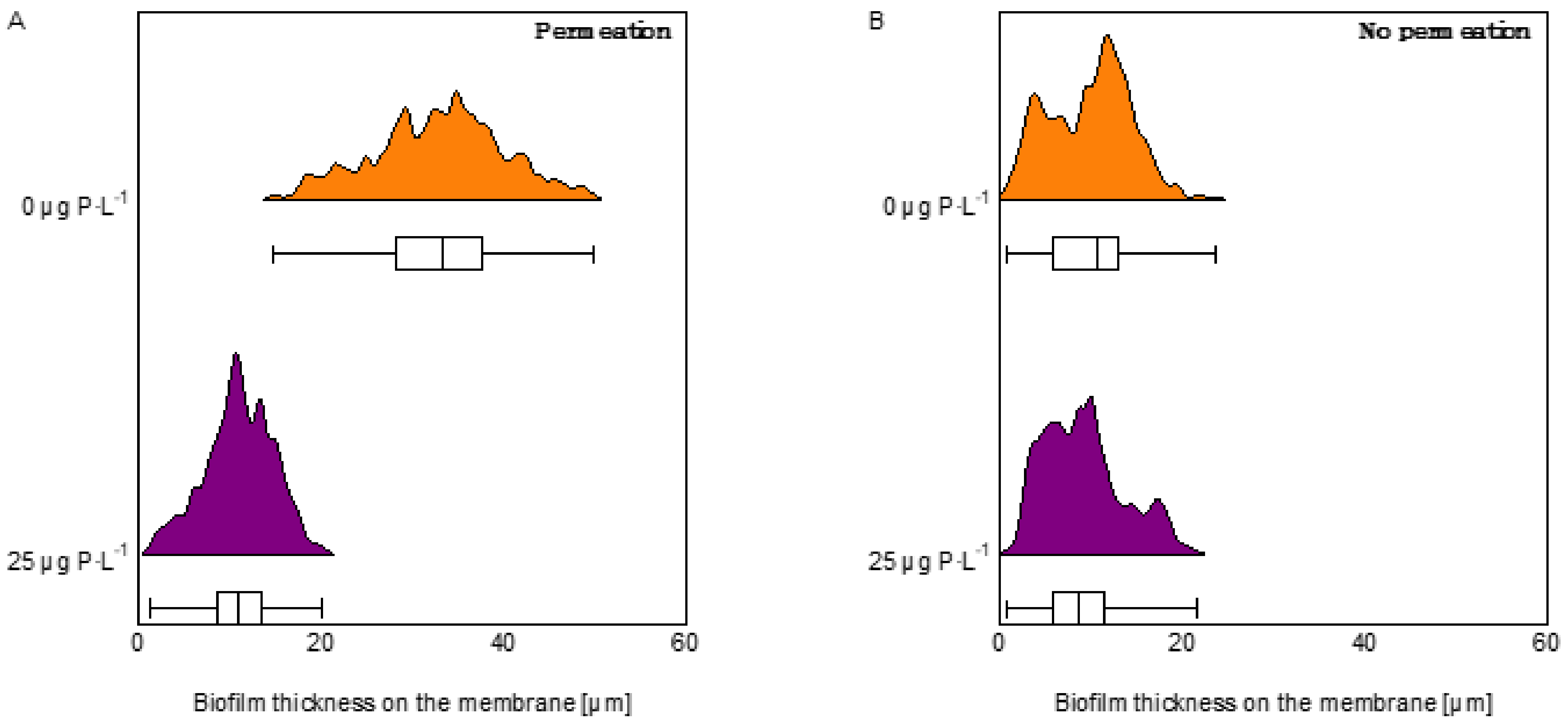
3.2. Optical Coherence Tomography Images and Biofilm Thickness on the Membrane
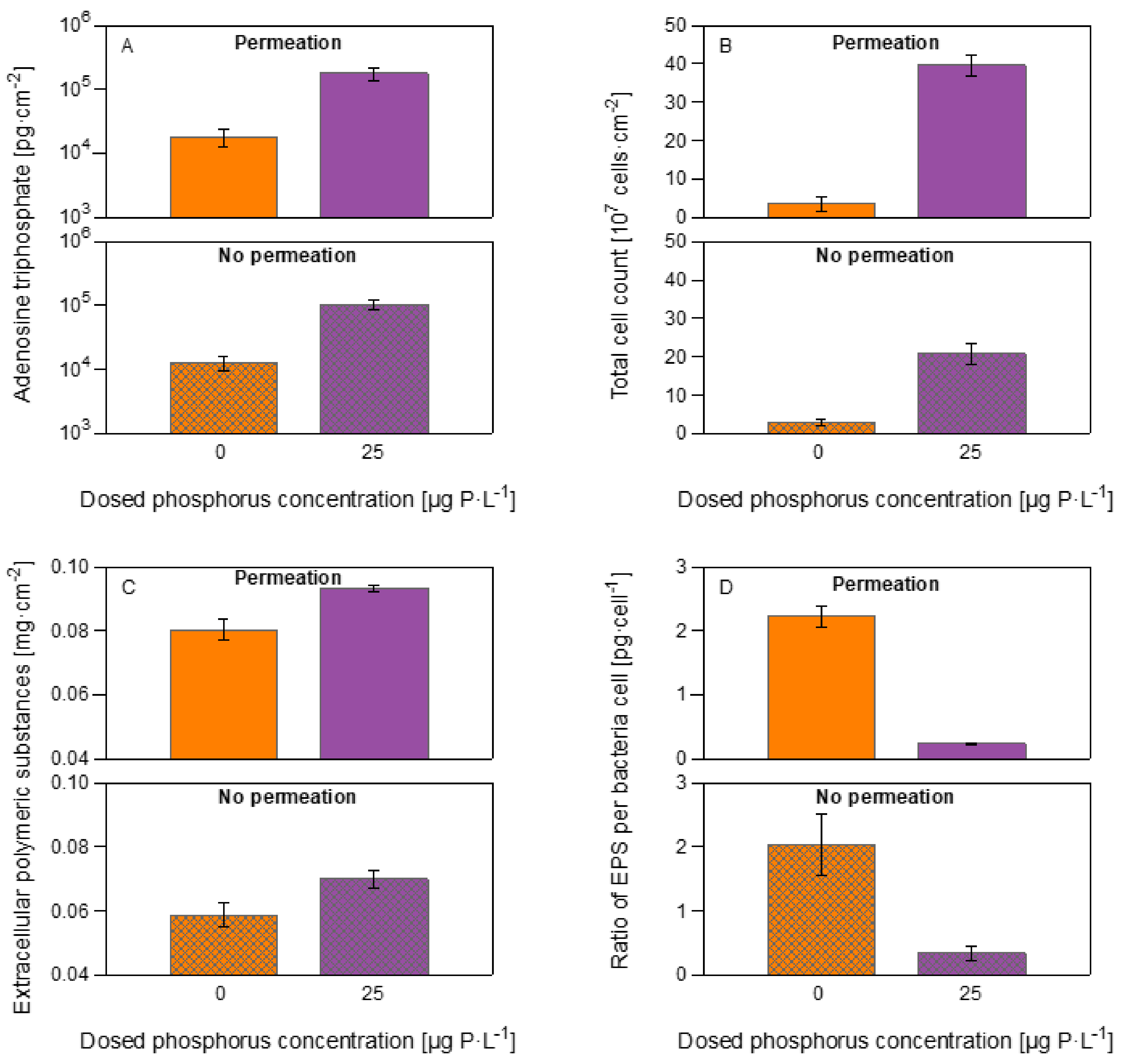
3.3. Biomass Quantification
3.4. Phosphorus Measurements
4. Discussion
4.1. Permeation Caused a Faster Decline in System Performance
4.2. Permeation Impact on Biofilm Development Varied Depending on the Nutrient Condition
4.3. Practical Implications and Future Research
5. Conclusions
- (i).
- Permeation resulted in a faster decline in system performance (faster feed channel pressure drop increase and higher transmembrane pressure increase).
- (ii).
