Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines
Abstract
:1. Introduction
2. Epidemiology of Influenza
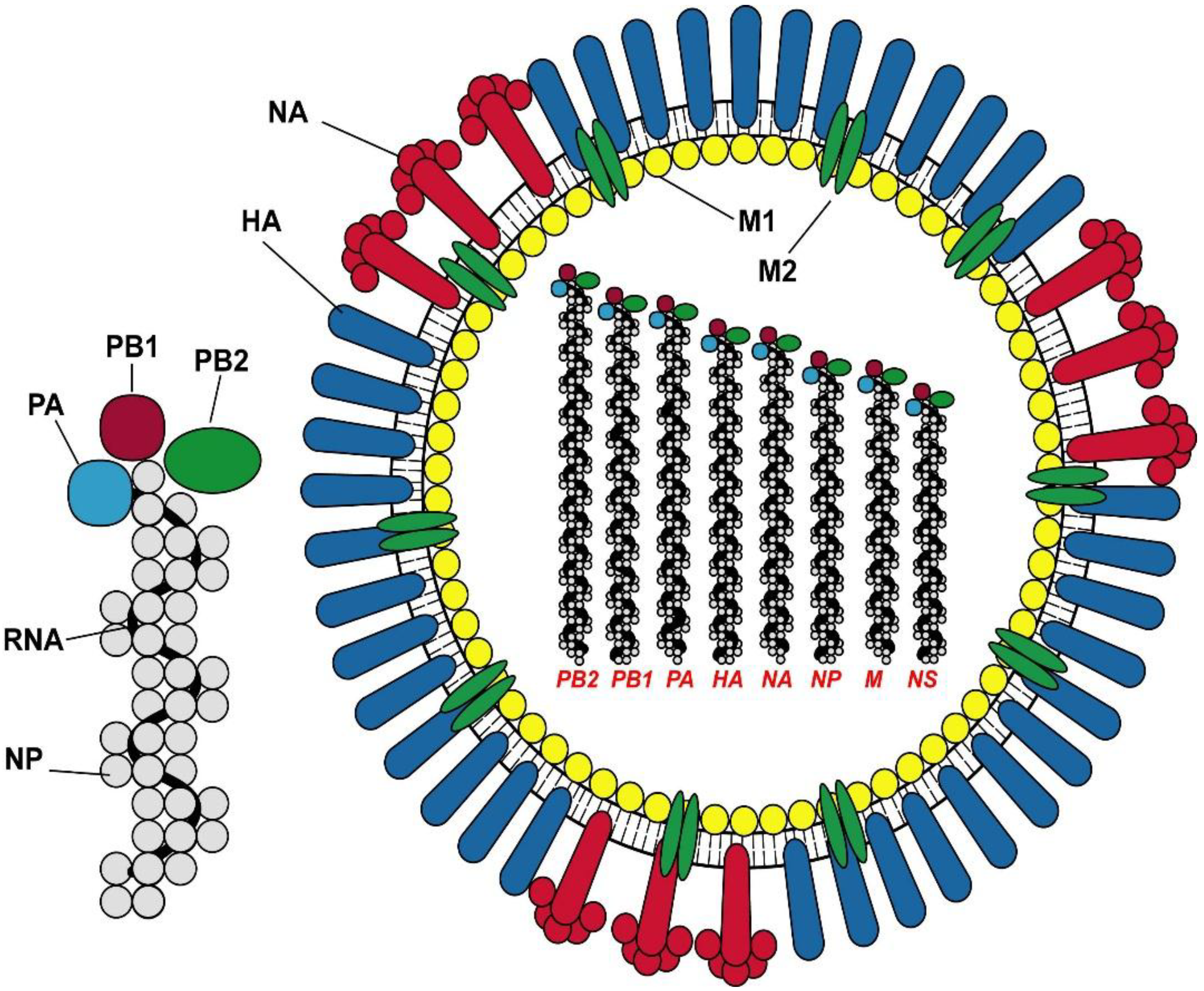
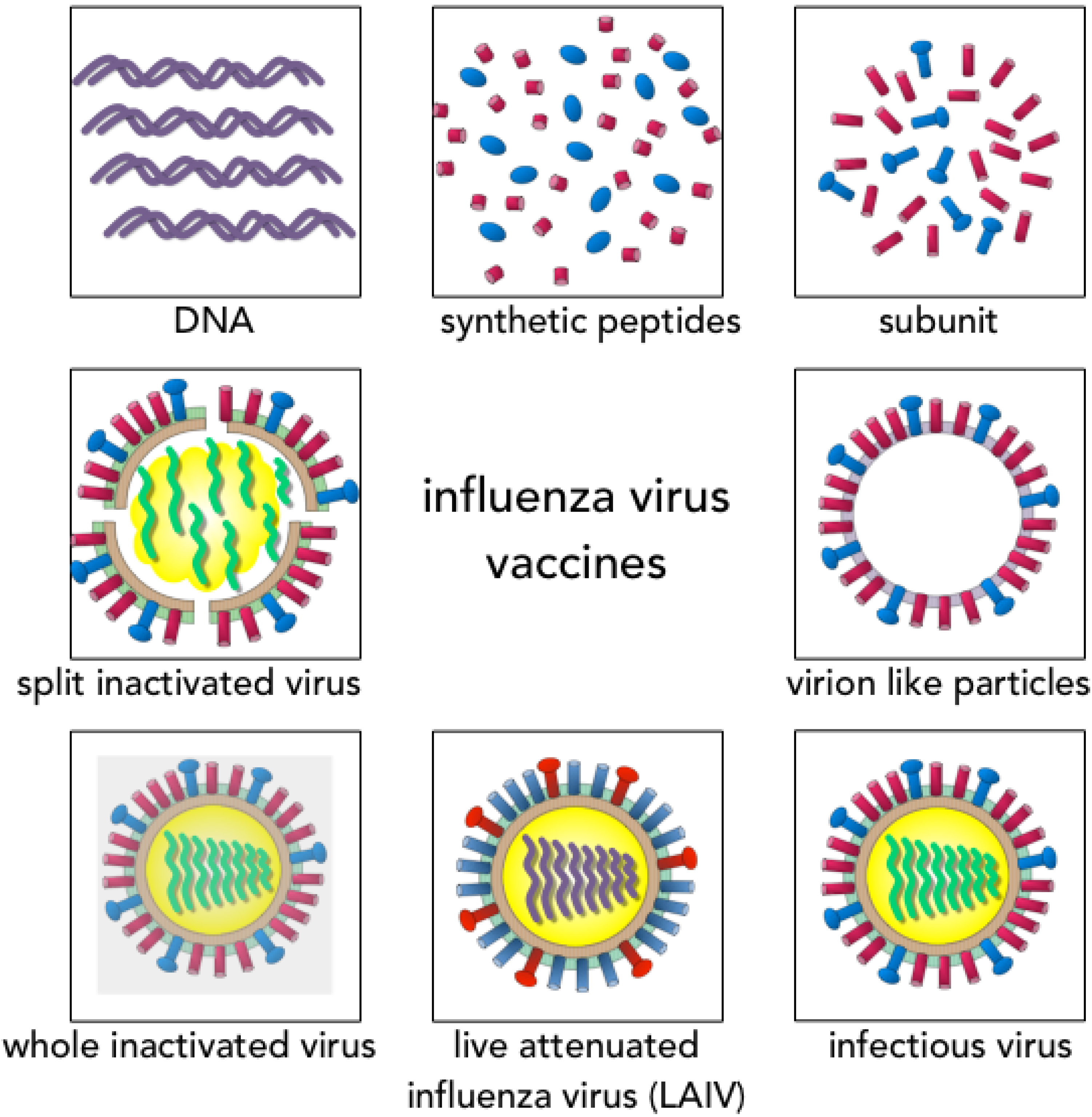
3. Vaccine Design and Recommendations
| Recommended Group | WHO Rationale for the Recommendation |
|---|---|
| Pregnant women | Increased risk of serious disease in mother |
| Increased risk of death in mother and unborn child | |
| Secondary effect of protection of child up to 6 m | |
| Globally applicable * | |
| Healthcare workers | Increased exposure to influenza |
| Reduces morbidity and mortality in patients | |
| Preserves integrity of health care systems | |
| Possible to implement | |
| Children <2 years old | Experience highest levels of serious illness |
| Responsible for spread in community | |
| Disadvantage costly to implement vaccination campaign | |
| Children 2–5 years old | Large burden of morbidity |
| Respond better to vaccines than younger children | |
| live attenuated influenza virus (LAIV) gives improved protection | |
| Children < 6 months | No available vaccines |
| Indirect protection through vaccination of mother during pregnancy | |
| Indirect protection through vaccination of close contacts | |
| Elderly > 65 years old | Highest risk of mortality |
| Vaccine is less effective | |
| Disadvantage annual immunization is costly to administer | |
| Patients with chronic conditions | Highest risk for serious disease |
