3.1. Enhancement of Systemic and Mucosal Antigen-Specific Immune Responses by α-GalCer after Intranasal Vaccination with Viral Vectored Antigens in Mice
Earlier studies from our group demonstrated the effectiveness of immunizations employing multiple doses of protein antigen admixed with α-GalCer delivered by the mucosal intranasal route to afford repeated activation of NKT cells as well as enhancement of adaptive immune responses [
17,
19]. In this investigation, we tested the effectiveness of α-GalCer adjuvant for improving immunity induced by antigens expressed from viral vectors employing the vesicular stomatitis viral vector encoding ovalbumin as the model antigen (VSV-OVA). Mice were immunized once by the intranasal route with the VSV-OVA in the presence or absence of the α-GalCer adjuvant and 14 days later analyzed for humoral and cellular immune responses in different systemic and mucosal tissues (
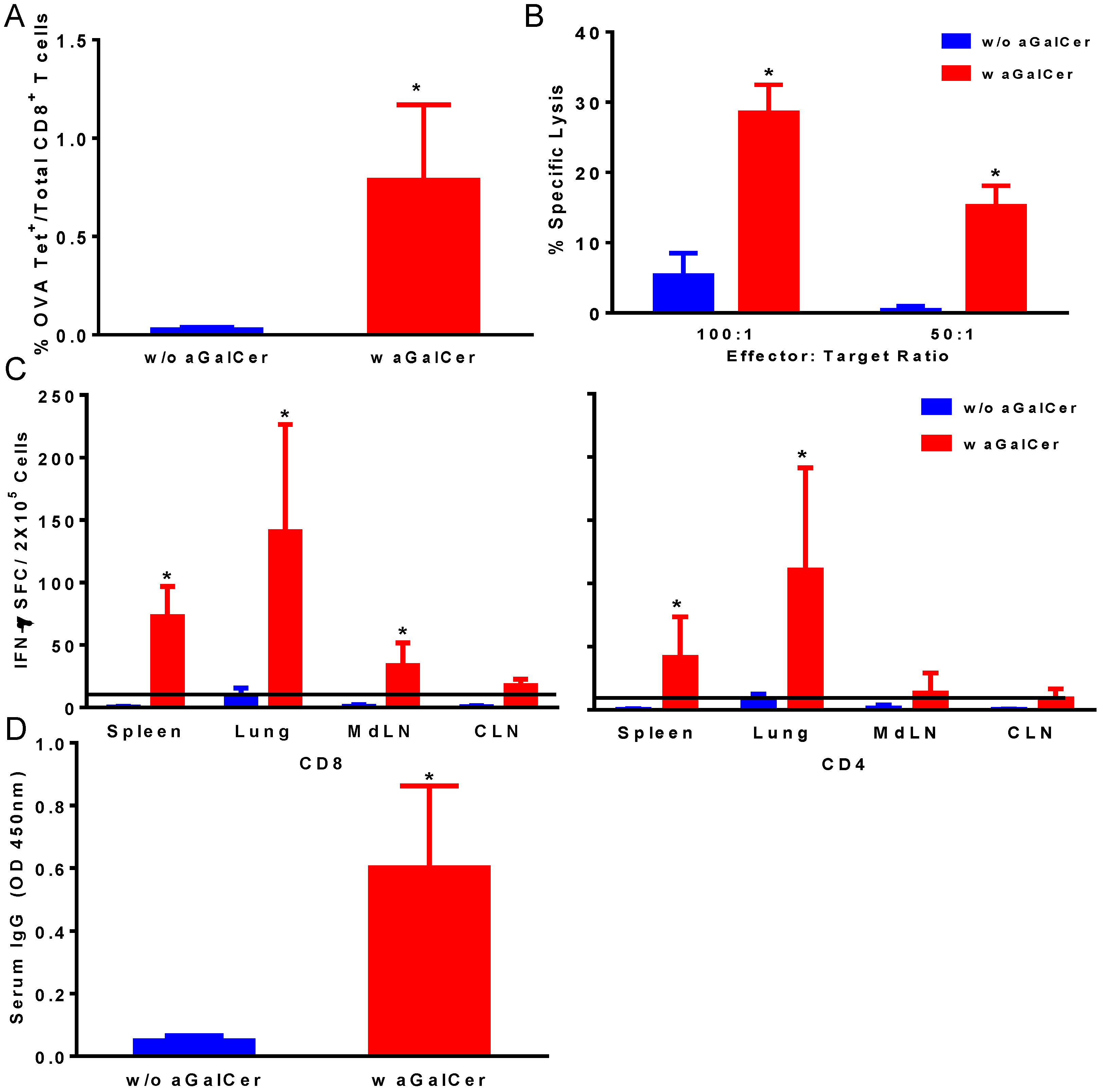
Figure 1). We observed significant enhancement in OVA tetramer positive CD8 T cells in the blood of mice that were immunized with the VSV-OVA in the presence of α-GalCer, relative to those with VSV-OVA alone (
Figure 1A). This is also reflected in the functional properties of the T cells in terms of significantly higher OVA-specific CTL responses in the spleen (
Figure 1B) and IFN-γ producing CD4 and CD8 T cells in spleen and multiple mucosal tissues in mice vaccinated with VSV-OVA + α-GalCer when compared to mice that received VSV-OVA only (
Figure 1C). A similar level of effectiveness of the α-GalCer adjuvant was observed for inducing antigen-specific humoral immunity in terms of significantly enhanced production of OVA-specific serum IgG antibodies (
Figure 1D).
