Is There Still Room for Cancer Vaccines at the Era of Checkpoint Inhibitors
Abstract
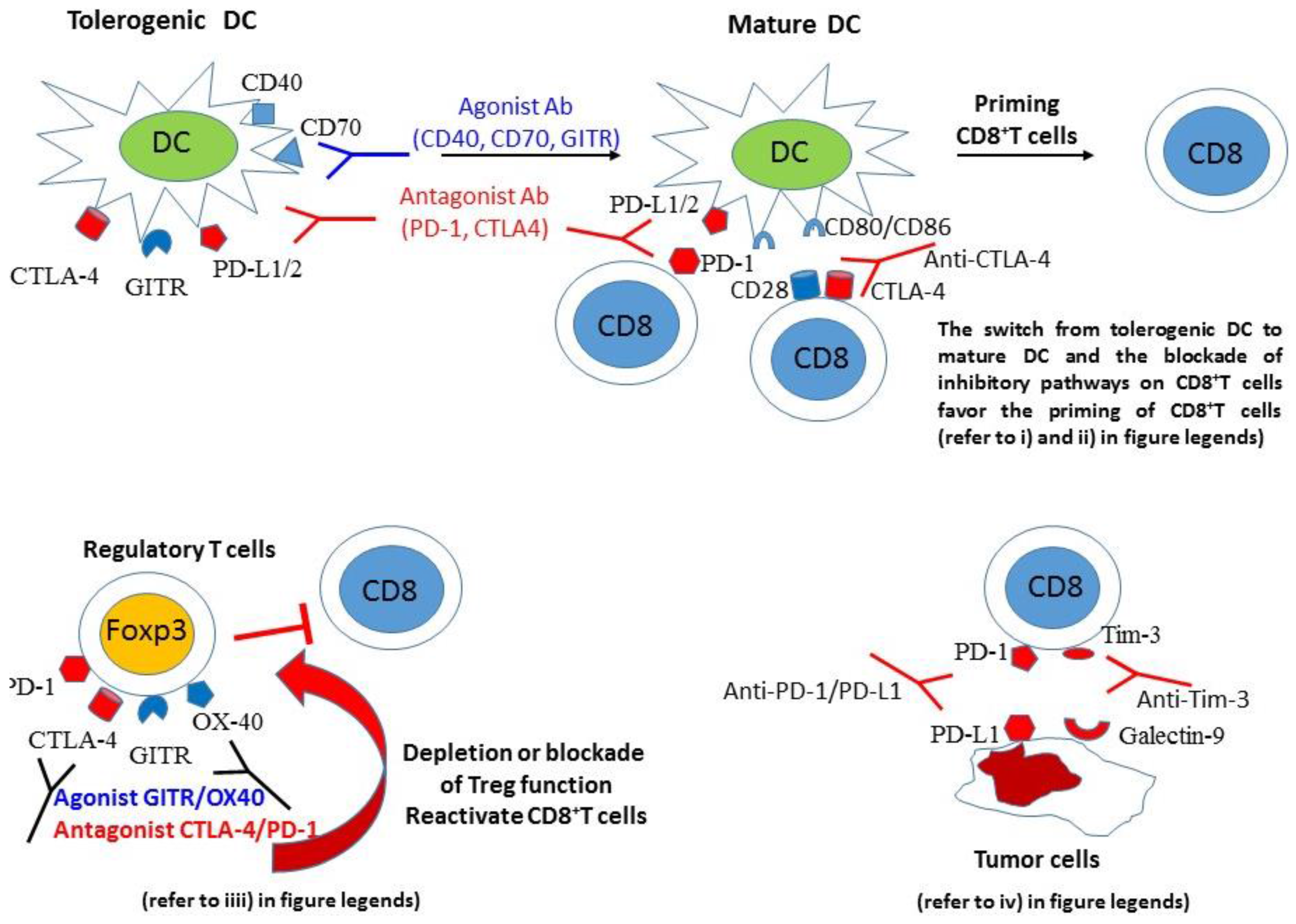
:1. Introduction
2. Rationale for the Combination of Cancer Vaccines AND Checkpoint Inhibitor Blockade
3. Combination of Cancer Vaccines and Modulation of Costimulatory Pathways in Preclinical Models (Table 1)
3.1. Inhibitory Pathway (PD-1/PD-L1-PD-L2, CTLA-4/CD80-CD86) Blockade Increases the Efficacy of Various Types of Cancer Vaccines
3.1.1. Peptide Vaccines
3.1.2. Live Vectors
3.1.3. Cellular Vaccines
3.1.4. Inert Vectors Targeting Dendritic Cells
3.1.5. DNA Vaccines
3.2. Synergy between Cancer Vaccines and Checkpoint Inhibitor Blockade Extends Beyond CTLA-4 and PD-1 Pathway Inhibition
3.3. Checkpoint Inhibitor Blockade is Not Limited to the Use of Antibodies
3.4. Agonist Antibodies Directed Against Costimulatory Molecules are also Synergistic with Cancer Vaccines
3.5. Multiple Targeting of Costimulatory or Inhibitory Receptors Dramatically Improved the Efficiency of Cancer Vaccines
3.5.1. Cancer Vaccines Combined with Multiple Inhibitory Receptor Blockade
3.5.2. Cancer Vaccines Combined with Agonist mAb Against Costimulatory Molecules and Antagonist mAb Against Checkpoint Inhibitors
4. Mechanisms Mediating the Efficacy of Cancer Vaccines Combined with Inhibitory Pathway Blockade or Costimulatory Pathway Activation
5. Combinations of Cancer Vaccine and Modulation of Inhibitory Pathways in Humans
6. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Van Poelgeest, M.I.; Welters, M.J.; Vermeij, R.; Stynenbosch, L.F.; Loof, N.M.; Berends-van der Meer, D.M.; Lowik, M.J.; Hamming, I.L.; van Esch, E.M.; Hellebrekers, B.W.; et al. Vaccination against Oncoproteins of HPV16 for Noninvasive Vulvar/Vaginal Lesions: Lesion Clearance Is Related to the Strength of the T-Cell Response. Clin. Cancer Res. 2016, 22, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Pere, H.; Badoual, C.; Tartour, E. Hope in the Long Road Toward the Development of a Therapeutic Human Papillomavirus Vaccine. Clin. Cancer Res. 2016, 22, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Skeate, J.G.; Woodham, A.W.; Einstein, M.H.; Da Silva, D.M.; Kast, W.M. Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum. Vaccin. Immunother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Jin, H.T.; Hur, S.Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.W.; Kim, S.; Woo, J.W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nature Commun. 2014. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L. HPV Infection-Associated Cancers: Next-Generation Technology for Diagnosis and Treatment. Cancer Immunol. Res. 2014, 2, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, F.; Terme, M.; Nizard, M.; Badoual, C.; Bureau, M.F.; Freyburger, L.; Clement, O.; Marcheteau, E.; Gey, A.; Fraisse, G.; et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci. Transl. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Peng, S.; Han, L.; Qiu, J.; Song, L.; Tsai, Y.; Yang, B.; Roden, R.B.; Trimble, C.L.; Hung, C.F.; et al. Local HPV Recombinant Vaccinia Boost Following Priming with an HPV DNA Vaccine Enhances Local HPV-Specific CD8+ T-cell-Mediated Tumor Control in the Genital Tract. Clin. Cancer Res. 2016, 22, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Roussel, H.; Tartour, E. Resident Memory T Cells as Surrogate Markers of the Efficacy of Cancer Vaccines. Clin. Cancer Res. 2016, 22, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.; van der Burg, S.H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nature Rev. Cancer 2008, 8, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Disis, M.L. Designing vaccines to prevent breast cancer recurrence or invasive disease. Immunotherapy 2015, 7, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Diniz, M.O.; Dransart, E.; Gey, A.; Merillon, N.; Lone, Y.C.; Godefroy, S.; Sibley, C.; Ferreira, L.C.; Medioni, J.; et al. A Therapeutic Her2/neu Vaccine Targeting Dendritic Cells Preferentially Inhibits the Growth of Low Her2/neu-Expressing Tumor in HLA-A2 Transgenic Mice. Clin. Cancer Res. 2016, 22, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, L.H. Cancer vaccines. Br. Med. J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Combe, P.; de Guillebon, E.; Thibault, C.; Granier, C.; Tartour, E.; Oudard, S. Trial Watch: Therapeutic vaccines in metastatic renal cell carcinoma. Oncoimmunology 2015. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, T.C.; van Duikeren, S.; Huppelschoten, S.; Jordanova, E.S.; Beyranvand Nejad, E.; Sloots, A.; Boon, L.; Smit, V.T.; Welters, M.J.; Ossendorp, F.; et al. Vaccine-induced tumor necrosis factor-producing T cells synergize with cisplatin to promote tumor cell death. Clin. Cancer Res. 2015, 21, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Lena, H.; Losonczy, G.; Forget, F.; Chouaid, C.; Papai, Z.; Gervais, R.; Ottensmeier, C.; Szczesna, A.; Kazarnowicz, A.; et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): Results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016, 17, 212–223. [Google Scholar] [CrossRef]
