Dendritic Cell-Based Adjuvant Vaccination Targeting Wilms’ Tumor 1 in Patients with Advanced Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacture of a DC Vaccine and Vaccination Technique
2.2. DC Vaccine Release Criteria
2.3. Application and Conditions for DC Vaccine Therapy Approved Under “Advanced Medical Care”
- (i)
- Adjuvant therapy after surgical resection or high risk of disease relapse.
- (ii)
- De novo cancer at an advanced stage or recurrent cancer after standard therapies.
2.3.1. Competent Standard for DC Therapy and Eligibility
2.3.2. Evaluation of Safety and Efficacy
2.4. Case Report
2.4.1. Case 1
2.4.2. Case 2
2.4.3. Case 3
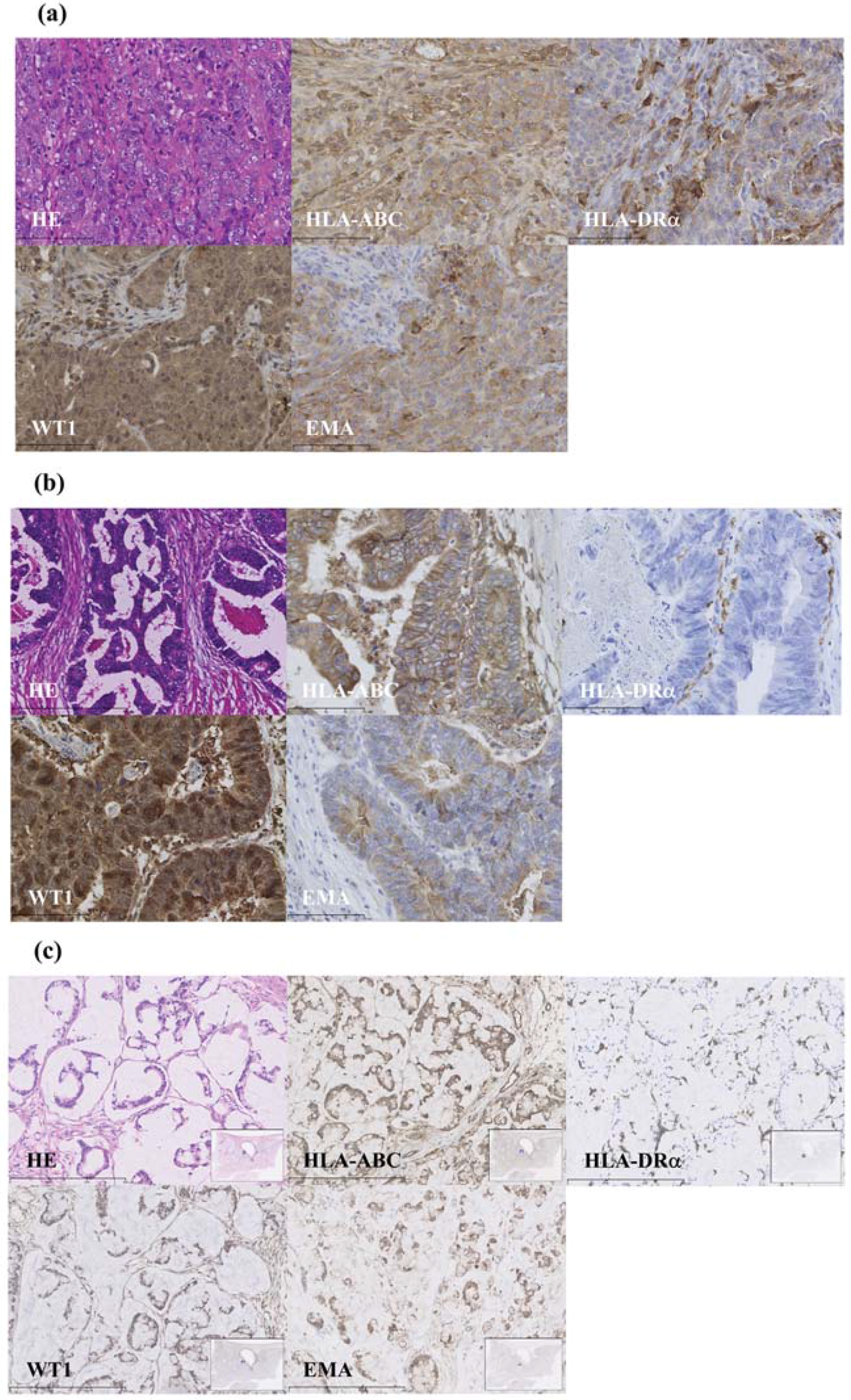
2.5. WT1 Expression Using Immunohistochemistry
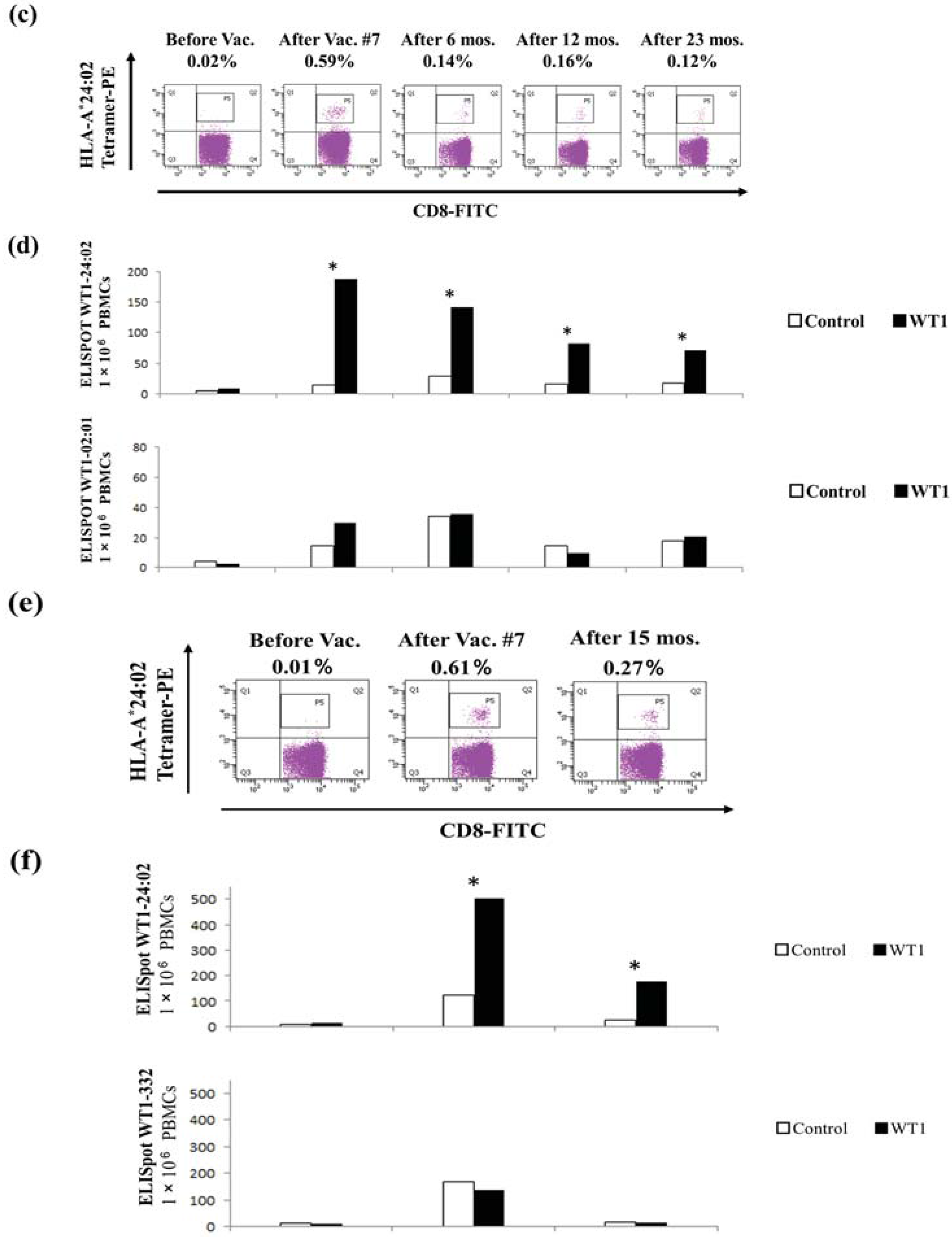
2.6. Immune Monitoring with Tetramer Analysis and Enzyme-Linked Immunosorbent Spot (ELISPOT) Assays
3. Results
3.1. WT1 Expression Using Immunohistochemistry
3.2. Immune Monitoring with Tetramer Analysis and ELISPOT Assays

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. 2010 Cancer statistics. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. Available online: http://ganjoho.jp/en/professional/statistics/table_download.html (accessed on 31 March 2015).