Based on the effectiveness of α-GalCer observed using the VSV vector expressing the model OVA antigen, we tested the adenoviral (Ad) vector platform used by our group for HIV vaccine formulations consisting of the first generation Ad vectors (FG-Ad) expressing the HIV envelope and gag antigens (FG-Ad HIV-env and FG-Ad HIV-Gag, respectively) in the presence and absence of α-GalCer adjuvant for intranasal immunization. Serum and vaginal-wash samples from mice in each of the groups collected prior to and at 14 days post-vaccination were analyzed for the presence of antibodies specific to HIV envelope and gag antigens in the vaccine using the standard ELISA protocol (
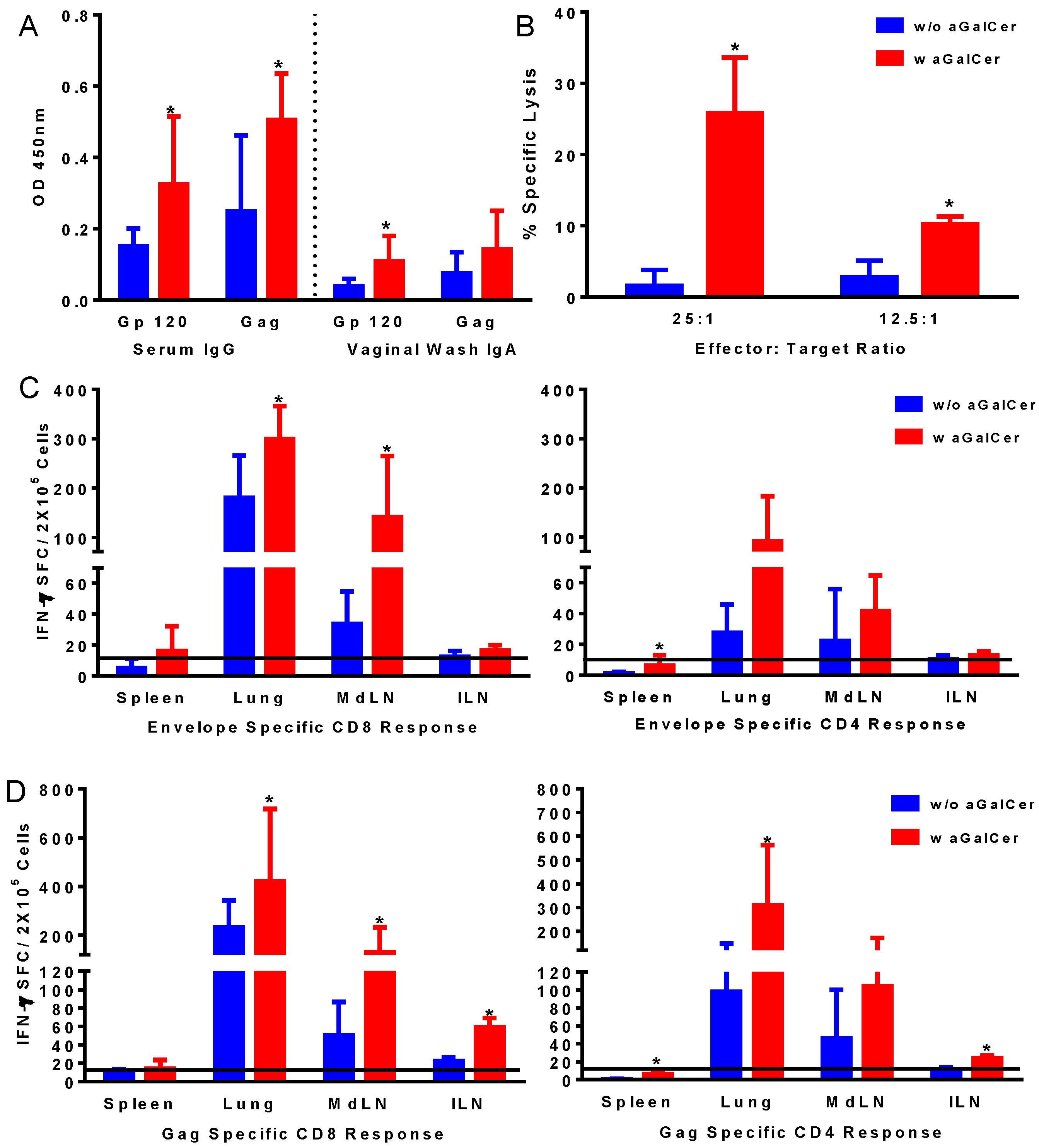
Figure 2A). Significantly higher levels of IgG and IgA antibodies in the serum and vaginal washes, respectively, were detected against both envelope and gag antigens in mice after vaccination in the presence of α-GalCer relative to that in mice vaccinated without the adjuvant, indicating the effectiveness of α-GalCer in enhancing antigen-specific systemic and mucosal antibody responses. Similarly, antigen-specific CTL responses, determined in the splenocytes using the standard chromium release assay, were significantly higher in mice administered the vaccine with α-GalCer relative to those without α-GalCer (
Figure 2B). Single cell suspensions collected from different tissues harvested on Day 14 were analyzed for antigen-specific T cells in terms of cytokine production using ELISpot assay (
Figure 2C,D). Significantly higher antigen-specific IFN-γ producing CD8 and CD4 T cells were detected in several tissues including lung, lung-draining mediastinal lymph nodes (MdLN) as well as Iliac lymph nodes (ILN), surrogates of genital immune responses (
Figure 2C,D).
Together, these results support α-GalCer to be an effective adjuvant to afford significant improvements for viral vectored vaccines in mice in terms of inducing antigen-specific humoral and cellular immune responses in systemic as well as mucosal tissues.
Figure 1.
