DNA/MVA Vaccines for HIV/AIDS
Abstract
:1. Introduction
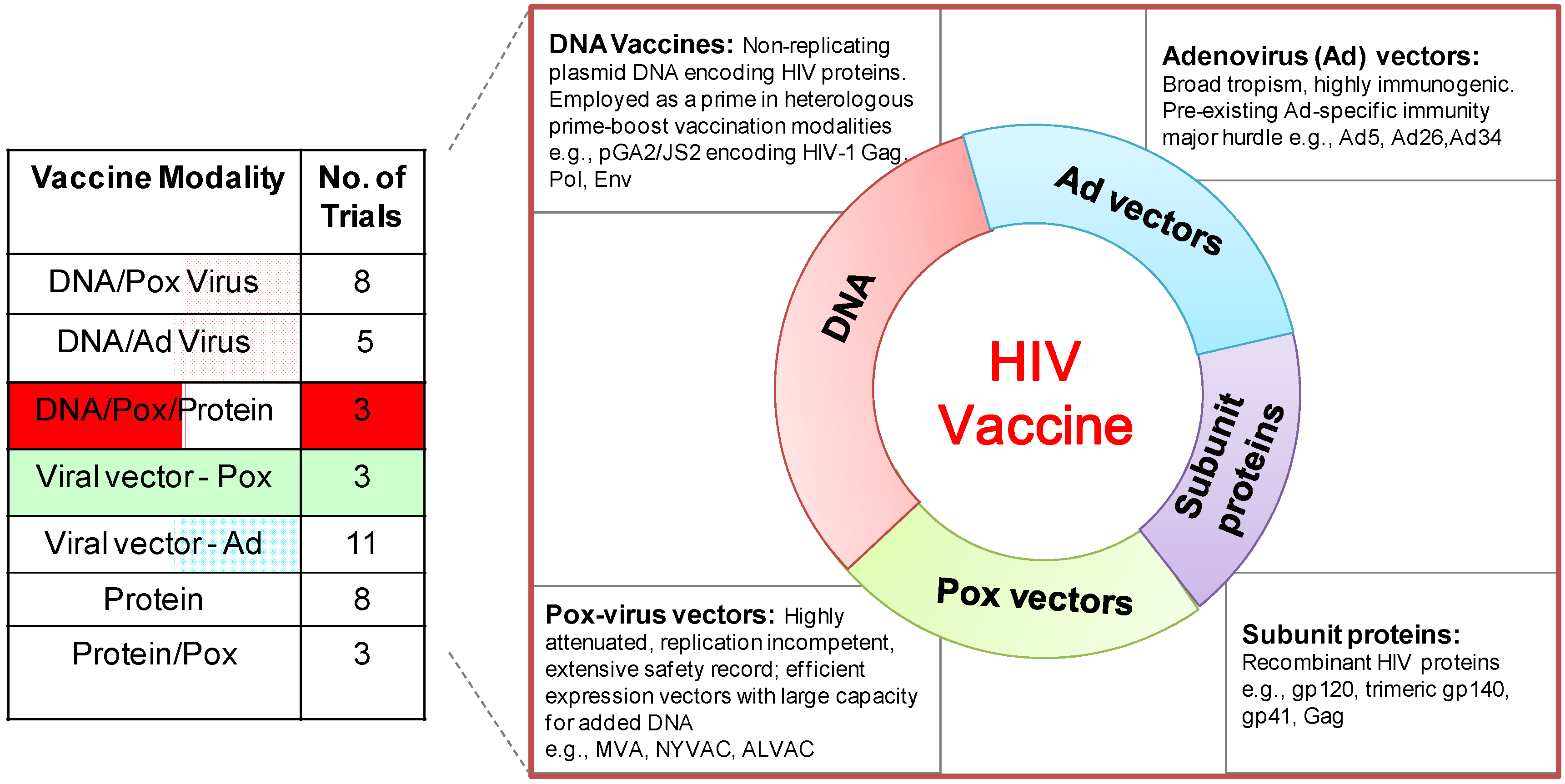
2. A Brief History of DNA Vaccines

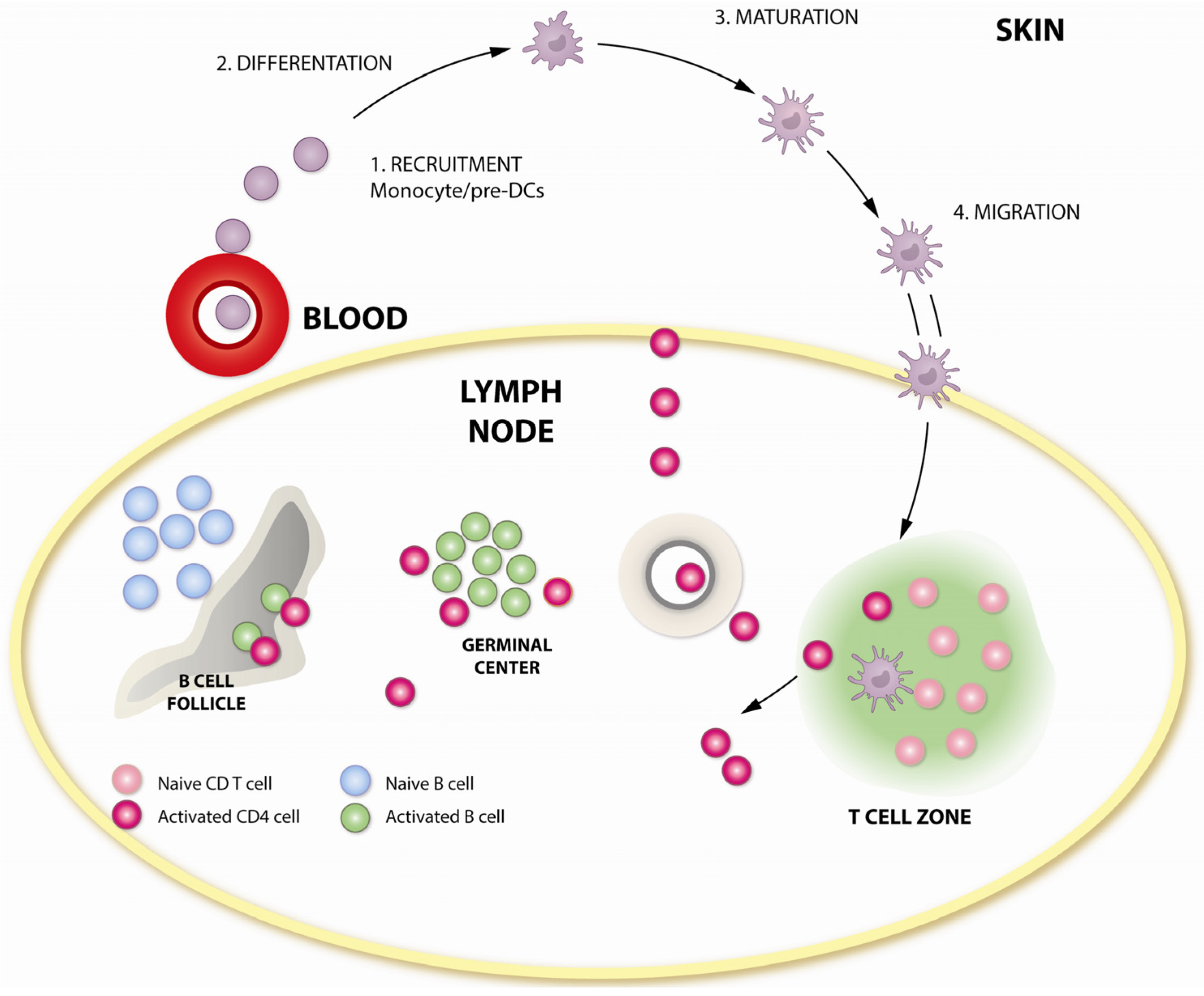
3. How Is DNA Immunogenic?
3.1. DNA As a Prime for an HIV Vaccine
3.2. Development of DNA Prime/MVA Boost Vaccine
4. DNA/MVA vs. MVA-Only Vaccines
5. Adjuvanted DNA Vaccines
5.1. GM-CSF Adjuvanted DNA Vaccine
5.2. CD40L Adjuvanted DNA/MVA Vaccines

6. DNA/MVA HIV Vaccines in Clinical Trials
7. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.P.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 2013, 369, 2083–2092. [Google Scholar] [CrossRef]
- Michael, N.L. Rare serotype adenoviral vectors for HIV vaccine development. J. Clin. Invest. 2012, 122, 25–27. [Google Scholar] [CrossRef]
- IAVI Report. Clinical Trials Database. November 2013. Available online: http://www.iavireport.org/Trials-Database/Pages/default.aspx (accessed on 31 January 2014).