- Matsuda, A.; Matsuda, T.; Shibata, A.; Katanoda, K.; Sobue, T.; Nishimoto, H. The Japan Cancer Surveillance Research Group. Cancer Incidence and Incidence Rates in Japan in 2008: A Study of 25 Population-based Cancer Registries for the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn. J. Clin. Oncol. 2013, 44, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.W.; Weber, J.S.; et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 2013, 369, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Kudchadkar, R.R.; Yu, B.; Gallenstein, D.; Horak, C.E.; Inzunza, H.D.; Zhao, X.; Martinez, A.J.; Wang, W.; Gibney, G.; et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J. Clin. Oncol. 2013, 31, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012, 30. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.K.; Boczkowski, D.; Morse, M.; Cumming, R.I.; Lyerly, H.K.; Gilboa, E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat. Biotechnol. 1998, 16, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Deng, Y.; Coleman, D.; Hull, S.; Kitrell-Fisher, E.; Nair, S.; Schlom, J.; Ryback, M.E.; Lyerly, H.K. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin. Cancer Res. 1999, 5, 1331–1338. [Google Scholar] [PubMed]
- Nair, S.K.; Hull, S.; Coleman, D.; Gilboa, E.; Lyerly, H.K.; Morse, M.A. Induction of carcinoembryonic antigen (CEA)-specific cytotoxic T-lymphocyte responses in vitro using autologous dendritic cells loaded with CEA peptide or CEA RNA in patients with metastatic malignancies expressing CEA. Int. J. Cancer 1999, 82, 121–124. [Google Scholar] [CrossRef]
- Fong, L.; Hou, Y.; Rivas, A.; Benike, C.; Yuen, A.; Fisher, G.A.; Davis, M.M.; Engleman, E.G. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 8809–8814. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Wang, C.C.; Chen, L.T.; Cheng, A.L.; Lin, D.T.; Wu, Y.C.; Yu, W.L.; Hung, Y.M.; Yang, H.Y.; Juang, S.H.; et al. Generation of carcinoembryonic antigen (CEA)-specific T-cell responses in HLA-A*0201 and HLA-A*2402 late-stage colorectal cancer patients after vaccination with dendritic cells loaded with CEA peptides. Clin. Cancer Res. 2004, 10, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Lesterhuis, W.J.; de Vries, I.J.; Schuurhuis, D.H.; Boullart, A.C.; Jacobs, J.F.; de Boer, A.J.; Scharenborg, N.M.; Brouwer, H.M.; van de Rakt, M.W.; Figdor, C.G.; et al. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: Antigen-specific T cell responses in DTH skin tests. Ann. Oncol. 2006, 17, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Elisseeva, O.A.; Tsuboi, A.; Ogawa, H.; Tamaki, H.; Li, H.; Oji, Y.; Kim, E.H.; Soma, T.; Asada, M.; et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms’ tumor gene (WT1) product. Immunogenetics 2000, 51, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ohminami, H.; Yasukawa, M.; Fujita, S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood 2000, 95, 286–293. [Google Scholar] [PubMed]
- Tsuboi, A.; Oka, Y.; Udaka, K.; Murakami, M.; Masuda, T.; Nakano, A.; Nakajima, H.; Yasukawa, M.; Hiraki, A.; Oji, Y.; et al. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol. Immunother. 