Induction of antigen-specific immune responses by intranasal immunization with vesicular stomatitis viral vector encoding ovalbumin as the model antigen (VSV-OVA) using alpha-galactosylceramide (α-GalCer) adjuvant. Single cell suspensions from spleens were analyzed for the presence of antigen-specific CD8+ T lymphocytes by staining with fluorescently labeled CD8 OVA tetramer and antibodies to mouse CD44 and CD8 along with Aqua live/dead stain (A). Antigen-specific cytolytic activity of splenocytes isolated from immunized mice was determined by the standard chromium-release assay employing the syngeneic EL-4 target cells pulsed with the OVA peptide, at two different effector to target cell ratios (B). Data were adjusted for background by subtracting control values (target cells not pulsed with the OVA peptide). IFN-γ production in response to stimulation with CD8 and CD4 T cell epitope peptides from OVA was determined by using a standard IFN-γ ELISpot assay (C). Data are shown as IFN-γ spot forming cells (SFC) per 2 × 105 input cells and OVA specific responses were adjusted to background medium control. Antigen specific IgG antibody responses were determined in serum samples by ELISA (D). Data are representative of two separate experiments and expressed as mean ± S.D. Statistical analyses between responses to different immunizations was performed using Mann Whitney Test to determine significant differences (* p ≤ 0.05) between immunizations with VSV-OVA alone and VSV-OVA admixed with α-GalCer.
Figure 1.
Induction of antigen-specific immune responses by intranasal immunization with vesicular stomatitis viral vector encoding ovalbumin as the model antigen (VSV-OVA) using alpha-galactosylceramide (α-GalCer) adjuvant. Single cell suspensions from spleens were analyzed for the presence of antigen-specific CD8+ T lymphocytes by staining with fluorescently labeled CD8 OVA tetramer and antibodies to mouse CD44 and CD8 along with Aqua live/dead stain (A). Antigen-specific cytolytic activity of splenocytes isolated from immunized mice was determined by the standard chromium-release assay employing the syngeneic EL-4 target cells pulsed with the OVA peptide, at two different effector to target cell ratios (B). Data were adjusted for background by subtracting control values (target cells not pulsed with the OVA peptide). IFN-γ production in response to stimulation with CD8 and CD4 T cell epitope peptides from OVA was determined by using a standard IFN-γ ELISpot assay (C). Data are shown as IFN-γ spot forming cells (SFC) per 2 × 105 input cells and OVA specific responses were adjusted to background medium control. Antigen specific IgG antibody responses were determined in serum samples by ELISA (D). Data are representative of two separate experiments and expressed as mean ± S.D. Statistical analyses between responses to different immunizations was performed using Mann Whitney Test to determine significant differences (* p ≤ 0.05) between immunizations with VSV-OVA alone and VSV-OVA admixed with α-GalCer.
![Vaccines 02 00686 g001]()
Figure 2.
Induction of antigen-specific immune responses by intranasal immunization with adenoviral vectors expressing HIV-env and Gag using α-GalCer adjuvant. Mice were immunized by intranasal route with first generation adenoviral vectors with deletion of E1 gene (FG-Ad) HIV-env and FG-Ad HIV-Gag either alone or admixed with α-GalCer and were sacrificed 14 days later to determine systemic and mucosal adaptive immune responses. Antigen specific IgG and IgA antibody responses to recombinant HIV envelope protein gp120 and HIV Gag (p55) were determined in serum and vaginal washes by enzyme-linked immunosorbent assay (ELISA) (A). Antigen-specific cytolytic activity of splenocytes isolated from immunized mice were determined by the standard chromium-release assay employing the syngeneic P815 target cells pulsed with the HIV-gp120 peptide, at different effector to target cell ratios (B). Data were adjusted for background by subtracting control values (target cells not pulsed with the peptide). Single cell suspensions from spleen, lungs, mediastinal lymph nodes (MdLN), and Iliac lymph nodes (ILN) were analyzed for interferon-gamma (IFN-γ) production in response to stimulation with CD8 T and CD4 T cell epitope peptides from HIV-env (C) and HIV-Gag (D) by using a standard IFN-γ ELISpot assay. Data are shown as IFN-γ spot forming cells (SFC) per 2 × 105 input cells and antigen specific responses were adjusted to background medium control. Data are representative of two separate experiments and expressed as mean ± S.D. Statistical analyses between responses to different immunizations were performed using Mann Whitney Test, * (p ≤ 0.05) between immunizations with Ad vectors alone and Ad vectors admixed with α-GalCer.
Figure 2.
Induction of antigen-specific immune responses by intranasal immunization with adenoviral vectors expressing HIV-env and Gag using α-GalCer adjuvant. Mice were immunized by intranasal route with first generation adenoviral vectors with deletion of E1 gene (FG-Ad) HIV-env and FG-Ad HIV-Gag either alone or admixed with α-GalCer and were sacrificed 14 days later to determine systemic and mucosal adaptive immune responses. Antigen specific IgG and IgA antibody responses to recombinant HIV envelope protein gp120 and HIV Gag (p55) were determined in serum and vaginal washes by enzyme-linked immunosorbent assay (ELISA) (A). Antigen-specific cytolytic activity of splenocytes isolated from immunized mice were determined by the standard chromium-release assay employing the syngeneic P815 target cells pulsed with the HIV-gp120 peptide, at different effector to target cell ratios (B). Data were adjusted for background by subtracting control values (target cells not pulsed with the peptide). Single cell suspensions from spleen, lungs, mediastinal lymph nodes (MdLN), and Iliac lymph nodes (ILN) were analyzed for interferon-gamma (IFN-γ) production in response to stimulation with CD8 T and CD4 T cell epitope peptides from HIV-env (C) and HIV-Gag (D) by using a standard IFN-γ ELISpot assay. Data are shown as IFN-γ spot forming cells (SFC) per 2 × 105 input cells and antigen specific responses were adjusted to background medium control. Data are representative of two separate experiments and expressed as mean ± S.D. Statistical analyses between responses to different immunizations were performed using Mann Whitney Test, * (p ≤ 0.05) between immunizations with Ad vectors alone and Ad vectors admixed with α-GalCer.