- Permeation impact on biofilm development varied depending on nutrient condition with a stronger impact at low phosphorous concentration:
- ○
- For the 0 μg P·L−1 under permeation conditions, the pressure drop increase is explained by “expanded” EPS in the flow channel, thus biofilm localization. The higher flux decline was explained by a thicker biofilm, resulting from a higher phosphorus accumulation on the membrane.
- ○
- For the 25 μg P·L−1 under permeation conditions, the pressure drop increase is explained by a higher quantity of “condensed” EPS around the spacer, thus biofilm structure and composition.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenten, G. Recent Development in Membrane Science and Its Industrial Applications. Membr. Sci. Technol. 2003, 24, 1009–1024. Available online: https://rdo.psu.ac.th/sjstweb/journal/24-Suppl-1/27membrane-science.pdf (accessed on 7 January 2022).
- Loeb, S. The Loeb-Sourirajan Membrane: How It Came About. 1981. Available online: https://pubs.acs.org/sharingguidelines (accessed on 7 August 2021).
- Fane, A.G.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–45. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Patra, T.; Ang, M.B.M.Y.; Chen, S.-T.; Almodovar, J.; Qian, X.; Wickramasinghe, S.R.; Hung, W.-S.; Huang, S.-H.; Chang, Y.; et al. Zwitterion Co-Polymer PEI-SBMA Nanofiltration Membrane Modified by Fast Second Interfacial Polymerization. Polymers 2020, 12, 269. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.-Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Membr. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol. Lett. 2017, 364, 120. [Google Scholar] [CrossRef] [Green Version]
- Unosson, E. Antibacterial Strategies for Titanium Biomaterials. 2015. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-249181 (accessed on 26 September 2021).
- Vrouwenvelder, J.; Manolarakis, S.; van der Hoek, J.; van Paassen, J.; van der Meer, W.; van Agtmaal, J.; Prummel, H.; Kruithof, J.; van Loosdrecht, M. Quantitative biofouling diagnosis in full scale nanofiltration and reverse osmosis installations. Water Res. 2008, 42, 4856–4868. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.-C. The perfect slime. Colloids Surf. B Biointerfaces 2011, 86, 251–259. [Google Scholar] [CrossRef]
- Jafari, M.; Vanoppen, M.; van Agtmaal, J.; Cornelissen, E.; Vrouwenvelder, J.; Verliefde, A.; van Loosdrecht, M.; Picioreanu, C. Cost of fouling in full-scale reverse osmosis and nanofiltration installations in the Netherlands. Desalination 2021, 500, 114865. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—the Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.; Beyer, F.; Dahmani, K.; Hasan, N.; Galjaard, G.; Kruithof, J.; van Loosdrecht, M. Phosphate limitation to control biofouling. Water Res. 2010, 44, 3454–3466. [Google Scholar] [CrossRef]
- Kim, C.-M.; Kim, S.-J.; Kim, L.H.; Shin, M.S.; Yu, H.-W.; Kim, I.S. Effects of phosphate limitation in feed water on biofouling in forward osmosis (FO) process. Desalination 2014, 349, 51–59. [Google Scholar] [CrossRef]