- Mondini, M.; Nizard, M.; Tran, T.; Mauge, L.; Loi, M.; Clemenson, C.; Dugue, D.; Maroun, P.; Louvet, E.; Adam, J.; et al. Synergy of Radiotherapy and a Cancer Vaccine for the Treatment of HPV-Associated Head and Neck Cancer. Mol. Cancer Ther. 2015, 14, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Rixe, O.; Beuselinck, B.; Linassier, C.; Banu, E.; Machiels, J.P.; Baudard, M.; Ringeisen, F.; Velu, T.; Lefrere-Belda, M.A.; et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol. Immunother. 2011, 60, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Tartour, E.; Pere, H.; Maillere, B.; Terme, M.; Merillon, N.; Taieb, J.; Sandoval, F.; Quintin-Colonna, F.; Lacerda, K.; Karadimou, A.; et al. Angiogenesis and immunity: A bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011, 30, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Ramlau, R.; Quoix, E.; Rolski, J.; Pless, M.; Lena, H.; Levy, E.; Krzakowski, M.; Hess, D.; Tartour, E.; Chenard, M.P.; et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J. Thorac. Oncol. 2008, 3, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Lyerly, H.K. Checkpoint blockade in combination with cancer vaccines. Vaccine 2015, 33, 7377–7385. [Google Scholar] [CrossRef] [PubMed]
- Gibney, G.; Hamid, O.; Lutzky, J.; Olszanski, A.; Gangadhar, T.C.; Gajewski, T.F.; Chmielewski, B.; Hanks, B.; Boasberg, P.; Zhao, Y.; et al. Updated results from a phase 1/2 study of epacadostat (INCB024360) in combination with ipilimumab in patients with metastatic melanoma. Eur. J. Cancer 2015, 51, S106–S107. [Google Scholar] [CrossRef]
- Gangadhar, T.; Hamid, O.; Smith, D.; Bauer, T.; Wasser, J.; Luke, J.; Balmanoukian, A.; Kaufman, D.R.; Zhao, Y.; Maleski, J.; et al. Preliminary results from a phase I/II study of epacadostat (incb024360) in combination with pembrolizumab in patients with selected advanced cancers. J. Immunother. Cancer 2015. [Google Scholar] [CrossRef]
- Vilgelm, A.E.; Johnson, D.B.; Richmond, A. Combinatorial approach to cancer immunotherapy: Strength in numbers. J. Leukoc. Biol. 2016, 100, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Pagliano, O.; Chauvin, J.M.; Sander, C.; Janjic, B.; Tarhini, A.A.; Tawbi, H.A.; Kirkwood, J.M.; Moschos, S.; et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res. 2014, 74, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sultan, H.; Fesenkova, V.I.; Addis, D.; Fan, A.E.; Kumai, T.; Wu, J.; Salazar, A.M.; Celis, E. Designing therapeutic cancer vaccines by mimicking viral infections. Cancer Immunol. Immunother. 2016. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Pere, H.; Roussel, H.; Si Mohamed, A.; Tartour, E. Cancers of the upper aerodigestive tract associated with human papillomavirus. Med. Sci. 2013, 29, 83–88. [Google Scholar]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Anders, R.A.; Young, G.D.; Xu, H.; Sharma, R.; McMiller, T.L.; Chen, S.; Klein, A.P.; Pardoll, D.M.; Topalian, S.L.; et al. Colocalization of inflammatory response with b7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012. [Google Scholar] [CrossRef] [PubMed]
- Tanchot, C.; Terme, M.; Pere, H.; Tran, T.; Benhamouda, N.; Strioga, M.; Banissi, C.; Galluzzi, L.; Kroemer, G.; Tartour, E. Tumor-Infiltrating Regulatory T Cells: Phenotype, Role, Mechanism of Expansion In Situ and Clinical Significance. Cancer Microenviron. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pere, H.; Montier, Y.; Bayry, J.; Quintin-Colonna, F.; Merillon, N.; Dransart, E.; Badoual, C.; Gey, A.; Ravel, P.; Marcheteau, E.; et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self-antigens. Blood 2011, 118, 4853–4862. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.Q.; Mills, K.H. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene 2014, 33, 4623–4631. [Google Scholar] [CrossRef] [PubMed]
- Pere, H.; Tanchot, C.; Bayry, J.; Terme, M.; Taieb, J.; Badoual, C.; Adotevi, O.; Merillon, N.; Marcheteau, E.; Quillien, V.R.; et al. Comprehensive analysis of current approaches to inhibit regulatory T cells in cancer. Oncoimmunology 2012, 1, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Bulliard, Y.; Jolicoeur, R.; Windman, M.; Rue, S.M.; Ettenberg, S.; Knee, D.A.; Wilson, N.S.; Dranoff, G.; Brogdon, J.L. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 2013, 210, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn-Cymerman, D.; Rizzuto, G.A.; Merghoub, T.; Cohen, A.D.; Avogadri, F.; Lesokhin, A.M.; Weinberg, A.D.; Wolchok, J.D.; Houghton, A.N. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J. Exp. Med. 2009, 206, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Voo, K.S.; Bover, L.; Harline, M.L.; Vien, L.T.; Facchinetti, V.; Arima, K.; Kwak, L.W.; Liu, Y.J. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J. Immunol. 2013, 191, 3641–3650. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, J.; Nishikawa, H.; Muraoka, D.; Wang, L.; Noguchi, T.; Sato, E.; Kondo, S.; Allison, J.P.; Sakaguchi, S.; Old, L.J.; et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin. Cancer Res. 2010, 16, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.Y.; Keam, B.; Kim, S.; Lee, J.S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Weinschenk, T.; Stenzl, A.; Zdrojowy, R.; Pluzanska, A.; Szczylik, C.; Staehler, M.; Brugger, W.; Dietrich, P.Y.; Mendrzyk, R.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012, 18, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.; Carroll, P.; Weinberg, V.; Chan, S.; Lewis, J.; Corman, J.; Amling, C.L.; Stephenson, R.A.; Simko, J.; Sheikh, N.A.; et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J. Natl. Cancer Inst. 2014. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, J.; Kaluza, K.M.; Freeman, G.J.; Coukos, G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013, 73, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.A.; Yu, T.F.; Leach, D.R.; Allison, J.P. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc. Natl. Acad. Sci. USA 1998, 95, 10067–10071. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, A.; Hurwitz, A.A.; Allison, J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999, 190, 355–366. [Google Scholar] [PubMed]
- Hurwitz, A.A.; Foster, B.A.; Kwon, E.D.; Truong, T.; Choi, E.M.; Greenberg, N.M.; Burg, M.B.; Allison, J.P. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000, 60, 2444–2448. [Google Scholar] [PubMed]
- Wada, S.; Jackson, C.M.; Yoshimura, K.; Yen, H.R.; Getnet, D.; Harris, T.J.; Goldberg, M.V.; Bruno, T.C.; Grosso, J.F.; Durham, N.; et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J. Transl. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Allison, J.P. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 2009, 69, 7747–7755. [Google Scholar] [CrossRef] [PubMed]
- Davila, E.; Kennedy, R.; Celis, E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003, 63, 3281–3288. [Google Scholar] [PubMed]
- Daftarian, P.; Song, G.Y.; Ali, S.; Faynsod, M.; Longmate, J.; Diamond, D.J.; Ellenhorn, J.D. Two distinct pathways of immuno-modulation improve potency of p53 immunization in rejecting established tumors. Cancer Res. 2004, 64, 5407–5414. [Google Scholar] [CrossRef] [PubMed]
- Met, O.; Wang, M.; Pedersen, A.E.; Nissen, M.H.; Buus, S.; Claesson, M.H. The effect of a therapeutic dendritic cell-based cancer vaccination depends on the blockage of CTLA-4 signaling. Cancer Lett. 2006, 231, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.E.; Buus, S.; Claesson, M.H. Treatment of transplanted CT26 tumour with dendritic cell vaccine in combination with blockade of vascular endothelial growth factor receptor 2 and CTLA-4. Cancer Lett. 2006, 235, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.I.; Jung, S.H.; Sohn, H.J.; Celis, E.; Kim, T.G. An optimized peptide vaccine strategy capable of inducing multivalent CD8+ T cell responses with potent antitumor effects. Oncoimmunology 2015. [Google Scholar] [CrossRef] [PubMed]
- Espenschied, J.; Lamont, J.; Longmate, J.; Pendas, S.; Wang, Z.; Diamond, D.J.; Ellenhorn, J.D. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J. Immunol. 2003, 170, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Schlom, J.; Hodge, J.W. The combined activation of positive costimulatory signals with modulation of a negative costimulatory signal for the enhancement of vaccine-mediated T-cell responses. Cancer Immunol. Immunother. 2007, 56, 1471–1484. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Duraiswamy, J.; Freeman, G.J.; Coukos, G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013, 73, 6900–6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, D.C.; Engels, B.; Arina, A.; Yu, P.; Slauch, J.M.; Fu, Y.X.; Karrison, T.; Burnette, B.; Idel, C.; Zhao, M.; et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol. Res. 2013, 1, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Linch, S.N.; Kasiewicz, M.J.; McNamara, M.J.; Hilgart-Martiszus, I.F.; Farhad, M.; Redmond, W.L. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc. Natl. Acad. Sci. USA 2016, 113, E319–E327. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xi, H.; Ju, S.; Zhang, X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett. 2013, 336, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Malm, I.J.; Kadayakkara, D.K.; Levitsky, H.; Pardoll, D.; Kim, Y.J. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014, 74, 4042–4052. [Google Scholar] [CrossRef] [PubMed]