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar]
- Tang, D.C.; deVit, M.; Johnston, S.A. Genetic immunization is a simple method for eliciting an immune response. Nature 1992, 356, 152–154. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; Rhodes, G.H.; Felgner, P.L.; Dwarki, V.J.; Gromkowski, S.H.; Deck, R.R.; DeWitt, C.M.; Feredman, A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993, 259, 1745–1749. [Google Scholar]
- Robinson, H.L.; Hunt, L.A.; Webster, R.G. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine 1993, 11, 957–960. [Google Scholar]
- Fynan, E.F.; Webster, R.G.; Fuller, D.H.; Haynes, J.R.; Santoro, J.C.; Robinson, H.L. DNA vaccines: Protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. USA 1993, 90, 11478–11482. [Google Scholar] [CrossRef]
- Wang, B.; Ugen, K.E.; Srikantan, V.; Agadjanyan, M.G.; Dang, K.; Refaeli, Y.; Sato, A.I.; Boyer, J.; Williams, W.V.; Weiner, D.B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1993, 90, 4156–4160. [Google Scholar] [CrossRef]
- Peet, N.M.; McKeating, J.A.; Ramos, B.; Klonisch, T.; de Souza, J.B.; Delves, P.J.; Lund, T. Comparison of nucleic acid and protein immunization for induction of antibodies specific for HIV-1 gp120. Clin. Exp. Immunol. 1997, 109, 226–232. [Google Scholar]
- Jiao, S.S.; Williams, P.; Berg, R.K.; Hodgeman, B.A.; Liu, L.J.; Repetto, G.; Wolff, J.A. Direct gene transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992, 3, 21–33. [Google Scholar] [CrossRef]
- Wang, B.; Boyer, J.; Srikantan, V.; Uqen, K.; Gilbert, L.; Phan, C.; Dang, K.; Merva, M.; Aqadjanyan, M.G. Induction of humoral and cellular immune responses to the human immunodeficiency type 1 virus in nonhuman primates by in vivo DNA inoculation. Virology 1995, 211, 102–112. [Google Scholar] [CrossRef]
- Boyer, J.D.; Uqen, K.E.; Wang, B.; Aqadjanyan, M.; Gilbert, L.; Baqarazzi, M.L.; Chatterqoon, M.; Frost, P.; Javadian, A.; Williams, W.V.; et al. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat. Med. 1997, 3, 526–532. [Google Scholar]
- Donnelly, J.J.; Ulmer, J.B.; Liu, M.A. DNA vaccines. Annu. Rev. Immunol. 1997, 15, 617–648. [Google Scholar] [CrossRef]
- Letvin, N.L.; Montefiori, D.C.; Yasutomi, Y.; Perry, H.C.; Davies, M.E.; Lekutis, C.; Alroy, M.; Freed, D.C.; Lord, C.I.; Handt, L.K.; et al. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 1997, 94, 9378–9383. [Google Scholar] [CrossRef]
- Fuller, D.H.; Simpson, L.; Cole, K.S.; Clements, J.E.; Panicali, D.L.; Montelaro, R.C.; Murphey-Corb, M.; Haynes, J.R. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunol. Cell Biol. 1997, 75, 389–396. [Google Scholar] [CrossRef]
- Schneider, J.; Gilbert, S.C.; Blanchard, T.J.; Hanke, T.; Robson, K.J.; Hannan, C.M.; Becker, M.; Sinden, R.; Smith, G.L.; Hill, A.V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 1998, 4, 397–402. [Google Scholar]
- Robinson, H.L.; Montefiori, D.C.; Johnson, P.; Manson, K.H.; Kalish, M.; Lifson, J.D.; Rizvi, T.A.; Lu, S.; Hu, S.L.; Mazzara, G.P.; et al. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 1999, 5, 526–534. [Google Scholar]
- Allen, T.M.; Voqel, T.U.; Fuller, D.H.; Mothé, B.R.; Steffen, S.; Boyson, J.E.; Shipley, T.; Fuller, J.; Hanke, T.; Sette, A.; et al. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 2000, 164, 4968–4978. [Google Scholar]
- Hanke, T.; McMichael, A. Pre-clinical development of a multi-CTL epitope-based DNA prime MVA boost vaccine for AIDS. Immunol. Lett. 1999, 66, 177–181. [Google Scholar] [CrossRef]