- Farhat, N.; Javier, L.; Van Loosdrecht, M.; Kruithof, J.; Vrouwenvelder, J. Role of feed water biodegradable substrate concentration on biofouling: Biofilm characteristics, membrane performance and cleanability. Water Res. 2019, 150, 1–11. [Google Scholar] [CrossRef]
- Javier, L.; Farhat, N.M.; Vrouwenvelder, J.S. Enhanced hydraulic cleanability of biofilms developed under a low phosphorus concentration in reverse osmosis membrane systems. Water Res. X 2021, 10, 100085. [Google Scholar] [CrossRef]
- Javier, L.; Farhat, N.M.; Desmond, P.; Linares, R.V.; Bucs, S.; Kruithof, J.C.; Vrouwenvelder, J.S. Biofouling control by phosphorus limitation strongly depends on the assimilable organic carbon concentration. Water Res. 2020, 183, 116051. [Google Scholar] [CrossRef]
- Monaghan, E.J.; Ruttenberg, K.C. Dissolved organic phosphorus in the coastal ocean: Reassessment of available methods and seasonal phosphorus profiles from the Eel River Shelf. Limnol. Oceanogr. 1999, 44, 1702–1714. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, M.M. Defining the Condensed Phosphates. J. Chem. Educ. 1956, 33, 36. [Google Scholar] [CrossRef]
- Estela, J.M.; Cerdà, V. Flow analysis techniques for phosphorus: An overview. Talanta 2005, 66, 307–331. [Google Scholar] [CrossRef]
- Mine, A.H.; Coleman, M.L.; Colman, A.S. Phosphorus Release and Regeneration Following Laboratory Lysis of Bacterial Cells. Front. Microbiol. 2021, 12, 641700. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, I.T.; Vartiainen, T.; Myllykangas, T.; Martikainen, P.J. Microbially Available Organic Carbon, Phosphorus, and Microbial Growth in Ozonated Drinking Water. Water Res. 2001, 35, 1635–1640. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Hinrichs, C.; Van Der Meer, W.G.; van Loosdrecht, M.; Kruithof, J.C. Pressure drop increase by biofilm accumulation in spiral wound RO and NF membrane systems: Role of substrate concentration, flow velocity, substrate load and flow direction. Biofouling 2009, 25, 543–555. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.; van Paassen, J.; van Agtmaal, J.; van Loosdrecht, M.; Kruithof, J. A critical flux to avoid biofouling of spiral wound nanofiltration and reverse osmosis membranes: Fact or fiction? J. Membr. Sci. 2009, 326, 36–44. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Neo. Low Phosphorus in Wastewater Treatment, Phosphorus Limit. 2020. Available online: https://www.neomaterials.com/phosphorus-reduction-in-wastewater-treatment/ (accessed on 3 August 2021).
- Yang, J.; Bai, J.; Liu, M.; Chen, Y.; Wang, S.; Yang, Q. Determination of Phosphorus in Soil by ICP-OES Using an Improved Standard Addition Method. J. Anal. Methods Chem. 2018, 2018, 1324751. [Google Scholar] [CrossRef]