- Mkrtichyan, M.; Najjar, Y.G.; Raulfs, E.C.; Abdalla, M.Y.; Samara, R.; Rotem-Yehudar, R.; Cook, L.; Khleif, S.N. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur. J. Immunol. 2011, 41, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Ahrends, T.; Babala, N.; Xiao, Y.; Yagita, H.; van Eenennaam, H.; Borst, J. CD27 Agonism Plus PD-1 Blockade Recapitulates CD4+ T-cell Help in Therapeutic Anticancer Vaccination. Cancer Res. 2016, 76, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Lasaro, M.O.; Sazanovich, M.; Giles-Davis, W.; Mrass, P.; Bunte, R.M.; Sewell, D.A.; Hussain, S.F.; Fu, Y.X.; Weninger, W.; Paterson, Y.; et al. Active immunotherapy combined with blockade of a coinhibitory pathway achieves regression of large tumor masses in cancer-prone mice. Mol. Ther. 2011, 19, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yoshikawa, T.; Shimomura, M.; Iwama, T.; Endo, I.; Nakatsura, T. Programmed death-1 blockade enhances the antitumor effects of peptide vaccine-induced peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2015, 46, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.L.; Dunn, S.N.; James, S.; Johnson, P.W.; Cragg, M.S.; Glennie, M.J.; Gray, J.C. Immunomodulatory monoclonal antibodies combined with peptide vaccination provide potent immunotherapy in an aggressive murine neuroblastoma model. Clin. Cancer Res. 2013, 19, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.I.; Galvao-Filho, B.; de Faria, P.C.; Junqueira, C.; Dutra, M.S.; Teixeira, S.M.; Rodrigues, M.M.; Ritter, G.; Bannard, O.; Fearon, D.T.; et al. Blockade of CTLA-4 promotes the development of effector CD8+ T lymphocytes and the therapeutic effect of vaccination with an attenuated protozoan expressing NY-ESO-1. Cancer Immunol. Immunother. 2015, 64, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Sierro, S.R.; Donda, A.; Perret, R.; Guillaume, P.; Yagita, H.; Levy, F.; Romero, P. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur. J. Immunol. 2011, 41, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.; Rucki, A.A.; Wu, A.A.; Olino, K.; Xiao, Q.; Chai, Y.; Wamwea, A.; Bigelow, E.; Lutz, E.; Liu, L.; et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J. Immunother. 2015. [Google Scholar] [CrossRef] [PubMed]
- Son, C.H.; Bae, J.H.; Shin, D.Y.; Lee, H.R.; Choi, Y.J.; Jo, W.S.; Ho Jung, M.; Kang, C.D.; Yang, K.; Park, Y.S. CTLA-4 blockade enhances antitumor immunity of intratumoral injection of immature dendritic cells into irradiated tumor in a mouse colon cancer model. J. Immunother. 2014, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Dumont, C.; Johnston, A.P.; Mintern, J.D. Targeting dendritic cells: A promising strategy to improve vaccine effectiveness. Clin. Transl. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.H.; Heger, L.; Heidkamp, G.F.; Baranska, A.; Luhr, J.J.; Hoffmann, A.; Dudziak, D. Direct Delivery of Antigens to Dendritic Cells via Antibodies Specific for Endocytic Receptors as a Promising Strategy for Future Therapies. Vaccines 2016. [Google Scholar] [CrossRef] [PubMed]
- Haicheur, N.; Bismuth, E.; Bosset, S.; Adotevi, O.; Warnier, G.; Lacabanne, V.; Regnault, A.; Desaymard, C.; Amigorena, S.; Ricciardi-Castagnoli, P.; et al. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J. Immunol. 2000, 165, 3301–3308. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Tartour, E.; van der Bruggen, P.; Vantomme, V.; Joyeux, I.; Goud, B.; Fridman, W.H.; Johannes, L. Major histocompatibility complex class I presentation of exogenous soluble tumor antigen fused to the B-fragment of Shiga toxin. Eur. J. Immunol. 1998, 28, 2726–2737. [Google Scholar] [CrossRef]
- Adotevi, O.; Vingert, B.; Freyburger, L.; Shrikant, P.; Lone, Y.C.; Quintin-Colonna, F.; Haicheur, N.; Amessou, M.; Herbelin, A.; Langlade-Demoyen, P.; et al. B Subunit of Shiga Toxin-Based Vaccines Synergize with α-Galactosylceramide to Break Tolerance against Self Antigen and Elicit Antiviral Immunity. J. Immunol. 2007, 179, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Rekoske, B.T.; Smith, H.A.; Olson, B.M.; Maricque, B.B.; McNeel, D.G. PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer Immunol. Res. 2015, 3, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Metheringham, R.L.; Brentville, V.A.; Gunn, B.; Symonds, P.; Yagita, H.; Ramage, J.M.; Durrant, L.G. SCIB2, an antibody DNA vaccine encoding NY-ESO-1 epitopes, induces potent antitumor immunity which is further enhanced by checkpoint blockade. Oncoimmunology 2016. [Google Scholar] [CrossRef] [PubMed]