2002, 51, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tsukada, J.; Tomoda, T.; Takahashi, H.; Imai, K.; Shimamura, K.; Sunamura, M.; Yonemitsu, Y.; Shimodaira, S.; Koido, S.; et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 2012, 41, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Okamoto, M.; Shimodaira, S.; Tsujitani, S.; Nagaya, M.; Ishidao, T.; Kishimoto, J.; Yonemitsu, Y. DC-Vaccine Study Group at the Japan Society of Innovative Cell Therapy (J-SICT). Impact of dendritic cell vaccines pulsed with Wilms’ tumour-1 peptide antigen on the survival of patients with advanced non-small cell lung cancers. Eur. J. Cancer 2013, 49, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sakabe, T.; Abe, H.; Tanii, M.; Takahashi, H.; Chiba, A.; Yanagida, E.; Shibamoto, Y.; Ogasawara, M.; Tsujitani, S.; et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J. Gastrointest. Surg. 2013, 17, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shimodaira, S.; Nagai, K.; Ogasawara, M.; Takahashi, H.; Abe, H.; Tanii, M.; Okamoto, M.; Tsujitani, S.; Yusa, S.; et al. Prognostic factors related to add-on dendritic cell vaccines on patients with inoperable pancreatic cancer receiving chemotherapy: A multicenter analysis. Cancer Immunol. Immunother. 2014, 63, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Koido, S.; Homma, S.; Okamoto, M.; Takakura, K.; Mori, M.; Yoshizaki, S.; Tsukinaga, S.; Odahara, S.; Koyama, S.; Imazu, H.; et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clin. Cancer Res. 2014, 20, 4228–4239. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chiba, A.; Izawa, H.; Yanagida, E.; Okamoto, M.; Shimodaira, S.; Yonemitsu, Y.; Shibamoto, Y.; Suzuki, N.; Nagaya, M.; et al. The feasibility and clinical effects of dendritic cell-based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. J. Ovarian Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sakabe, T.; Chiba, A.; Nakajima, A.; Okamoto, M.; Shimodaira, S.; Yonemitsu, Y.; Shibamoto, Y.; Suzuki, N.; Nagaya, M. Therapeutic effect of intratumoral injections of dendritic cells for locally recurrent gastric cancer: A case report. World J. Surg. Oncol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Shimodaira, S.; Maejima, S.; Udagawa, N.; Sano, K.; Higuchi, Y.; Koya, T.; Ochiai, T.; Koide, M.; Uehara, S.; et al. Dendritic cell-based immunotherapy targeting Wilms’ Tumor 1 (WT1) in patients with relapsed malignant glioma. J. Neurosurg. 2015, 123, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, F.; Oka, Y.; Tsuboi, A.; Kawakami, M.; Kawakatsu, M.; Nakajima, H.; Elisseeva, O.A.; Harada, Y.; Ito, K.; Li, Z.; et al. Identification and characterization of a WT1 (Wilms Tumor Gene) protein-derived HLA-DRB1*0405-restricted 16-mer helper peptide that promotes the induction and activation of WT1-specific cytotoxic T lymphocytes. J. Immunother. 2007, 30, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A. Evolution of end points for cancer immunotherapy trials. Ann. Oncol. 2012, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Schlom, J.; Arlen, P.M.; Gulley, J.L. Cancer vaccines: Moving beyond current paradigms. Clin. Cancer Res. 2007, 13, 3776–3782. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Greten, T.F. Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann. Oncol. 2014, 25, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, Y.; Okamoto, M.; Kobayashi, M.; Ayakawa, S.; Iwata, H.; Sugie, C.; Mitsuishi, Y.; Takahashi, H. Immune-maximizing (IMAX) therapy for cancer: Combination of dendritic cell vaccine and intensity-modulated radiation. Mol. Clin. Oncol. 2013, 1, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, S.; Kobayashi, T.; Hirabayashi, K.; Horiuchi, K.; Koya, T.; Mizuno, Y.; Yamaoka, N.; Yuzawa, M.; Ishikawa, S.; Higuchi, Y.; et al. Induction of antigen-specific cytotoxic T lymphocytes by chemoradiotherapy in patients receiving Wilms’ tumor 1-targetted dendritic cell vaccinations for pancreatic cancer. OMICS J. Radiol. 2015. [Google Scholar] [CrossRef]