- Belila, A.; El-Chakhtoura, J.; Otaibi, N.; Muyzer, G.; Gonzalez-Gil, G.; Saikaly, P.; van Loosdrecht, M.; Vrouwenvelder, J. Bacterial community structure and variation in a full-scale seawater desalination plant for drinking water production. Water Res. 2016, 94, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Sanawar, H.; Siddiqui, A.; Bucs, S.; Farhat, N.; van Loosdrecht, M.; Kruithof, J.; Vrouwenvelder, J. Applicability of short-term accelerated biofouling studies to predict long-term biofouling accumulation in reverse osmosis membrane systems. Desalin. Water Treat. 2017, 97, 72–78. [Google Scholar] [CrossRef]
- Farhat, N.; Staal, M.; Bucs, S.; Van Loosdrecht, M.; Vrouwenvelder, J. Spatial heterogeneity of biofouling under different cross-flow velocities in reverse osmosis membrane systems. J. Membr. Sci. 2016, 520, 964–971. [Google Scholar] [CrossRef]
- Sevcenco, A.-M.; Paravidino, M.; Vrouwenvelder, J.S.; Wolterbeek, H.T.; van Loosdrecht, M.C.; Hagen, W.R. Phosphate and arsenate removal efficiency by thermostable ferritin enzyme from Pyrococcus furiosus using radioisotopes. Water Res. 2015, 76, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Sanawar, H.; Pinel, I.; Farhat, N.; Bucs, S.; Zlopasa, J.; Kruithof, J.; Witkamp, G.; van Loosdrecht, M.; Vrouwenvelder, J. Enhanced biofilm solubilization by urea in reverse osmosis membrane systems. Water Res. X 2018, 1, 100004. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Javier, L.; Pulido-Beltran, L.; Kruithof, J.; Vrouwenvelder, J.S.; Farhat, N.M. Phosphorus Concentration in Water Affects the Biofilm Community and the Produced Amount of Extracellular Polymeric Substances in Reverse Osmosis Membrane Systems. Membranes 2021, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Vrouwenvelder, J.; Bakker, S.; Cauchard, M.; Le Grand, R.; Apacandié, M.; Idrissi, M.; Lagrave, S.; Wessels, L.; Van Paassen, J.; Kruithof, J.; et al. The membrane fouling simulator: A suitable tool for prediction and characterisation of membrane fouling. Water Sci. Technol. 2007, 55, 197–205. [Google Scholar] [CrossRef]
- Siddiqui, A.; Lehmann, S.; Bucs, S.S.; Fresquet, M.; Fel, L.; Prest, E.; Ogier, J.; Schellenberg, C.; van Loosdrecht, M.; Kruithof, J.; et al. Predicting the impact of feed spacer modification on biofouling by hydraulic characterization and biofouling studies in membrane fouling simulators. Water Res. 2017, 110, 281–287. [Google Scholar] [CrossRef]
- Koyuncu, I.; Topacik, D. Effect of Cross Flow Velocity, Feed Concentration, and Pressure on the Salt Rejection of Nanofiltration Membranes in Reactive Dye Having Two Sodium Salts and NaCl Mixtures: Model Application. J. Environ. Sci. Health Part A 2004, 39, 1055–1068. [Google Scholar] [CrossRef]
- Dreszer, C.; Flemming, H.-C.; Zwijnenburg, A.; Kruithof, J.; Vrouwenvelder, J. Impact of biofilm accumulation on transmembrane and feed channel pressure drop: Effects of crossflow velocity, feed spacer and biodegradable nutrient. Water Res. 2014, 50, 200–211. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Van Paassen, J.A.M.; Wessels, L.P.; Van Dam, A.F.; Bakker, S.M. The Membrane Fouling Simulator: A practical tool for fouling prediction and control. J. Membr. Sci. 2006, 281, 316–324. [Google Scholar] [CrossRef]