- Grosso, J.F.; Kelleher, C.C.; Harris, T.J.; Maris, C.H.; Hipkiss, E.L.; De Marzo, A.; Anders, R.; Netto, G.; Getnet, D.; Bruno, T.C.; et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 2007, 117, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.; Nagao, H.; Yoshiyama, H.; Akiba, H.; Yagita, H.; Dosaka-Akita, H.; Jinushi, M. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol. Immunother. 2013, 62, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Sempere, L.F.; Broughton, T.; Wang, L.; Noelle, R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol. Res. 2014, 2, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Park, S.H.; Nam, H.J.; Choi, D.H.; Sung, Y.C. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J. Immunother. 2011, 34, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ertl, H.C. The effect of adjuvanting cancer vaccines with herpes simplex virus glycoprotein D on melanoma-driven CD8+ T cell exhaustion. J. Immunol. 2014, 193, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, W.; Lu, J.; Kong, F.; Ma, G.; Zhu, Y.; Zhao, D.; Zhu, J.; Shuai, W.; Zhou, Q.; et al. AAV-sBTLA facilitates HSP70 vaccine-triggered prophylactic antitumor immunity against a murine melanoma pulmonary metastasis model in vivo. Cancer Lett. 2014, 354, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, W.; Fang, Y.; Feng, Z.; Liao, S.; Li, W.; Li, Y.; Li, C.; Maitituoheti, M.; Dong, H.; et al. Soluble B and T lymphocyte attenuator possesses antitumor effects and facilitates heat shock protein 70 vaccine-triggered antitumor immunity against a murine TC-1 cervical cancer model in vivo. J. Immunol. 2009, 183, 7842–7850. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Diab, A.; Perales, M.A.; Wolchok, J.D.; Rizzuto, G.; Merghoub, T.; Huggins, D.; Liu, C.; Turk, M.J.; Restifo, N.P.; et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006, 66, 4904–4912. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Merghoub, T.; Maughan, M.F.; Hirschhorn-Cymerman, D.; Morris, J.; Ritter, E.; Olmsted, R.; Houghton, A.N.; Wolchok, J.D. Alphavirus replicon particles expressing TRP-2 provide potent therapeutic effect on melanoma through activation of humoral and cellular immunity. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4–1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290–E5299. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Huddleston, C.A.; Dullforce, P.A.; Montfort, M.J.; Weinberg, A.D.; Parker, D.C. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J. Immunol. 2004, 172, 6735–6743. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.I.; McAdam, A.J.; Buhlmann, J.E.; Scott, S.; Lupher, M.L., Jr.; Greenfield, E.A.; Baum, P.R.; Fanslow, W.C.; Calderhead, D.M.; Freeman, G.J.; et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity 1999, 11, 689–698. [Google Scholar] [CrossRef]
- Murata, K.; Ishii, N.; Takano, H.; Miura, S.; Ndhlovu, L.C.; Nose, M.; Noda, T.; Sugamura, K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000, 19, 365–374. [Google Scholar] [CrossRef]
- den Boer, A.T.; Diehl, L.; van Mierlo, G.J.; van der Voort, E.I.; Fransen, M.F.; Krimpenfort, P.; Melief, C.J.; Offringa, R.; Toes, R.E. Longevity of antigen presentation and activation status of APC are decisive factors in the balance between CTL immunity versus tolerance. J. Immunol. 2001, 167, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Carlring, J.; Szabo, M.J.; Dickinson, R.; De Leenheer, E.; Heath, A.W. Conjugation of lymphoma idiotype to CD40 antibody enhances lymphoma vaccine immunogenicity and antitumor effects in mice. Blood 2012, 119, 2056–2065. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.I.; Celis, E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res. 2009, 69, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Smyth, L.; Wang, J.T.; Costa, P.M.; Ratnasothy, K.; Diebold, S.S.; Lombardi, G.; Al-Jamal, K.T. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials 2016, 104, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Ladle, B.H.; Kim, P.S.; Lutz, E.R.; Wolpoe, M.E.; Ivie, S.E.; Smith, H.M.; Armstrong, T.D.; Emens, L.A.; Jaffee, E.M.; et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J. Immunol. 2006, 176, 974–983. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, T.; Smith, J.; Baum, P.; Fanslow, W.; Butz, E.; Maliszewski, C. Ox40 costimulation enhances the development of T cell responses induced by dendritic cells in vivo. J. Immunol. 2002, 168, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Verma, R.; Sznol, M.; Boddupalli, C.S.; Gettinger, S.N.; Kluger, H.; Callahan, M.; Wolchok, J.D.; Halaban, R.; Dhodapkar, M.V.; et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 2015, 194, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.R.; Holst, P.J.; Steffensen, M.A.; Christensen, J.P.; Thomsen, A.R. Adenoviral vaccination combined with CD40 stimulation and CTLA-4 blockage can lead to complete tumor regression in a murine melanoma model. Vaccine 2010, 28, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Ogasawara, K.; Matsushita, K.; Morohashi, T.; Namba, K.; Matsuki, N.; Kitaichi, N.; Inuyama, Y.; Hosokawa, M.; Nakayama, E.; et al. Effective priming of cytotoxic T lymphocyte precursors by subcutaneous administration of peptide antigens in liposomes accompanied by anti-CD40 and anti-CTLA-4 antibodies. Immunobiology 2000, 201, 527–540. [Google Scholar] [CrossRef]