- Shimodaira, S.; Hirabayashi, K.; Kobayashi, T.; Higuchi, Y.; Yokokawa, K. Future Prospective of Cancer Vaccination Technology in Japan. Pharmaceut. Reg. Affairs 2015, 4. [Google Scholar] [CrossRef]
- Shimodaira, S.; Higuchi, Y.; Koya, T.; Kobayashi, T.; Yanagisawa, R.; Hirabayashi, K.; Ito, K.; Koizumi, T.; Maejima, S.; Udagawa, N. Smoking influences the yield of dendritic cells for cancer immunotherapy. Pharmaceut. Reg. Affairs 2015. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Oji, Y.; Horiuchi, T.; Kanda, T.; Kitagawa, M.; Takeuchi, T.; Kawano, K.; Kuwae, Y.; Yamauchi, A.; Okumura, M.; et al. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod. Pathol. 2006, 19, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Koya, T.; Yuzawa, M.; Yamaoka, N.; Mizuno, Y.; Yoshizawa, K.; Hirabayashi, K.; Kobayashi, T.; Sano, K.; Shimodaira, S. Enzyme-linked immunosorbent spot assay for the detection of Wilms’ tumor 1-specific T cells induced by dendritic cell vaccination. Biomedicines 2015, 3, 304–315. [Google Scholar] [CrossRef]
- Koido, S.; Ohkusa, T.; Homma, S.; Namiki, Y.; Takakura, K.; Saito, K.; Ito, Z.; Kobayashi, H.; Kajihara, M.; Uchiyama, K.; et al. Immunotherapy for colorectal cancer. World J. Gastroenterol. 2013, 19, 8531–8542. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin. Biol. Ther. 2014, 14, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Gabrilovich, D.I. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008, 68, 2561–2563. [Google Scholar] [CrossRef] [PubMed]
- Ezzelarab, M.; Thomson, A.W. Tolerogenic dendritic cells and their role in transplantation. Semin. Immunol. 2011, 23, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Rozman, P. Tolerogenic dendritic cells: Molecular and cellular mechanisms in transplantation. J. Leukoc. Biol. 2014, 95, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.; Arkenau, H.T.; Infante, J.R. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 2014, 74, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Oji, Y.; Ogawa, H.; Tamaki, H.; Oka, Y.; Tsuboi, A.; Kim, E.H.; Soma, T.; Tatekawa, T.; Kawakami, M.; Asada, M.; et al. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn. J. Cancer Res. 1999, 90, 194–204. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimodaira, S.; Sano, K.; Hirabayashi, K.; Koya, T.; Higuchi, Y.; Mizuno, Y.; Yamaoka, N.; Yuzawa, M.; Kobayashi, T.; Ito, K.; et al. Dendritic Cell-Based Adjuvant Vaccination Targeting Wilms’ Tumor 1 in Patients with Advanced Colorectal Cancer. Vaccines 2015, 3, 1004-1018. https://doi.org/10.3390/vaccines3041004
Shimodaira S, Sano K, Hirabayashi K, Koya T, Higuchi Y, Mizuno Y, Yamaoka N, Yuzawa M, Kobayashi T, Ito K, et al. Dendritic Cell-Based Adjuvant Vaccination Targeting Wilms’ Tumor 1 in Patients with Advanced Colorectal Cancer. Vaccines. 2015; 3(4):1004-1018. https://doi.org/10.3390/vaccines3041004
Chicago/Turabian StyleShimodaira, Shigetaka, Kenji Sano, Koichi Hirabayashi, Terutsugu Koya, Yumiko Higuchi, Yumiko Mizuno, Naoko Yamaoka, Miki Yuzawa, Takashi Kobayashi, Kenichi Ito, and et al. 2015. "Dendritic Cell-Based Adjuvant Vaccination Targeting Wilms’ Tumor 1 in Patients with Advanced Colorectal Cancer" Vaccines 3, no. 4: 1004-1018. https://doi.org/10.3390/vaccines3041004