- Amara, R.R.; Villinger, F.; Altman, J.D.; Lydy, S.L.; O’Neil, S.P.; Staprans, S.I.; Montefiori, D.C.; Xu, Y.; Herndon, J.G.; Wyatt, L.S.; et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 2001, 292, 69–74. [Google Scholar] [CrossRef]
- Mwau, M.; Cebere, I.; Sutton, J.; Chikoti, P.; Winstone, N.; Wee, E.G.; Beattie, T.; Chen, Y.H.; Dorrell, L.; McShane, H.; et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: Stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J. Gen. Virol. 2004, 85, 911–919. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Russell, N.D.; Celum, C.; Kahn, J.; Noonan, E.; Montefiori, D.C.; Ferrari, G.; Weinhold, K.J.; Smith, J.M.; Amara, R.R.; et al. Excellent safety and tolerability of the human immunodeficiency virus type 1 pGA2/JS2 plasmid DNA priming vector vaccine in HIV type 1 uninfected adults. AIDS Res. Hum. Retrovir. 2006, 22, 678–683. [Google Scholar] [CrossRef]
- Ondondo, B.O.; Yang, H.; Dong, T.; di Gleria, K.; Suttill, A.; Conlon, C.; Brown, D.; Williams, P.; Rowland-Jones, S.L.; Hanke, T.; et al. Immunisation with recombinant modified vaccinia virus Ankara expressing HIV-1 gag in HIV-1-infected subjects stimulates broad functional CD4+ T cell responses. Eur. J. Immunol. 2006, 36, 2585–2594. [Google Scholar] [CrossRef]
- Goonetilleke, N.; Moore, S.; Dally, L.; Winstone, N.; Cebere, I.; Mahmoud, A.; Pinheiro, S.; Gillespie, G.; Brown, D.; Loach, V.; et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 2006, 80, 4717–4728. [Google Scholar] [CrossRef]
- Sandstrom, E.; Nilsson, C.; Hejdeman, B.; Brave, A.; Bratt, G.; Robb, M.; Cox, J.; Vancott, T.; Marovich, M.; Stout, R.; et al. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J. Infect Dis. 2008, 198, 1482–1490. [Google Scholar] [CrossRef]
- Cebere, I.; Dorrell, L.; McShane, H.; Simmons, A.; McCormack, S.; Schmidt, C.; Smith, C.; Brooks, M.; Roberts, J.E.; Darwin, S.C.; et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine 2006, 24, 417–425. [Google Scholar] [CrossRef]
- Hanke, T.; Goonetilleke, N.; McMichael, A.J.; Dorrell, L. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J. Gen. Virol. 2007, 88, 1–12. [Google Scholar] [CrossRef]
- Guimaraes-Walker, A.; Mackie, N.; McCormack, S.; Hanke, T.; Schmidt, C.; Gilmour, J.; Barin, B.; McMichael, A.; Weber, J.; Legg, K.; et al. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine 2008, 26, 6671–6677. [Google Scholar] [CrossRef]
- Aboud, S.; Nilsson, C.; Karlen, K.; Marovich, M.; Wahren, B.; Sandstrom, E.; Gaines, H.; Biberfeld, G.; Godoy-Ramirez, K. Strong HIV-specific CD4+ and CD8+ T-lymphocyte proliferative responses in healthy individuals immunized with an HIV-1 DNA vaccine and boosted with recombinant modified vaccinia virus ankara expressing HIV-1 genes. Clin. Vaccine Immunol. 2010, 17, 1124–1131. [Google Scholar] [CrossRef]
- Goepfert, P.A.; Elizaga, M.L.; Sato, A.; Qin, L.; Cardinali, M.; Hay, C.M.; Hural, J.; DeRosa, S.C.; DeFawe, O.D.; Tomaras, G.D.; et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J. Infect Dis. 2011, 203, 610–619. [Google Scholar] [CrossRef]
- Nilsson, C.; Godoy-Ramirez, K.; Hejdeman, B.; Brave, A.; Gudmundsdotter, L.; Hallengard, D.; Currier, J.R.; Wieczorek, L.; Hasselrot, K.; Earl, P.L.; et al. Broad and potent cellular and humoral immune responses after a second late HIV-modified vaccinia virus Ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-Boosted Swedish vaccinees. AIDS Res. Hum. Retrovir. 2013. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Liu, M.A.; Ulmer, J.B. Antigen presentation and DNA vaccines. Am. J. Respir. Crit. Care Med. 2000, 162, S190–S193. [Google Scholar] [CrossRef]