- McGray, A.J.; Bernard, D.; Hallett, R.; Kelly, R.; Jha, M.; Gregory, C.; Bassett, J.D.; Hassell, J.A.; Pare, G.; Wan, Y.; et al. Combined vaccination and immunostimulatory antibodies provides durable cure of murine melanoma and induces transcriptional changes associated with positive outcome in human melanoma patients. Oncoimmunology 2012, 1, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Chatterjee, S.K. Combination of CTL-associated antigen-4 blockade and depletion of CD25 regulatory T cells enhance tumour immunity of dendritic cell-based vaccine in a mouse model of colon cancer. Scand. J. Immunol. 2010, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Sutmuller, R.P.; van Duivenvoorde, L.M.; van Elsas, A.; Schumacher, T.N.; Wildenberg, M.E.; Allison, J.P.; Toes, R.E.; Offringa, R.; Melief, C.J. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 2001, 194, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Liakou, C.I.; Kamat, A.; Tang, D.N.; Chen, H.; Sun, J.; Troncoso, P.; Logothetis, C.; Sharma, P. CTLA-4 blockade increases IFNgamma-producing CD4+ ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl. Acad. Sci. USA 2008, 105, 14987–14992. [Google Scholar] [CrossRef] [PubMed]
- Sarnaik, A.A.; Yu, B.; Yu, D.; Morelli, D.; Hall, M.; Bogle, D.; Yan, L.; Targan, S.; Solomon, J.; Nichol, G.; et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin. Cancer Res. 2011, 17, 896–906. [Google Scholar] [CrossRef] [PubMed]
- O′Mahony, D.; Morris, J.C.; Quinn, C.; Gao, W.; Wilson, W.H.; Gause, B.; Pittaluga, S.; Neelapu, S.; Brown, M.; Fleisher, T.A.; et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin. Cancer Res. 2007, 13, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, B.; O′Brien, S.; Lee, D.; Hou, Y.; Weinberg, V.; Rini, B.; Allison, J.P.; Small, E.J.; Fong, L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood 2008, 112, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.; Miyakawa, R.; Suzuki, K.; Narumi, K.; Udagawa, T.; Yamamoto, Y.; Chikaraishi, T.; Yoshida, T.; Aoki, K. Suppression of Tregs by anti-glucocorticoid induced TNF receptor antibody enhances the antitumor immunity of interferon-alpha gene therapy for pancreatic cancer. Cancer Sci. 2014, 105, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Mateus, C.; Grohmann, U.; Caillat-Zucman, S.; Zitvogel, L.; Robert, C. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin. Cancer Res. 2008, 14, 5242–5249. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Kudchadkar, R.R.; Yu, B.; Gallenstein, D.; Horak, C.E.; Inzunza, H.D.; Zhao, X.; Martinez, A.J.; Wang, W.; Gibney, G.; et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013, 31, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Avogadri, F.; Zappasodi, R.; Yang, A.; Budhu, S.; Malandro, N.; Hirschhorn-Cymerman, D.; Tiwari, S.; Maughan, M.F.; Olmsted, R.; Wolchok, J.D.; et al. Combination of alphavirus replicon particle-based vaccination with immunomodulatory antibodies: therapeutic activity in the B16 melanoma mouse model and immune correlates. Cancer Immunol. Res. 2014, 2, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.D.; Xiao, X.; Gao, W.; Degauque, N.; Chen, M.; Kroemer, A.; Killeen, N.; Ishii, N.; Li, X.C. OX40 costimulation turns off Foxp3+ Tregs. Blood 2007, 110, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Pico de Coana, Y.; Poschke, I.; Gentilcore, G.; Mao, Y.; Nystrom, M.; Hansson, J.; Masucci, G.V.; Kiessling, R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol. Res. 2013, 1, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Feder-Mengus, C.; Schultz-Thater, E.; Oertli, D.; Marti, W.R.; Heberer, M.; Spagnoli, G.C.; Zajac, P. Nonreplicating recombinant vaccinia virus expressing CD40 ligand enhances APC capacity to stimulate specific CD4+ and CD8+ T cell responses. Hum. Gene Ther. 2005, 16, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, S.K.; Boczkowski, D.; de Rosa, N.; Haley, N.R.; Morse, M.A.; Tyler, D.S.; Dannull, J.; Nair, S. Enhancement of anti-tumor immunity through local modulation of CTLA-4 and GITR by dendritic cells. Eur. J. Immunol. 2011, 41, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Bonehill, A.; Tuyaerts, S.; Van Nuffel, A.M.; Heirman, C.; Bos, T.J.; Fostier, K.; Neyns, B.; Thielemans, K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol. Ther. 2008, 16, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Wilgenhof, S.; Van Nuffel, A.M.; Benteyn, D.; Corthals, J.; Aerts, C.; Heirman, C.; Van Riet, I.; Bonehill, A.; Thielemans, K.; Neyns, B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann. Oncol. 2013, 24, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- Hodi, F.S.; O′Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Bjoern, J.; Iversen, T.Z.; Nitschke, N.J.; Andersen, M.H.; Svane, I.M. Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy 2016, 18, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Attia, P.; Phan, G.Q.; Maker, A.V.; Robinson, M.R.; Quezado, M.M.