| Disadvantage requires considerable resources to identify individuals |
| Children | General Contradictions in all Groups |
|---|---|
| < 24 months of age Receiving aspirin or aspirin-containing therapy 1 | Hypersensitivity to gelatin, gentamicin or ovalbumin Pregnant women in USA and Canada Older adults (USA >50 & Canada >60 years old) |
| Clinical immunodeficiency due to conditions or immunosuppressive therapy 2 |
4. Natural Immunity To Influenza
5. Inactivated Influenza Vaccines (IIV)
6. Live Attenuated Influenza Vaccine (LAIV)

7. Conclusions
| Inactivated Influenza Vaccine | Live Attenuated Influenza Vaccine | |
|---|---|---|
| HAI response | +++ | + |
| Antibody secreting cells | ++ | + |
| Memory B cells | + | + |
| Nasal IgA | −/+ | +++ |
| NA antibody | −/+ | ++ |
| CD4 T cells | ++ | +++ |
| CD8 T cells | − | +? |
| Cross protective immunity | −/+ | ++ |
Author Contributions
Conflicts of Interest
References
- Molinari, N.A.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; di Pietrantonj, C.; Rivetti, A.; Bawazeer, G.A.; Al-Ansary, L.A.; Ferroni, E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Rumke, H.C.; Richardus, J.H.; Rombo, L.; Pauksens, K.; Plassmann, G.; Durand, C.; Devaster, J.M.; Dewe, W.; Oostvogels, L. Selection of an adjuvant for seasonal influenza vaccine in elderly people: Modelling immunogenicity from a randomized trial. BMC Infect. Dis. 2013. [Google Scholar] [CrossRef]