- Corr, M.; Lee, D.J.; Carson, D.A.; Tighe, H. Gene vaccination with naked plasmid DNA: Mechanism of CTL priming. J. Exp. Med. 1996, 184, 1555–1560. [Google Scholar] [CrossRef]
- Asakura, Y.; Liu, L.J.; Shono, N.; Hinkula, J.; Kjerrstrom, A.; Aoki, I.; Okuda, K.; Wahren, B.; Fukushima, J. Th1-biased immune responses induced by DNA-based immunizations are mediated via action on professional antigen-presenting cells to up-regulate IL-12 production. Clin. Exp. Immunol. 2000, 119, 130–139. [Google Scholar] [CrossRef]
- Feltquate, D.M.; Heaney, S.; Webster, R.G.; Robinson, H.L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 1997, 158, 2278–2784. [Google Scholar]
- Pillai, V.B.; Hellerstein, M.; Yu, T.; Amara, R.R.; Robinson, H.L. Comparative studies on in vitro expression and in vivo immunogenicity of supercoiled and open circular forms of plasmid DNA vaccines. Vaccine 2008, 26, 1136–1141. [Google Scholar] [CrossRef]
- Flingai, S.; Czerwonko, M.; Goodman, J.; Kudchodkar, S.B.; Muthumani, K.; Weiner, D.B. Synthetic DNA vaccines: Improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol. 2013, 4, 354. [Google Scholar]
- MacGregor, R.R.; Ginsberg, R.; Ugen, K.E.; Baine, Y.; Kang, C.U.; Tu, X.M.; Higgins, T.; Weiner, D.B.; Boyer, J.D. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. AIDS 2002, 16, 2137–2143. [Google Scholar] [CrossRef]
- Sardesai, N.Y.; Weiner, D.B. Electroporation delivery of DNA vaccines: Prospects for success. Curr. Opin. Immunol. 2011, 23, 421–429. [Google Scholar] [CrossRef]
- Luckay, A.; Sidhu, M.K.; Kjeken, R.; Megati, S.; Chong, S.Y.; Roopchand, V.; Garcia-Hand, D.; Abdullah, R.; Braun, R.; Montefiori, D.C.; et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 2007, 81, 5257–5269. [Google Scholar] [CrossRef]
- Sutter, G.; Moss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851. [Google Scholar] [CrossRef]
- Carroll, M.W.; Moss, B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 1997, 238, 198–211. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Wyatt, L.S.; Eller, L.A.; Whitbeck, J.C.; Cohen, G.H.; Eisenberg, R.J.; Hartmann, C.J.; Jackson, D.L.; Kulesh, D.A.; et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 2004, 428, 182–185. [Google Scholar] [CrossRef]
- Seth, A.; Ourmanov, I.; Kuroda, M.J.; Schmitz, J.E.; Carroll, M.W.; Wyatt, L.S.; Moss, B.; Forman, M.A.; Hirsch, V.M.; Letvin, N.L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc. Natl. Acad. Sci. USA 1998, 95, 10112–10116. [Google Scholar] [CrossRef]
- Sadagopal, S.; Amara, R.R.; Montefiori, D.C.; Wyatt, L.S.; Staprans, S.I.; Kozyr, N.L.; McClure, H.M.; Moss, B.; Robinson, H.L. Signature for long-term vaccine-mediated control of a Simian and human immunodeficiency virus 89.6P challenge: Stable low-breadth and low-frequency T-cell response capable of coproducing gamma interferon and interleukin-2. J. Virol. 2005, 79, 3243–3253. [Google Scholar] [CrossRef]
- Amara, R.R.; Smith, J.M.; Staprans, S.I.; Montefiori, D.C.; Villinger, F.; Altman, J.D.; O’Neil, S.P.; Kozyr, N.L.; Xu, Y.; Wyatt, L.S.; et al. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 2002, 76, 6138–6146. [Google Scholar] [CrossRef]
- Sadagopal, S.; Amara, R.R.; Kannanganat, S.; Sharma, S.; Chennareddi, L.; Robinson, H.L. Expansion and exhaustion of T-cell responses during mutational escape from long-term viral control in two DNA/modified vaccinia virus Ankara-vaccinated and simian-human immunodeficiency virus SHIV-89.6P-challenged macaques. J. Virol. 2008, 82, 4149–4153. [Google Scholar] [CrossRef]