![Vaccines 02 00686 g002]()
3.2. Induction of NKT Cells Responses in Rhesus Macaques by α-GalCer
Since immune responses generated by vaccination in mice may not truly predict applicability to humans and also since rhesus macaques represent a suitable nonhuman primate model close to humans, specifically for HIV vaccine approaches, we tested the effectiveness of α-GalCer to induce NKT cells responses in macaques. However, in general the populations of NKT cells in circulation as well as within tissues are relatively low in primates when compared to those in rodents, and systemic administration of α-GalCer as free drug can result in the NKT cell anergy under some conditions [
26,
27]. Because of this potential problem, most studies rely on administering dendritic cells (DCs) loaded
ex vivo with α-GalCer to ensure a stimulatory effect [
26,
28,
29]. While DC-mediated vaccination has a role in certain situations, this is not a practical option for gene-based vector-mediated vaccines against infectious diseases like HIV-AIDS that require mass scale immunization campaigns. Given this, the ability to stimulate NKT cells in non-human rhesus macaques was explored. We tested delivering α-GalCer to macaques by four different routes to activate NKT cells: intramuscular (IM), intravenous (IV), intranasal (Nasal), and sublingual (
Figure 3). Blood and liver NKT cell populations were analyzed using the CD1d tetramer (
Figure 3A). Consistent with previous reports, NKT cell populations were low in the macaques prior to α-GalCer administration. However, IM injection of α-GalCer stimulated robust increases in CD1d-positive NKT cells (
Figure 3B). In contrast, α-GalCer administration by the other routes stimulated only weak responses. Since the hall mark for
in vivo functionality of activated NKT cells is production of high amounts of IL4 and IFN-γ, we tested peripheral blood mononuclear cells (PBMC) isolated at different time points after α-GalCer administration by the four different routes for IFN-γ producing cells and observed the strongest responses from sublingual and IN routes (
Figure 3C), which is also reflected in lymph node cells isolated at 24 h post-administration of α-GalCer (
Figure 3D). Analyses of serum levels of cytokines at several time points after α-GalCer administration by the four different routes for IL4 and IFN-γ production showed low but detectable levels of IL-4 and IFN-γ after IM and sublingual routes, respectively, relative to other routes (data not shown) [
30].
Together, these results show for the first time functional activation of NKT cells in nonhuman primates after directly administering α-GalCer as free reagent without having to pre-load ex vivo onto presenting cells such as the DC.
3.3. Intranasal Administration of α-GalCer to Rhesus Macaques Induces NKT Cells Activation and Induces Adaptive Immune Responses to Co-Administered Antigen
To further investigate the
in vivo adjuvant potential of α-GalCer, we tested in a separate pilot study the immunization of single rhesus monkeys each by the intranasal route with the nucleoprotein (NP) of the influenza virus as a model antigen in the presence and absence of α-GalCer and analyzed for NP-specific T cell and antibody responses (
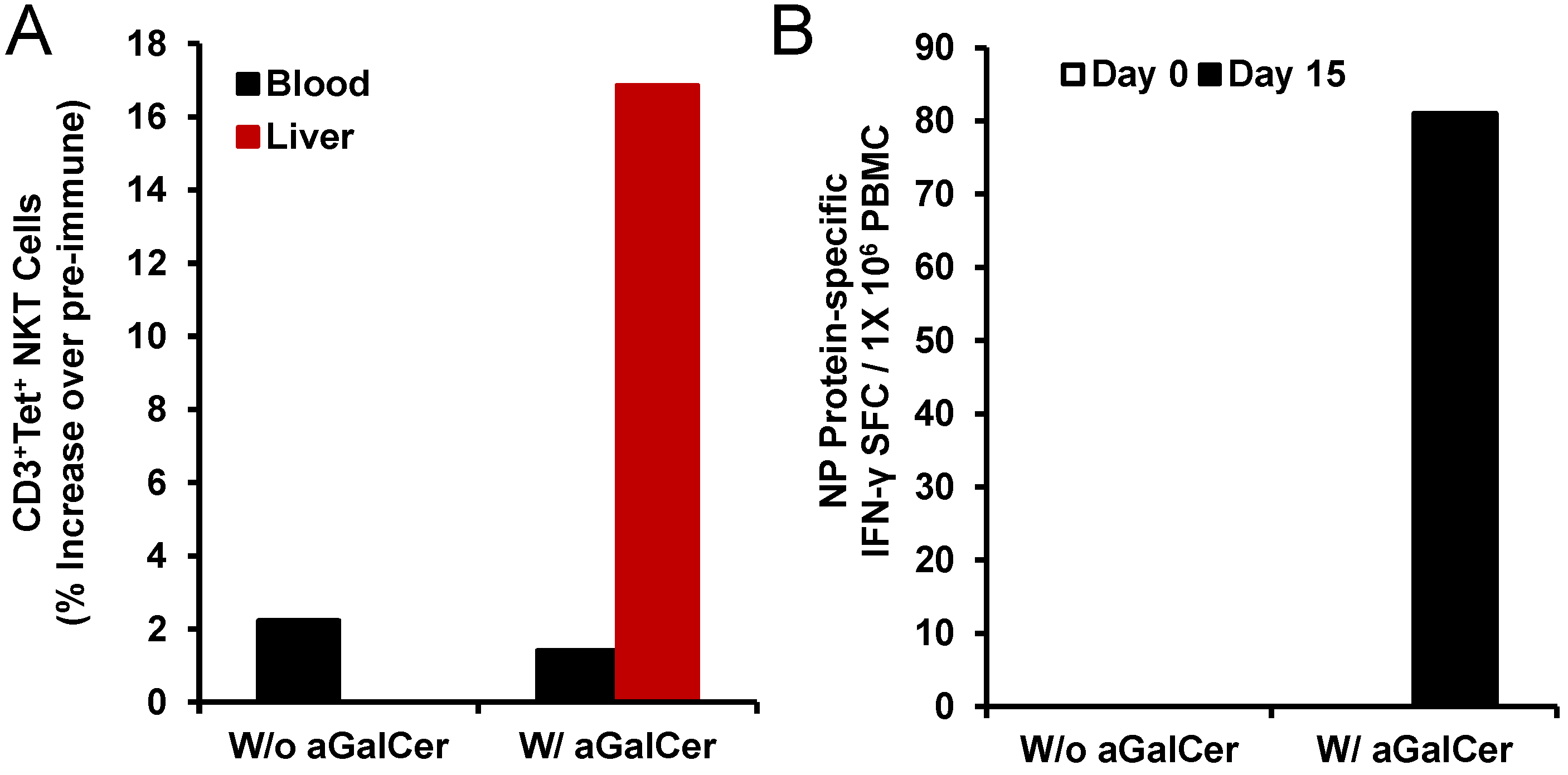
Figure 4). We observed induction of higher numbers of NKT cells in the liver after intranasal immunization with NP in the presence of α-GalCer but not NP alone (
Figure 4A). Only in the PBMC isolated from the animal immunized with NP and α-GalCer, we observed induction of NP-specific IFN-γ producing cells (
Figure 4B). Immunization with NP along with α-GalCer, but not NP alone resulted in the production of NP-specific IgG antibodies in the blood and IgA antibodies in the bronchial alveolar lavage (BAL) that were above background pre-immune levels (data not shown) [
30].
These results from the pilot study are suggestive of the in vivo effectiveness of α-GalCer as an adjuvant in the nonhuman primate model of rhesus macaques, similar to our data from mice, for improving adaptive immune responses to co-administered antigens.