- Hoffmann, E.; Mahmood, K.; Chen, Z.; Yang, C.F.; Spaete, J.; Greenberg, H.B.; Herlocher, M.L.; Jin, H.; Kemble, G. Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J. Virol. 2005, 79, 11014–11021. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lu, B.; Zhou, H.; Ma, C.; Zhao, J.; Yang, C.F.; Kemble, G.; Greenberg, H. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 2003, 306, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.A.; Kao, K.; Zhao, J.; Fast, P.E.; Mendelman, P.M.; Arvin, A. Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J. Clin. Microbiol. 2000, 38, 839–845. [Google Scholar] [PubMed]
- Belshe, R.; Lee, M.S.; Walker, R.E.; Stoddard, J.; Mendelman, P.M. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev. Vaccines 2004, 3, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Vaccination and Immunisation, Department of Health. Minute of meeting on Monday 19 September 2011. Available online: http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_131105.pdf (accessed on 1 March 2015).
- Falkenhorst, G.; Harder, T.; Remschmidt, C.; Terhardt, M.; Zepp, F.; Ledig, T.; Wicker, S.; Keller-Stanislawski, B.; Mertens, T. Background paper to the recommendation for the preferential use of live-attenuated influenza vaccine in children aged 2–6 years in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Advisory Committee on Immunization Practices (ACIP). Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). Available online: http://www.cdc.gov/flu/about/qa/nasalspray.htm (accessed on 26 February 2015).