- Amara, R.R.; Ibegbu, C.; Villinger, F.; Montefiori, D.C.; Sharma, S.; Nigam, P.; Xu, Y.; McClure, H.M.; Robinson, H.L. Studies using a viral challenge and CD8 T cell depletions on the roles of cellular and humoral immunity in the control of an SHIV-89.6P challenge in DNA/MVA-vaccinated macaques. Virology 2005, 343, 246–255. [Google Scholar] [CrossRef]
- Amara, R.R.; Villinger, F.; Staprans, S.I.; Altman, J.D.; Montefiori, D.C.; Kozyr, N.L.; Xu, Y.; Wyatt, L.S.; Earl, P.L.; Herndon, J.G.; et al. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 2002, 76, 7625–7631. [Google Scholar] [CrossRef]
- Lai, L.; Kwa, S.F.; Kozlowski, P.A.; Montefiori, D.C.; Nolen, T.L.; Hudgens, M.G.; Johnson, W.E.; Ferrari, G.; Hirsch, V.M.; Felber, B.K.; et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine 2012, 30, 1737–1745. [Google Scholar] [CrossRef]
- Geissler, M.; Gesien, A.; Tokushige, K.; Wands, J.R. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J. Immunol. 1997, 158, 1231–1237. [Google Scholar]
- Barouch, D.H.; Santra, S.; Schmitz, J.E.; Kuroda, M.J.; Fu, T.M.; Wagner, W.; Bilska, M.; Craiu, A.; Zheng, X.X.; Krivulka, G.R.; et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 2000, 290, 486–492. [Google Scholar] [CrossRef]
- Baden, L.R.; Blattner, W.A.; Morgan, C.; Huang, Y.; Defawe, O.D.; Sobieszczyk, M.E.; Kochar, N.; Tomaras, G.D.; McElrath, M.J.; Russell, N.; et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J. Infect Dis. 2011, 204, 1541–1549. [Google Scholar] [CrossRef]
- Morrissey, P.J.; Bressler, L.; Park, L.S.; Alpert, A.; Gillis, S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J. Immunol. 1987, 139, 1113–1119. [Google Scholar]
- Elliott, M.J.; Strasser, A.; Metcalf, D. Selective up-regulation of macrophage function in granulocyte-macrophage colony-stimulating factor transgenic mice. J. Immunol. 1991, 147, 2957–2963. [Google Scholar]
- Haddad, D.; Ramprakash, J.; Sedegah, M.; Charoenvit, Y.; Baumgartner, R.; Kumar, S.; Hoffman, S.L.; Weiss, W.R. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J. Immunol. 2000, 165, 3772–3781. [Google Scholar]
- Robinson, H.L.; Montefiori, D.C.; Villinger, F.; Robinson, J.E.; Sharma, S.; Wyatt, L.S.; Earl, P.L.; McClure, H.M.; Moss, B.; Amara, R.R. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology 2006, 352, 285–294. [Google Scholar] [CrossRef]
- Lai, L.; Vodros, D.; Kozlowski, P.A.; Montefiori, D.C.; Wilson, R.L.; Akerstrom, V.L.; Chennareddi, L.; Yu, T.; Kannanganat, S.; Ofielu, L.; et al. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 2007, 369, 153–167. [Google Scholar] [CrossRef]
- Lai, L.; Kwa, S.; Kozlowski, P.A.; Montefiori, D.C.; Ferrari, G.; Johnson, W.E.; Hirsch, V.; Villinger, F.; Chennareddi, L.; Earl, P.L.; et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J. Infect Dis. 2011, 204, 164–173. [Google Scholar] [CrossRef]
- Hellerstein, M.; Xu, Y.; Marino, T.; Lu, S.; Yi, H.; Wright, E.R.; Robinson, H.L. Co-expression of HIV-1 virus-like particles and granulocyte-macrophage colony stimulating factor by GEO-D03 DNA vaccine. Hum. Vaccin. Immunother. 2012, 8, 1654–1658. [Google Scholar] [CrossRef]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar]
- Van Mierlo, G.J.; den Boer, A.T.; Medema, J.P.; van der Voort, E.I.; Fransen, M.F.; Offringa, R.; Melief, C.J.; Toes, R.E. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl. Acad. Sci. USA 2002, 99, 5561–5566. [Google Scholar] [CrossRef]