- Shaaban, A.M.F.; Hafez, A.I.; Abdel-Fatah, M.A.; Abdel-Monem, N.M.; Mahmoud, M.H. Process engineering optimization of nanofiltration unit for the treatment of textile plant effluent in view of solution diffusion model. Egypt. J. Pet. 2016, 25, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Derlon, N.; Peter-Varbanets, M.; Scheidegger, A.; Pronk, W.; Morgenroth, E. Predation influences the structure of biofilm developed on ultrafiltration membranes. Water Res. 2012, 46, 3323–3333. [Google Scholar] [CrossRef]
- Desmond, P.; Morgenroth, E.; Derlon, N. Physical structure determines compression of membrane biofilms during Gravity Driven Membrane (GDM) ultrafiltration. Water Res. 2018, 143, 539–549. [Google Scholar] [CrossRef]
- Neu, L.; Proctor, C.R.; Walser, J.-C.; Hammes, F. Small-Scale Heterogeneity in Drinking Water Biofilms. Front. Microbiol. 2019, 10, 2446. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, H.H.P. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Holden, M.J.; Rabb, S.A.; Tewari, Y.B.; Winchester, M.R. Traceable Phosphorus Measurements by ICP-OES and HPLC for the Quantitation of DNA. Anal. Chem. 2007, 79, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Eshed, L.; Yaron, S.; Dosoretz, C.G. Effect of Permeate Drag Force on the Development of a Biofouling Layer in a Pressure-Driven Membrane Separation System. Appl. Environ. Microbiol. 2008, 74, 7338–7347. [Google Scholar] [CrossRef] [Green Version]
- Chong, T.H.; Wong, F.; Fane, A. The effect of imposed flux on biofouling in reverse osmosis: Role of concentration polarisation and biofilm enhanced osmotic pressure phenomena. J. Membr. Sci. 2008, 325, 840–850. [Google Scholar] [CrossRef]
- Suwarno, S.; Chen, X.; Chong, T.H.; McDougald, D.; Cohen, Y.; Rice, S.; Fane, A. Biofouling in reverse osmosis processes: The roles of flux, crossflow velocity and concentration polarization in biofilm development. J. Membr. Sci. 2014, 467, 116–125. [Google Scholar] [CrossRef]
- Farhat, N.; Staal, M.; Siddiqui, A.; Borisov, S.; Bucs, S.; Vrouwenvelder, H. Early non-destructive biofouling detection and spatial distribution: Application of oxygen sensing optodes. Water Res. 2015, 83, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Vrouwenvelder, J.S.; Von Der Schulenburg, D.G.; Kruithof, J.C.; Johns, M.L.; Van Loosdrecht, M.C.M. Biofouling of spiral-wound nanofiltration and reverse osmosis membranes: A feed spacer problem. Water Res. 2009, 43, 583–594. [Google Scholar] [CrossRef]
- Ghosh, P.; Mondal, J.; Ben-Jacob, E.; Levine, H. Mechanically-driven phase separation in a growing bacterial colony. Proc. Natl. Acad. Sci. USA 2015, 112, E2166–E2173. [Google Scholar] [CrossRef] [Green Version]