Figure 3.
In vivo expansion of natural killer T (NKT) cells with α-GalCer delivered by different routes in rhesus macaques: Individual animals were administered α-GalCer as free drug (125 µg) by sublingual, intranasal (IN), intravenous (IV), and intramuscular (IM) routes followed by determining the percentages of NKT cells in the blood by staining with allophycocyanin (APC)-labeled CD1d tetramer pre-loaded with α-GalCer. The gating scheme (
A) for the analyses of the NKT cells in the peripheral blood mononuclear cells (PBMC) from a representative animal included first selecting the lymphocytes based on forward scatter (FCS) versus side scatter (SSC), and then live lymphocytes were identified based on staining with Aqua Live/Dead reagent (Invitrogen, Carlsbad, CA, USA). The T cells were then positively identified by CD3 expression followed by the detection of the CD1d tetramer
+ populations within the CD3
+ T cells. Percentages of CD1d tetramer
+ NKT cells in PBMC isolated from individual monkeys at 0, 6, 8 and 24 h after delivering α-GalCer by the different routes are shown in
Figure 3B. Numbers of IFN-γ producing cells within the blood (
C) and lymph nodes (
D) after administering α-GalCer by the different routes in individual monkeys as an indication of activated NKT cells are enumerated by the standard cytokine ELISpot assay. Data are shown as IFN-γ spot forming cells (SFC) per 1 × 10
5 input cells and antigen specific responses were adjusted to background medium control.
Figure 3.
In vivo expansion of natural killer T (NKT) cells with α-GalCer delivered by different routes in rhesus macaques: Individual animals were administered α-GalCer as free drug (125 µg) by sublingual, intranasal (IN), intravenous (IV), and intramuscular (IM) routes followed by determining the percentages of NKT cells in the blood by staining with allophycocyanin (APC)-labeled CD1d tetramer pre-loaded with α-GalCer. The gating scheme (
A) for the analyses of the NKT cells in the peripheral blood mononuclear cells (PBMC) from a representative animal included first selecting the lymphocytes based on forward scatter (FCS) versus side scatter (SSC), and then live lymphocytes were identified based on staining with Aqua Live/Dead reagent (Invitrogen, Carlsbad, CA, USA). The T cells were then positively identified by CD3 expression followed by the detection of the CD1d tetramer
+ populations within the CD3
+ T cells. Percentages of CD1d tetramer
+ NKT cells in PBMC isolated from individual monkeys at 0, 6, 8 and 24 h after delivering α-GalCer by the different routes are shown in
Figure 3B. Numbers of IFN-γ producing cells within the blood (
C) and lymph nodes (
D) after administering α-GalCer by the different routes in individual monkeys as an indication of activated NKT cells are enumerated by the standard cytokine ELISpot assay. Data are shown as IFN-γ spot forming cells (SFC) per 1 × 10
5 input cells and antigen specific responses were adjusted to background medium control.
![Vaccines 02 00686 g003]()
Figure 4.
Activation of NKT cell and adaptive immune responses after intranasal delivery of α-GalCer to rhesus macaques: Intranasal immunization with the recombinant nucleoprotein of influenza (NP protein 20 µg) along with, but not without, α-GalCer (125 µg) induced expansion of NKT cells in the blood and liver tissues as determined by flow cytometry staining with APC CD1d tetramer (panel A). Data are calculated as percent increase of CD3+, CD1d tetramer+ NKT cells relative to pre-immune values for one monkey each immunized with the NP protein in the absence or presence of α-GalCer. Adaptive immune responses to the NP protein were determined in terms of IFN-γ producing cells in the PBMC by the cytokine ELISpot assay (panel B). Data are shown as IFN-γ spot forming cells (SFC) per 1 × 105 input cells and antigen specific responses were adjusted to background medium control.