- French, R.R.; Chan, H.T.; Tutt, A.L.; Glennie, M.J. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 1999, 5, 548–553. [Google Scholar]
- Quezada, S.A.; Jarvinen, L.Z.; Lind, E.F.; Noelle, R.J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 2004, 22, 307–328. [Google Scholar] [CrossRef]
- Kishi, Y.; Aiba, Y.; Higuchi, T.; Furukawa, K.; Tokuhisa, T.; Takemori, T.; Tsubata, T. Augmented antibody response with premature germinal center regression in CD40L transgenic mice. J. Immunol. 2010, 185, 211–219. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Dutcher, J.P.; Anderson, J.E.; Eckhardt, S.G.; Stephans, K.F.; Razvillas, B.; Garl, S.; Butine, M.D.; Perry, V.P.; Armitage, R.J.; et al. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 2001, 19, 3280–3287. [Google Scholar]
- Stone, G.W.; Barzee, S.; Snarsky, V.; Kee, K.; Spina, C.A.; Yu, X.F.; Kornbluth, R.S. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J. Virol. 2006, 80, 1762–1772. [Google Scholar] [CrossRef]
- Gomez, C.E.; Najera, J.L.; Sanchez, R.; Jimenez, V.; Esteban, M. Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus vectors MVA and NYVAC expressing HIV antigens. Vaccine 2009, 27, 3165–3174. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Stone, G.W.; Yue, F.Y.; Ngai, N.; Jones, R.B.; Kornbluth, R.S.; Ostrowski, M.A. CD40L expressed from the canarypox vector, ALVAC, can boost immunogenicity of HIV-1 canarypox vaccine in mice and enhance the in vitro expansion of viral specific CD8+ T cell memory responses from HIV-1-infected and HIV-1-uninfected individuals. Vaccine 2008, 26, 4062–4072. [Google Scholar] [CrossRef]
- Wyzgol, A.; Yu, Q.; Stone, G.W.; Yue, F.Y.; Ngai, N.; Jones, R.B.; Kornbluth, R.S.; Ostrowski, M.A. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J. Immunol. 2009, 183, 1851–1861. [Google Scholar] [CrossRef]
- Calarota, S.A.; Leandersson, A.C.; Bratt, G.; Hinkula, J.; Klinman, D.M.; Weinhold, K.J.; Sandstrom, E.; Wahren, B. Immune responses in asymptomatic HIV-1-infected patients after HIV-DNA immunization followed by highly active antiretroviral treatment. J. Immunol. 1999, 163, 2330–2338. [Google Scholar]
- Harari, A.; Bart, P.A.; Stohr, W.; Tapia, G.; Garcia, M.; Medjitna-Rais, E.; Burnet, S.; Cellerai, C.; Erlwein, O.; Barber, T.; et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 2008, 205, 63–77. [Google Scholar] [CrossRef]
- Mehendale, S.; Thakar, M.; Sahay, S.; Kumar, M.; Shete, A.; Sathyamurthi, P.; Verma, A.; Kurle, S.; Shrotri, A.; Gilmour, J.; et al. Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: a phase I randomised Trial in HIV-uninfected Indian volunteers. PLoS One 2013, 8, e55831. [Google Scholar]
- Hayes, P.; Gilmour, J.; von Lieven, A.; Gill, D.; Clark, L.; Kopycinski, J.; Cheeseman, H.; Chung, A.; Alter, G.; Dally, L.; et al. Safety and immunogenicity of DNA prime and modified vaccinia ankara virus-HIV subtype C vaccine boost in healthy adults. Clin. Vaccine Immunol. 2013, 20, 397–408. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iyer, S.S.; Amara, R.R. DNA/MVA Vaccines for HIV/AIDS. Vaccines 2014, 2, 160-178. https://doi.org/10.3390/vaccines2010160
Iyer SS, Amara RR. DNA/MVA Vaccines for HIV/AIDS. Vaccines. 2014; 2(1):160-178. https://doi.org/10.3390/vaccines2010160
Chicago/Turabian StyleIyer, Smita S., and Rama R. Amara. 2014. "DNA/MVA Vaccines for HIV/AIDS" Vaccines 2, no. 1: 160-178. https://doi.org/10.3390/vaccines2010160
APA StyleIyer, S. S., & Amara, R. R. (2014). DNA/MVA Vaccines for HIV/AIDS. Vaccines, 2(1), 160-178. https://doi.org/10.3390/vaccines2010160