- Belshe, R.B.; Edwards, K.M.; Vesikari, T.; Black, S.V.; Walker, R.E.; Hultquist, M.; Kemble, G.; Connor, E.M.; Group, C.-T.C.E.S. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 2007, 356, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J. Correlates of protection to influenza virus, where do we go from here? Hum. Vaccine Immunother. 2013, 9, 405–408. [Google Scholar] [CrossRef]
- Ekiert, D.C.; Kashyap, A.K.; Steel, J.; Rubrum, A.; Bhabha, G.; Khayat, R.; Lee, J.H.; Dillon, M.A.; O’Neil, R.E.; Faynboym, A.M.; et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature 2012, 489, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.M.; et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011, 12, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Atmar, R.L.; Franco, L.M.; Quarles, J.M.; Wells, J.; Arden, N.; Nino, D.; Belmont, J.W. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J. Infect. Dis. 2013, 207, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Couch, R.B.; Kasel, J.A.; Gerin, J.L.; Schulman, J.L.; Kilbourne, E.D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J. Infect. Dis. 1974, 129, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.L.; Mitcham, J.L.; Koller, T.D.; Bonavia, A.; Usner, D.W.; Balaratnam, G.; Fredlund, P.; Swiderek, K.M. Efficacy and safety of treatment with an anti-M2e monoclonal antibody in experimental human influenza. J. Infect. Dis. 2014. [Google Scholar] [CrossRef]
- Weinfurter, J.T.; Brunner, K.; Capuano, S.V., 3rd; Li, C.; Broman, K.W.; Kawaoka, Y.; Friedrich, T.C. Cross-reactive T cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLOS Pathog. 2011, 7, e1002381. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Kelso, A. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol. Cell Biol. 2009, 87, 300–308. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Gotch, F.M.; Dongworth, D.W.; Clark, A.; Potter, C.W. Declining T-cell immunity to influenza, 1977–1982. Lancet 1983, 2, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Li, C.K.; Chui, C.S.; Huang, A.K.; Perkins, M.; Liebner, J.C.; Lambkin-Williams, R.; Gilbert, A.; Oxford, J.; Nicholas, B.; et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 2012, 18, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Strutt, T.M.; McKinstry, K.K.; Marshall, N.B.; Vong, A.M.; Dutton, R.W.; Swain, S.L. Multipronged CD4+ T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol. Rev. 2013, 255, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Moser, B.; Schaerli, P.; Loetscher, P. CXCR5(+) T cells: Follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002, 23, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.L.; Betts, R.F.; Tierney, E.L.; Murphy, B.R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J. Clin. Microbiol. 1986, 24, 157–160. [Google Scholar] [PubMed]
- Turner, D.L.; Farber, D.L. Mucosal resident memory CD4 T cells in protection and immunopathology. Front. Immunol. 2014. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrancois, L.; Farber, D.L. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Cookenham, T.; Miller, S.C.; Roberts, A.D.; Christensen, J.P.; Thomsen, A.R.; Woodland, D.L. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J. Immunol. 2009, 183, 4378–4384. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Rivetti, A.; di Pietrantonj, C.; Demicheli, V.; Ferroni, E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Jefferson, T.; di Pietrantonj, C.; Al-Ansary, L.A.; Ferroni, E.; Thorning, S.; Thomas, R.E. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Note for guidance on harmonization of requirements for influenza vaccines. 12 March 1997. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf (accessed on 1 March 2015).
- Committee for Medicinal Products for Human Use (CHMP). Guideline on influenza vaccines; Non-Clinical and Clinical Module. 25 July 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/07/WC500170300.pdf (accessed on 1 March 2015).