- NI, B.-J.; Yu, H.-Q. Microbial Products of Activated Sludge in Biological Wastewater Treatment Systems: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2011, 42, 187–223. [Google Scholar] [CrossRef]
- Jin, X.; Marshall, J.S. Mechanics of biofilms formed of bacteria with fimbriae appendages. PLoS ONE 2020, 15, e0243280. [Google Scholar] [CrossRef]
- Song, Z.W.; Ning, T.T.; Chen, Y.P.; Cheng, X.X.; Ren, N.Q. Bioaugmentation of Aerobic Granular Sludge with the Addition of a Bioflocculant-Producing Consortium. Adv. Mater. Res. 2013, 726-731, 2530–2535. [Google Scholar] [CrossRef]
- Szabó, E.; Liébana, R.; Hermansson, M.; Modin, O.; Persson, F.; Wilén, B.-M. Microbial Population Dynamics and Ecosystem Functions of Anoxic/Aerobic Granular Sludge in Sequencing Batch Reactors Operated at Different Organic Loading Rates. Front. Microbiol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Weissbrodt, D.G.; Shani, N.; Holliger, C. Linking bacterial population dynamics and nutrient removal in the granular sludge biofilm ecosystem engineered for wastewater treatment. FEMS Microbiol. Ecol. 2014, 88, 579–595. [Google Scholar] [CrossRef] [Green Version]
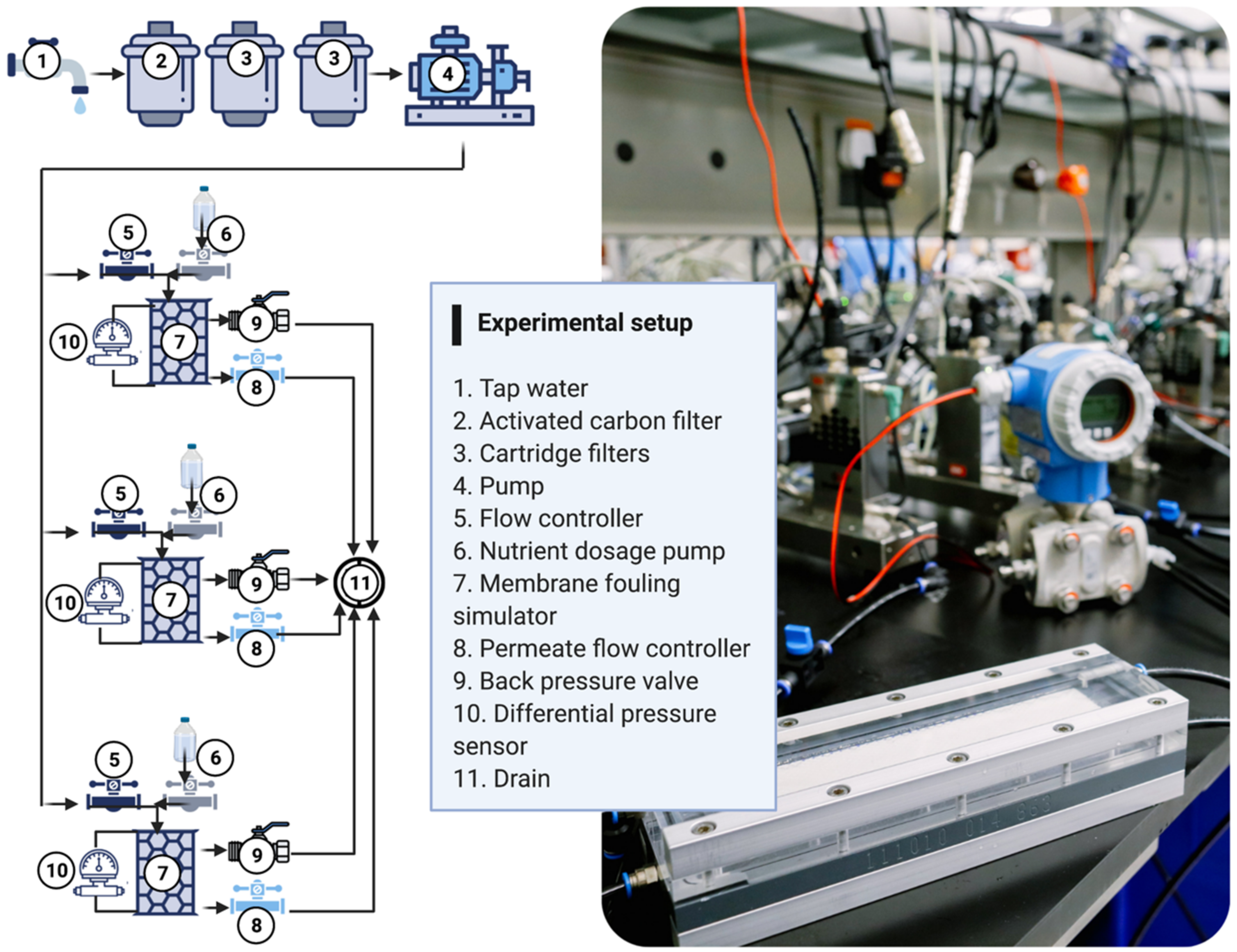



| Dosed C Concentration (µg C·L−1) | Dosed N Concentration (µg N·L−1) | Dosed P Concentration (µg P·L−1) | Permeation |
|---|---|---|---|
| 250 | 50 | 0 | Yes |
| 250 | 50 | 0 | No |
| 250 | 50 | 25 | Yes |
| 250 | 50 | 25 | No |
| Dosed Phosphorus Concentration | 0 µg P·L−1 | 25 µg P·L−1 |
|---|---|---|
| Phosphorus IN | ||
| Reactive phosphate as phosphorus in the feed water, PR-FW [µg PO4-PR] | 559 | 35,287 |
| Reactive phosphate as phosphorus in the tap water PR-TAP [µg PO4-PR] | 559 | 559 |
| Reactive phosphate as phosphorus dosed, PR-DSD [µg PO4-PR] | 0 | 34,728 |
| Phosphorus in the MFS under permeation conditions | ||
| Total phosphorus (reactive and non-reactive) accumulated on the membrane and spacer, PMFS [µg P] | 4943 | 31,716 |
| Phosphorus in the MFS under no permeation conditions | ||
| Total phosphorus (reactive and non-reactive) accumulated on the membrane and spacer, PMFS [µg P] | 4946 | 16,204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javier, L.; Pulido-Beltran, L.; Vrouwenvelder, J.S.; Farhat, N.M. Permeation Increases Biofilm Development in Nanofiltration Membranes Operated with Varying Feed Water Phosphorous Concentrations. Membranes 2022, 12, 335. https://doi.org/10.3390/membranes12030335
Javier L, Pulido-Beltran L, Vrouwenvelder JS, Farhat NM. Permeation Increases Biofilm Development in Nanofiltration Membranes Operated with Varying Feed Water Phosphorous Concentrations. Membranes. 2022; 12(3):335. https://doi.org/10.3390/membranes12030335
Chicago/Turabian StyleJavier, Luisa, Laura Pulido-Beltran, Johannes S. Vrouwenvelder, and Nadia M. Farhat. 2022. "Permeation Increases Biofilm Development in Nanofiltration Membranes Operated with Varying Feed Water Phosphorous Concentrations" Membranes 12, no. 3: 335. https://doi.org/10.3390/membranes12030335