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Kammula, U.S.; Royal, R.E.; et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. 2005, 23, 6043–6053. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Comin-Anduix, B.; Chmielowski, B.; Jalil, J.; de la Rocha, P.; McCannel, T.A.; Ochoa, M.T.; Seja, E.; Villanueva, A.; Oseguera, D.K.; et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin. Cancer Res. 2009, 15, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Wilgenhof, S.; Corthals, J.; Heirman, C.; van Baren, N.; Lucas, S.; Kvistborg, P.; Thielemans, K.; Neyns, B. Phase II Study of Autologous Monocyte-Derived mRNA Electroporated Dendritic Cells (TriMixDC-MEL) Plus Ipilimumab in Patients With Pretreated Advanced Melanoma. J. Clin. Oncol. 2016, 34, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfield, W.; Schadendorf, D.; Smylie, M.; Guthrie, T., Jr.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- O′Day, S.J.; Maio, M.; Chiarion-Sileni, V.; Gajewski, T.F.; Pehamberger, H.; Bondarenko, I.N.; Queirolo, P.; Lundgren, L.; Mikhailov, S.; Roman, L.; et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 2010, 21, 1712–1717. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.A.; Mohebtash, M.; Arlen, P.M.; Vergati, M.; Rauckhorst, M.; Steinberg, S.M.; Tsang, K.Y.; Poole, D.J.; Parnes, H.L.; Wright, J.J.; et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 501–508. [Google Scholar] [CrossRef]
- Jochems, C.; Tucker, J.A.; Tsang, K.Y.; Madan, R.A.; Dahut, W.L.; Liewehr, D.J.; Steinberg, S.M.; Gulley, J.L.; Schlom, J. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates. Cancer Immunol. Immunother. 2014, 63, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Schuetz, T.J.; Blumenstein, B.A.; Glode, L.M.; Bilhartz, D.L.; Wyand, M.; Manson, K.; Panicali, D.L.; Laus, R.; Schlom, J.; et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010, 28, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Nesselhut, J.; Marx, D.; Lange, H.; Regalo, G.; Cillien, N.; Chang, R.Y.; Nesselhut, T. Systemic treatment with anti-PD-1 antibody nivolumab in combination with vaccine therapy in advanced pancreatic cancer. In Proceedings of the ASCO Annual Meeting, Chicago, IL, USA, 2–6 June 2016.
- Gibney, G.T.; Kudchadkar, R.R.; DeConti, R.C.; Thebeau, M.S.; Czupryn, M.P.; Tetteh, L.; Eysmans, C.; Richards, A.; Schell, M.J.; Fisher, K.J.; et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin. Cancer Res. 2015, 21, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, H.; Wang, W.; Fu, Y.X.; Zhu, M. A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice. Oncoimmunology 2016. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Ann. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Dorfman, D.M.; Ma, F.R.; Sullivan, E.L.; Munoz, O.; Wood, C.R.; Greenfield, E.A.; Freeman, G.J. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003, 170, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Gregor, P.D.; Wolchok, J.D.; Ferrone, C.R.; Buchinshky, H.; Guevara-Patino, J.A.; Perales, M.A.; Mortazavi, F.; Bacich, D.; Heston, W.; Latouche, J.B.; et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self-antigens in animal and cellular model systems. Vaccine 2004, 22, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Manlove, L.S.; Schenkel, J.M.; Manlove, K.R.; Pauken, K.E.; Williams, R.T.; Vezys, V.; Farrar, M.A. Heterologous Vaccination and Checkpoint Blockade Synergize To Induce Antileukemia Immunity. J. Immunol. 2016, 196, 4793–4804. [Google Scholar] [CrossRef] [PubMed]
| Type of Checkpoint Inhibitor Blocked | Type of Tumor | Type of Vaccine | Clinical Benefit and Toxicity | Ref. |
|---|---|---|---|---|
| Anti-CTLA-4 | SM1 cell line (mammary tumor) | 1 × 106 irradiated GM-CSF transduced SM1 cells (s.c.) | Complete regression of tumor volume and enhanced survival of mice | [52] |
| B16-BL6 cell line (melanoma) | 1 × 106 irradiated GM-CSF–producing B16-BL6 and B16-F10 (sc) | Eradication of established tumors in 80% (68/85) of the cases. Development of depigmentation, starting at the site of vaccination or challenge and in most cases progressing to distant sites. | [53] | |
| TRAMP-C2 cell line (prostate cancer) | 1 × 106 cells irradiated GMTRAMP-C1/C2 | Reduction in tumor incidence and tumor grade. Development of prostatitis accompanied by destruction of glandular epithelium of the male reproductive tract | [54] | |
| SP1 cell line (Prostate cancer) | GVAX: 1 × 106 TRMPC-2HA admixed with 5 × 104 B78H1-GM then irradiated | Dramatic increase in effector CD8+T cells in the prostate gland, and enhanced tumor-antigen directed lytic function and decreased tumor burden and histologic grade in ProHA × TRAMP mice | [55] | |
| B16/BL6 (melanoma TRAMP-C2 cell line (prostate cancer) | Fl3vax: irradiated 1 × 106 Flt3L-expressing B16/BL6 (i.d.) | Rejection of established TRAMP prostate adenocarcinomas. Significantly greater prevention of the outgrowth of 5-day implanted B16-BL6 tumors than Gvax | [56] | |