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 1972, 70, 767–777. [Google Scholar] [CrossRef]
- Potter, C.W.; Oxford, J.S. Determinants of immunity to influenza infection in man. Br. Med. Bull. 1979, 35, 69–75. [Google Scholar] [PubMed]
- Al-Khayatt, R.; Jennings, R.; Potter, C.W. Interpretation of responses and protective levels of antibody against attenuated influenza A viruses using single radial haemolysis. J. Hyg. (Lond.) 1984, 93, 301–312. [Google Scholar] [CrossRef]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: Development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 2010. [Google Scholar] [CrossRef]
- Davies, J.R.; Grilli, E.A. Natural or vaccine-induced antibody as a predictor of immunity in the face of natural challenge with influenza viruses. Epidemiol. Infect. 1989, 102, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Gopfert, C.; Hammann, J.; Neumann, B.; Wood, J.; Newman, R.; Wallis, C.; Alex, N.; Pfleiderer, M. Enhancing the reproducibility of serological methods used to evaluate immunogenicity of pandemic H1N1 influenza vaccines-an effective EU regulatory approach. Vaccine 2012, 30, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Major, D.; Heath, A.; Newman, R.W.; Hoschler, K.; Stephenson, I.; Clark, T.; Katz, J.M.; Zambon, M.C. Reproducibility of serology assays for pandemic influenza H1N1: Collaborative study to evaluate a candidate WHO International Standard. Vaccine 2012, 30, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, I.; Heath, A.; Major, D.; Newman, R.W.; Hoschler, K.; Junzi, W.; Katz, J.M.; Weir, J.P.; Zambon, M.C.; Wood, J.M. Reproducibility of serologic assays for influenza virus A (H5N1). Emerg. Infect. Dis. 2009, 15, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Gaines-Das, R.E.; Taylor, J.; Chakraverty, P. Comparison of influenza serological techniques by international collaborative study. Vaccine 1994, 12, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J.; Brokstad, K.A.; Zuckerman, M.A.; Wood, J.M.; Haaheim, L.R.; Oxford, J.S. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 1994, 12, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.L.; Mendelman, P.M.; Mallon, K.P.; Jackson, L.A.; Gorse, G.J.; Belshe, R.B.; Glezen, W.P.; Wittes, J. Effectiveness of Live, Attenuated Intranasal Influenza Virus Vaccine in Healthy, Working Adults: A Randomized Controlled Trial. JAMA 1999, 282, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Powers, D.C.; Smith, G.E.; Anderson, E.L.; Kennedy, D.J.; Hackett, C.S.; Wilkinson, B.E.; Volvovitz, F.; Belshe, R.B.; Treanor, J.J. Influenza A virus vaccines containing purified recombinant H3 hemagglutinin are well tolerated and induce protective immune responses in healthy adults. J. Infect. Dis. 1995, 171, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Potter, C.W.; Jennings, R.; Nicholl, J.P.; Langrick, A.F.; Schild, G.C.; Wood, J.M.; Tyrrell, D.A. A comparison of live and inactivated influenza A (H1N1) virus vaccines. 1. Short-term immunity. J. Hyg. (Lond.) 1983, 90, 351–359. [Google Scholar] [CrossRef]
- El-Madhun, A.S.; Cox, R.J.; Haaheim, L.R. The effect of age and natural priming on the IgG and IgA subclass responses after parenteral influenza vaccination. J. Infect. Dis. 1999, 180, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- el-Madhun, A.S.; Cox, R.J.; Soreide, A.; Olofsson, J.; Haaheim, L.R. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J. Infect. Dis. 1998, 178, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Brokstad, K.A.; Eriksson, J.C.; Cox, R.J.; Tynning, T.; Olofsson, J.; Jonsson, R.; Davidsson, A. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J. Infect. Dis. 2002, 185, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Brokstad, K.A.; Cox, R.J.; Olofsson, J.; Jonsson, R.; Haaheim, L.R. Parenteral influenza vaccination induces a rapid systemic and local immune response. J. Infect. Dis. 1995, 171, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.D.; Bredholt, G.; Akselsen, P.E.; Pedersen, G.K.; Cox, R.J. A(H1N1)pdm09 vaccination of health care workers: Improved immune responses in low responders following revaccination. J. Infect. Dis. 2012, 206, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Halliley, J.L.; Kyu, S.; Kobie, J.J.; Walsh, E.E.; Falsey, A.R.; Randall, T.D.; Treanor, J.; Feng, C.; Sanz, I.; Lee, F.E. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine 2010, 28, 3582–3587. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Smith, K.; Miller, J.; Langley, W.A.; Kokko, K.; Larsen, C.; Zheng, N.Y.; Mays, I.; Garman, L.; Helms, C.; et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.C.; Cox, R.J.; Szyszko, E.; Davidsson, A.; Brokstad, K.A. Local and systemic cytokine and chemokine responses after parenteral influenza vaccination. Influenza Other Respir. Viruses 2007, 1, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Holmes, T.H.; Albrecht, R.A.; Garcia-Sastre, A.; Dekker, C.L.; He, X.S.; Greenberg, H.B. Distinct cross-reactive B-cell responses to live attenuated and inactivated influenza vaccines. J Infect Dis 2014, 210, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; He, J.; Weinstein, J.A.; Penland, L.; Sasaki, S.; He, X.S.; Dekker, C.L.; Zheng, N.Y.; Huang, M.; Sullivan, M.; et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci. Transl Med. 2013. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.M.; et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 2011, 12, 786–95. [Google Scholar] [CrossRef] [PubMed]
- He, X.S.; Holmes, T.H.; Mahmood, K.; Kemble, G.W.; Dekker, C.L.; Arvin, A.M.; Greenberg, H.B. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J. Infect. Dis. 2008, 197, 803–811. [Google Scholar] [CrossRef] [PubMed]
- He, X.S.; Holmes, T.H.; Zhang, C.; Mahmood, K.; Kemble, G.W.; Lewis, D.B.; Dekker, C.L.; Greenberg, H.B.; Arvin, A.M. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 2006, 80, 11756–11766. [Google Scholar] [CrossRef] [PubMed]
- Long, B.R.; Michaelsson, J.; Loo, C.P.; Ballan, W.M.; Vu, B.A.; Hecht, F.M.; Lanier, L.L.; Chapman, J.M.; Nixon, D.F. Elevated frequency of gamma interferon-producing NK cells in healthy adults vaccinated against influenza virus. Clin. Vaccine Immunol. 2008, 15, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Subbramanian, R.A.; Basha, S.; Shata, M.T.; Brady, R.C.; Bernstein, D.I. Pandemic and seasonal H1N1 influenza hemagglutinin-specific T cell responses elicited by seasonal influenza vaccination. Vaccine 2010, 28, 8258–8267. [Google Scholar] [CrossRef] [PubMed]
- Bentebibel, S.E.; Lopez, S.; Obermoser, G.; Schmitt, N.; Mueller, C.; Harrod, C.; Flano, E.; Mejias, A.; Albrecht, R.A.; Blankenship, D.; et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 2013. [Google Scholar] [CrossRef]
- Spensieri, F.; Borgogni, E.; Zedda, L.; Bardelli, M.; Buricchi, F.; Volpini, G.; Fragapane, E.; Tavarini, S.; Finco, O.; Rappuoli, R.; et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc. Natl. Acad. Sci. USA 2013, 110, 14330–14335. [Google Scholar] [CrossRef] [PubMed]
- Baz, M.; Luke, C.J.; Cheng, X.; Jin, H.; Subbarao, K. H5N1 vaccines in humans. Virus Res. 2013, 178, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Mohn, K.G.; Bredholt, G.; Brokstad, K.A.; Pathirana, R.D.; Aarstad, H.J.; Tondel, C.; Cox, R.J. Longevity of B-Cell and T-Cell responses after live attenuated influenza vaccination in children. J. Infect. Dis. 2014. [Google Scholar] [CrossRef]
- Forrest, B.D.; Pride, M.W.; Dunning, A.J.; Capeding, M.R.Z.; Chotpitayasunondh, T.; Tam, J.S.; Rappaport, R.; Eldridge, J.H.; Gruber, W.C. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin. Vaccine Immunol. 2008, 15, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Hoft, D.F.; Babusis, E.; Worku, S.; Spencer, C.T.; Lottenbach, K.; Truscott, S.M.; Abate, G.; Sakala, I.G.; Edwards, K.M.; Creech, C.B.; et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J. Infect. Dis. 2011, 204, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.E.; Palache, A.M.; de Jong, J.C.; Osterhaus, A.D. Cold-adapted live influenza vaccine versus inactivated vaccine: Systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine 2002, 20, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines 2015, 3, 373-389. https://doi.org/10.3390/vaccines3020373
Sridhar S, Brokstad KA, Cox RJ. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines. 2015; 3(2):373-389. https://doi.org/10.3390/vaccines3020373
Chicago/Turabian StyleSridhar, Saranya, Karl A. Brokstad, and Rebecca J. Cox. 2015. "Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines" Vaccines 3, no. 2: 373-389. https://doi.org/10.3390/vaccines3020373
APA StyleSridhar, S., Brokstad, K. A., & Cox, R. J. (2015). Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines, 3(2), 373-389. https://doi.org/10.3390/vaccines3020373