Figure 4.
Activation of NKT cell and adaptive immune responses after intranasal delivery of α-GalCer to rhesus macaques: Intranasal immunization with the recombinant nucleoprotein of influenza (NP protein 20 µg) along with, but not without, α-GalCer (125 µg) induced expansion of NKT cells in the blood and liver tissues as determined by flow cytometry staining with APC CD1d tetramer (panel A). Data are calculated as percent increase of CD3+, CD1d tetramer+ NKT cells relative to pre-immune values for one monkey each immunized with the NP protein in the absence or presence of α-GalCer. Adaptive immune responses to the NP protein were determined in terms of IFN-γ producing cells in the PBMC by the cytokine ELISpot assay (panel B). Data are shown as IFN-γ spot forming cells (SFC) per 1 × 105 input cells and antigen specific responses were adjusted to background medium control.
3.4. Enhancement of Systemic and Mucosal Immune Responses after Sublingual Mucosal Vaccination of Rhesus Macaques with Adenoviral Vectored HIV Antigen in the Presence of α-GalCer Adjuvant
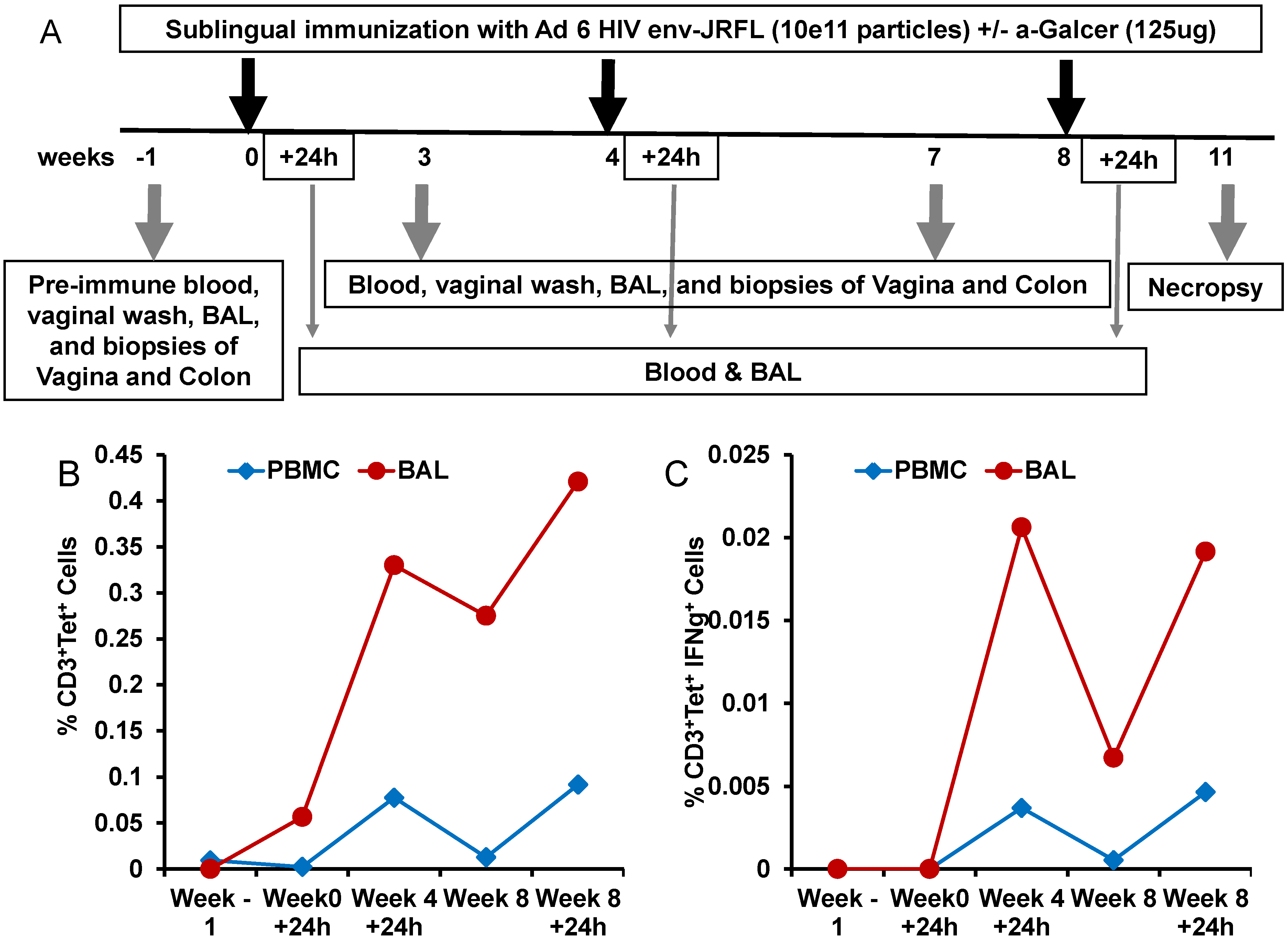
To determine whether the potency of α-GalCer to enhance immune responses to viral vectored antigens observed in mice will also translate into the nonhuman primate model of rhesus macaques, we conducted another pilot study testing α-GalCer as an adjuvant for mucosal sublingual immunization with the HD-Ad vector encoding the HIV envelope antigen (HD-Ad HIV-env). The immunization regimen included sublingual delivery of HD-Ad HIV-env alone or along with α-GalCer to one animal each at weeks 0, 4 and 8 (
Figure 5A). The animal immunized with HD-Ad HIV-env in the presence of α-GalCer showed pronounced enhancement of NKT cells numbers as well as IFN-γ production by NKT cells, relative to pre-immune time point, in the bronchia alveolar lavage (BAL) samples and to a lesser extent in the blood (
Figure 5B,C).