| B16 cell line (Melanoma) | Synthetic peptide+ CpG-ODN adjuvant | Increased survival of mice with poorly immunogenic B16 melanoma in a therapeutic protocol, but ineffective in the prophylactic mode. | [57] | |
| 11A-1 & MC-38 cell line (mammary and colon carcinoma | Modified vaccinia Ankara-expressing murine p53 +CpG-ODN | The combination of CpG ODN and CTLA-4 blockade synergized for the rejection of palpable 11A-1 and MC-38 tumors | [58] | |
| EG7-OVA cells (Thymoma) | 2 × 106 SIINFEKL-pulsed or unpulsed DCs (i.d.) | Rejection or slowed tumor growth in more than 60% of mice. | [59] | |
| CT26 cells (Colon cancer) | 106 cells peptide pulsed DCs (i.d.) | Increased mean survival of mice. | [60] | |
| MC-38 (Colon cancer) | 2–3 × 105 cells peptide pulsed DCs (i.d.) | Improvement of tumor-free survival of mice Immunized mice appeared healthy and maintained normal weight compared to mice mock-vaccinated with PBS. | [61] | |
| Meth A cells (Sarcoma) | Modified vaccinia Ankara (MVA) poxvirus encoding mutated p53 protein vaccine (i.p.) | Improvement of tumor-free survival (11/14) of mice receiving combination therapy | [62] | |
| MC-38 cells expressing CEA (Colon cancer) | 1 × 107 recombinant vaccinia vector carrying the genes for CEA, B7.1, ICAM-1, and LFA-3 | Reduction of tumor volume complete eradication of the tumor (4 out of 20 mice). No signs of autoimmunity | [63] | |
| Anti-CTLA-4 + anti-PD-1 | B16-BL6 cells (Melanoma) | 1 × 106 B16 melanoma cells expressing either GM-CSF (Gvax) or Flt3-ligand (Fvax) | Reduction of tumor volume enhanced survival (75% survival with combined regimen versus 25% with monotherapy) | [64] |
| CT26 cell line & ID8-VEGF cell line (colon and ovarian cancer) | 1 × 106 irradiated (150Gy) CT26-GVAX or ID8-VEGF-GVAX | Reduction of tumor volume and increased tumor rejection (between 75%–100% of tumor-free mice with combined therapy versus 30%–50% with monotherapy) | [65] | |
| B16-OVA cell line (melanoma) | Bacterial vaccine: The leucine–arginine auxotrophic S. Typhimurium A1-R strain 2 x 107 colony formation units | Eradication of established tumor in 80% of mice receiving the combined regimen | [66] | |
| anti-CTLA-4 + anti-OX40 | TUBO cell line (Breast cancer) | Anti–DEC-205/HER2 monoclonal antibodies | 4-fold reduction of tumor volume and survival enhancement (40% survival versus 0%) | [67] |
| Anti-PDL-1 | ID8 cell line (i.p.) (Ovarian cancer) | 106 irradiated ID8 cells expressing murine GM-CSF (ID8-GVAX) or Flt3-ligand (ID8-FVAX) | Rejection of ID8 tumor in 75% of tumor bearing mice increased proliferation and function of tumor antigen CD8+T cells | [65] |
| MDA-MB-231 & MDA-MB-435 (breast cancer) | DC vaccine | Reduction of tumor volume and enhanced survival (60% of mice survived in the groups treated with DC vaccines versus 100% mortality with vaccine alone or anti-PDL1 alone) | [68] | |
| anti-PD-1 | B16F10, EL4, RMA-S cells (melanoma, thymoma, lymphoma) | DC vaccine+Poly-IC+anti-CD40 (Trivax) | Eradication of established tumor enhanced survival | [61] |
| B16 cell line (melanoma) | A tumor cell based vaccine to create TLR agonists (TEGVAX) | Reduction of tumor volume (50% regression) appearance of vitiligo after treatment | [69] | |
| TC-1 cell line (lung cancer) | vaccine (E7/GM-CSF/anti-CD40) | Reduction of tumor volume (10-fold reduction) and enhanced survival (50%) | [70] | |
| CD27 agonist + anti-PD-1 | TC-1 cell line (lung cancer) | HELP-E7SH & E7SH DNA vaccines | Eradication of established tumor, resulting in 100% survival of the mice treated with combined therapy | [71] |
| Anti-BTLA | Transgenic mouse model mimicking thyroid adenocarcinoma | E7 expressed by herpes simplex virus (HSV)-1 glycoprotein D (gD) | Slowing of tumor progression resulting in 50% reduction of thyroid weight | [72] |
| VISTA blockade | B16-BL6 cells (melanoma) | Peptide-based cancer vaccine with TLR agonists as adjuvants | Eradication of established tumor protection against tumor rechallenge in 50% of mice | [73] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaki, S.; Anson, M.; Tran, T.; Giusti, D.; Blanc, C.; Oudard, S.; Tartour, E. Is There Still Room for Cancer Vaccines at the Era of Checkpoint Inhibitors. Vaccines 2016, 4, 37. https://doi.org/10.3390/vaccines4040037
Karaki S, Anson M, Tran T, Giusti D, Blanc C, Oudard S, Tartour E. Is There Still Room for Cancer Vaccines at the Era of Checkpoint Inhibitors. Vaccines. 2016; 4(4):37. https://doi.org/10.3390/vaccines4040037
Chicago/Turabian StyleKaraki, Soumaya, Marie Anson, Thi Tran, Delphine Giusti, Charlotte Blanc, Stephane Oudard, and Eric Tartour. 2016. "Is There Still Room for Cancer Vaccines at the Era of Checkpoint Inhibitors" Vaccines 4, no. 4: 37. https://doi.org/10.3390/vaccines4040037