In this pilot study, we also compared the levels of induction and expansion of antigen-specific IL2 and/or IFN-γ producing CD4
+ T cells (
Figure 6A–C) and CD8
+ T cells (
Figure 6D–F) in the blood, colon, and vagina samples at different time points post-immunization. Immunization with HD-Ad HIV-env in the presence of α-GalCer in general yielded higher percentages of CD4
+ T cells producing IFN-γ in blood, relative to that in the monkey immunized without α-GalCer adjuvant. Similarly, we observed higher percentages of CD8
+ T cells producing IFN-γ in colon and vagina, and IL-2 producing cells in the blood and colon when HD-Ad HIV-env vaccination was administered with α-GalCer, relative to without α-GalCer.
The immunization regimen involving HD-Ad HIV-env immunization in the presence and absence of α-GalCer in the two rhesus macaques also resulted in the establishment of differential levels of antigen-specific IL2 and/or IFN-γ producing effector and central memory subsets of CD4
+ T cells (
Figure 7A–C) and CD8
+ T cells subsets (
Figure 7D–F). Overall, in the animal immunized with HD-Ad HIV-env in the presence of α-GalCer, relative to that in the animal immunized in the absence of α-GalCer, there are more CD4 Tem cells producing IFN-γ alone or both IFN-γ and IL-2 in the blood as well as colon, while single cytokine producing CD4 T
cm cells were observed in the blood. In case of CD8 T cell memory subsets, immunization employing the α-GalCer adjuvant resulted in higher percentages of cells producing either one of the cytokines or both cytokines in the colon and vagina tissues. Overall, this data from single monkey pilot studies show a trend towards higher percentages of antigen-specific memory CD4 and CD8 T cells subsets (both effector and central memory cells) in the mucosal tissues when the vaccine was delivered in the presence of α-GalCer when compared to that in the absence of the adjuvant.
Figure 5.
Expansion and activation of NKT cells after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: In this pilot study, a single rhesus monkey was immunized by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (1011vp) along with α-GalCer (125 µg) as shown in the scheme (A). Prior to immunization and at different time points subsequently, blood and bronchial alveolar lavage (BAL) samples were collected and lymphocytes were analyzed by flow cytometry for the percentages of NKT cells in terms of CD3+, CD1d tetramer+ cells (B) and percentages of IFN-γ producing NKT cells in terms CD3+, CD1d tetramer+, IFN-γ+ cells (C).
Figure 5.
Expansion and activation of NKT cells after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: In this pilot study, a single rhesus monkey was immunized by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (1011vp) along with α-GalCer (125 µg) as shown in the scheme (A). Prior to immunization and at different time points subsequently, blood and bronchial alveolar lavage (BAL) samples were collected and lymphocytes were analyzed by flow cytometry for the percentages of NKT cells in terms of CD3+, CD1d tetramer+ cells (B) and percentages of IFN-γ producing NKT cells in terms CD3+, CD1d tetramer+, IFN-γ+ cells (C).
Figure 6.
Enhancement of antigen-specific CD4 and CD8 T cells responses after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: Single cell suspensions prepared from biopsy tissues of colon and vagina along with PBMC from two rhesus macaques immunized by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (1011vp) alone (n = 1) or along with α-GalCer (125 µg; n = 1), open and closed symbols, respectively, were analyzed by flow cytometry for IFN-γ and/or IL-2 producing CD4 and CD8 T cells. Various tissues at different time points post immunization showing percentages of CD4 T cells (A–C) and CD8 T cells (D–F) producing IFN-γ alone (IFN-γ+IL2−), IL-2 alone (IFN-γ−IL2+) or both (IFN-γ+IL2+).
Figure 6.
Enhancement of antigen-specific CD4 and CD8 T cells responses after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: Single cell suspensions prepared from biopsy tissues of colon and vagina along with PBMC from two rhesus macaques immunized by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (1011vp) alone (n = 1) or along with α-GalCer (125 µg; n = 1), open and closed symbols, respectively, were analyzed by flow cytometry for IFN-γ and/or IL-2 producing CD4 and CD8 T cells. Various tissues at different time points post immunization showing percentages of CD4 T cells (A–C) and CD8 T cells (D–F) producing IFN-γ alone (IFN-γ+IL2−), IL-2 alone (IFN-γ−IL2+) or both (IFN-γ+IL2+).
Figure 7.
Enhancement of antigen-specific memory subsets of CD4 and CD8 T cells responses after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: Single cell suspensions prepared from biopsy tissues of colon and vagina along with PBMC from single rhesus monkey immunized by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (10
11vp) alone or along with α-GalCer (125 µg), open and closed symbols, respectively, were analyzed by flow cytometry for IFN-γ and/or IL-2 producing effector and central memory subsets (Tem and Tcm, respectively) of CD4 (
A–
C) and CD8 (
D–
F) T cells. Various tissues at different time points post immunization showing percentages of CD4 T cells and CD8 T cells producing IFN-γ alone (IFN-γ
+IL2
−;
Figure 7A,D), IL-2 alone (IFN-γ
−IL2
+;
Figure 7B,E) or both (IFN-γ
+IL2
+;
Figure 7C,F).
Figure 7.
Enhancement of antigen-specific memory subsets of CD4 and CD8 T cells responses after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: Single cell suspensions prepared from biopsy tissues of colon and vagina along with PBMC from single rhesus monkey immunized by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (10
11vp) alone or along with α-GalCer (125 µg), open and closed symbols, respectively, were analyzed by flow cytometry for IFN-γ and/or IL-2 producing effector and central memory subsets (Tem and Tcm, respectively) of CD4 (
A–
C) and CD8 (
D–
F) T cells. Various tissues at different time points post immunization showing percentages of CD4 T cells and CD8 T cells producing IFN-γ alone (IFN-γ
+IL2
−;
Figure 7A,D), IL-2 alone (IFN-γ
−IL2
+;
Figure 7B,E) or both (IFN-γ
+IL2
+;
Figure 7C,F).
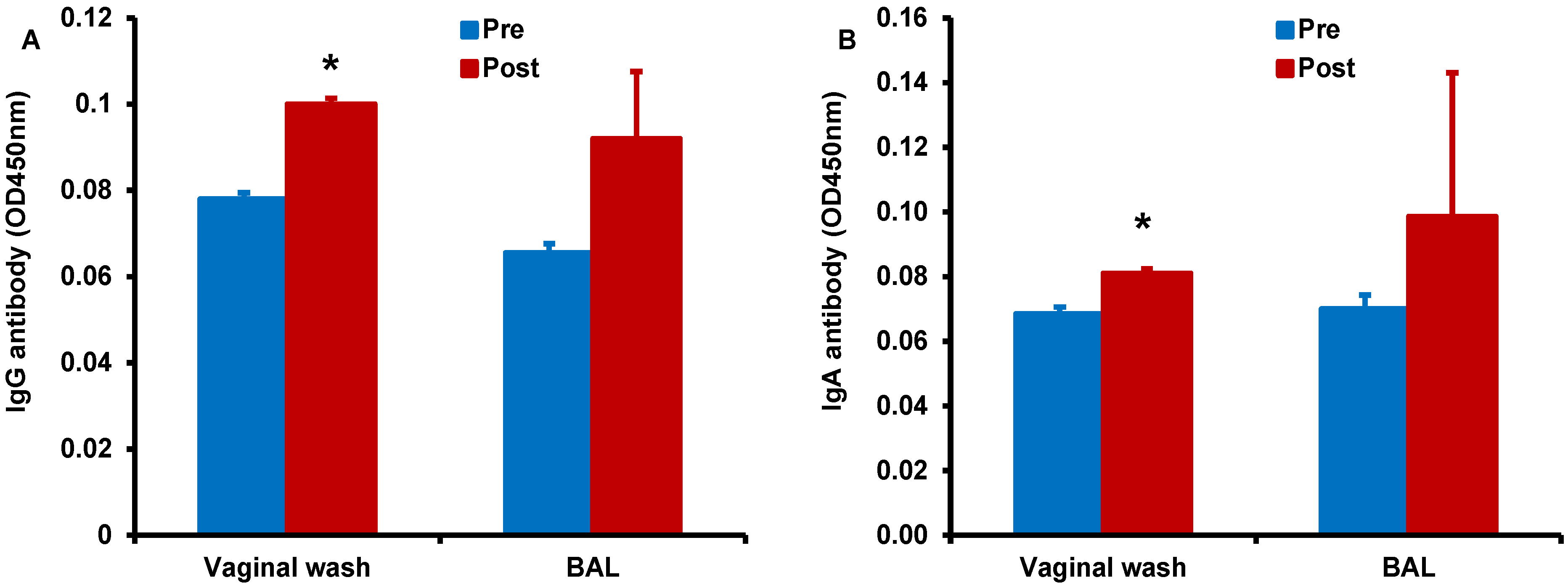
Finally, we determined antigen-specific antibody responses in serum, vaginal wash, and BAL samples collected form the monkey immunized by the sublingual route with HD-Ad HIV-env in the presence of α-GalCer adjuvant (
Figure 8). We observed higher IgG (
Figure 8A) and IgA (
Figure 8B) responses in the vaginal wash samples collected after vaccination when compared to pre-immune base level values. Similarly, increases for both IgG and IgA antibodies were observed in the BAL for vaccination including α-GalCer adjuvant relative to pre-immune levels, but these differences did not reach statistical significance. Serum levels of IgG and IgA antibodies were not significantly different between samples pre- and post-immunization (data not shown) [
30]. In the monkey immunized with HD-Ad HIV-env in the absence of α-GalCer adjuvant no substantial IgG or IgA responses were observed (data not shown) [
30].
Together, these data showing enhanced adaptive responses in terms of T cells and antibodies specific to HIV env in mice as well as rhesus macaques support sublingual immunization employing the α-GalCer adjuvant to be a potentially useful vaccination strategy for enhancing adaptive immune responses.
Figure 8.
Enhancement of antigen-specific antibody responses after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: Vaginal wash and BAL samples collected from monkeys prior to and after immunization (pre and post, respectively) by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (1011vp) along with α-GalCer (125 µg) were analyzed by the standard ELISA for IgG (A) and IgA (B) antibodies specific to the HIV envelope protein gp160. Levels of both types of antibodies were significantly higher in the vaginal wash samples when compared to those in pre-immune samples as determined by the student’s t test (* p < 0.05).
Figure 8.
Enhancement of antigen-specific antibody responses after sublingual immunization with HD-Ad HIV envelope in the presence of α-GalCer adjuvant: Vaginal wash and BAL samples collected from monkeys prior to and after immunization (pre and post, respectively) by the sublingual route with the HD-Ad vector encoding the HIV-1 envelope protein (1011vp) along with α-GalCer (125 µg) were analyzed by the standard ELISA for IgG (A) and IgA (B) antibodies specific to the HIV envelope protein gp160. Levels of both types of antibodies were significantly higher in the vaginal wash samples when compared to those in pre-immune samples as determined by the student’s t test (* p < 0.05).