Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation
Abstract
:1. Introduction
2. Experimental
2.1. Animals
2.2. Treatment and Tissue Processing
2.3. Immunohistochemistry
2.4. Imaging
3. Results and Discussion
3.1. Gene Delivery Enhanced by Dermal Electroporation Induces Sustained Expression on the Surface of the Skin

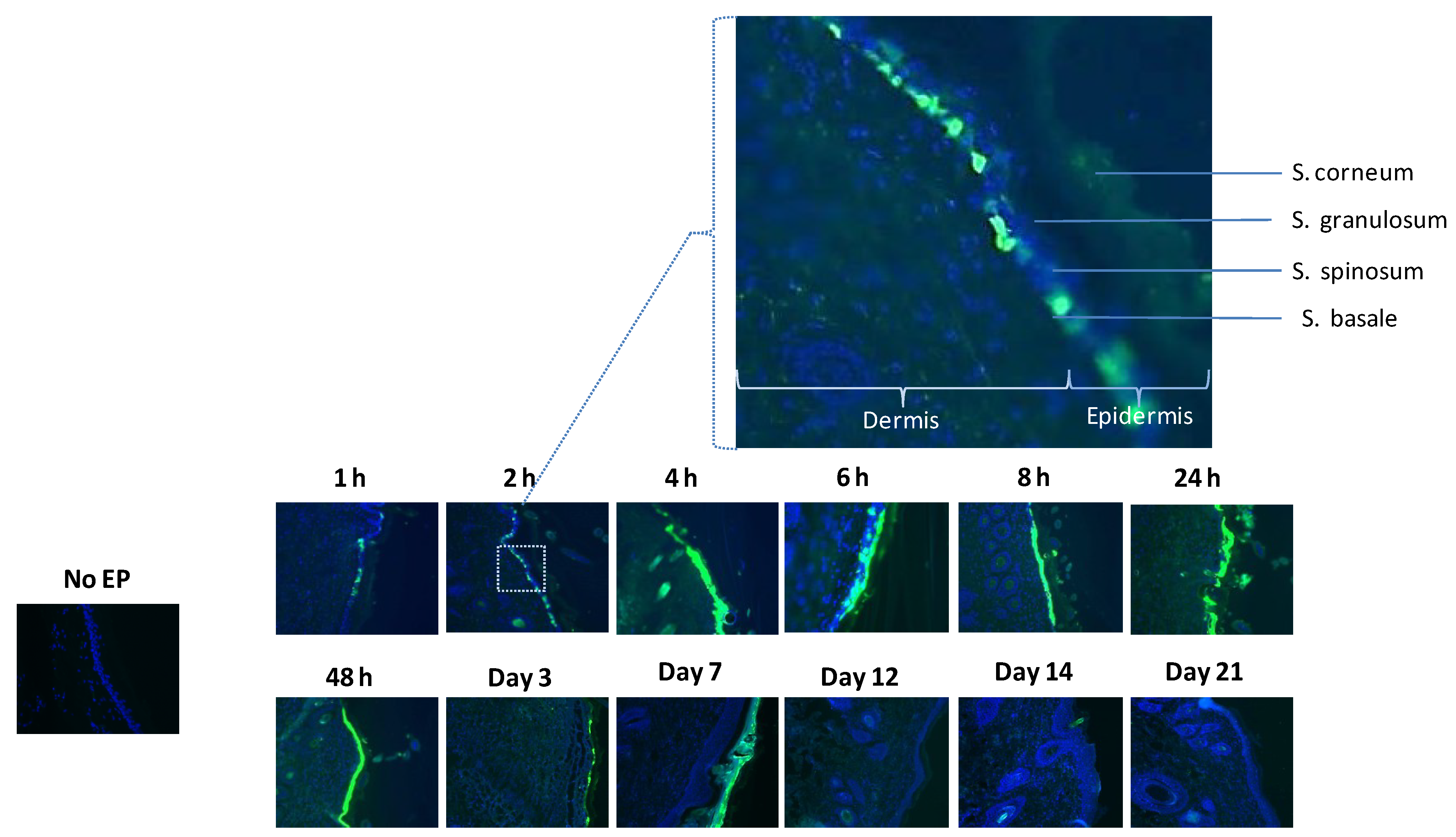
3.2. Histological Analysis Reveals GFP Expression Appearing after One Hour

3.3. Infiltration at the Electroporation Treatment Site is Fast and Persistent
3.4. Pronounced Migration of Lymphocytic Cells Is Observed at the Site of Gene Transfer Enhanced by Electroporation


4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tobin, D.J. Biochemistry of human skin—Our brain on the outside. Chem. Soc. Rev. 2006, 35, 52–67. [Google Scholar] [CrossRef]
- Toebak, M.J.; Gibbs, S.; Bruynzeel, D.P.; Scheper, R.J.; Rustemeyer, T. Dendritic cells: Biology of the skin. Contact Derm. 2009, 60, 2–20. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Turka, L.A.; Mitra, R.S.; Nestle, F.O. Direct and indirect control of T-cell activation by keratinocytes. J. Invest. Dermatol. 1995, 105, 25S–29S. [Google Scholar] [CrossRef]
- Romani, N.; Holzmann, S.; Tripp, C.H.; Koch, F.; Stoitzner, P. Langerhans cells—Dendritic cells of the epidermis. APMIS 2003, 111, 725–740. [Google Scholar]
- Weiner, D.B. DNA vaccines: Crossing a line in the sand. Introduction to special issue. Vaccine 2008, 26, 5073–5074. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Ulmer, J.B.; Liu, M.A. DNA vaccines. Life Sci. 1997, 60, 163–172. [Google Scholar] [CrossRef]
- Andre, S.; Seed, B.; Eberle, J.; Schraut, W.; Bultmann, A.; Haas, J. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 1998, 72, 1497–1503. [Google Scholar]
- Sardesai, N.Y.; Weiner, D.B. Electroporation delivery of DNA vaccines: Prospects for success. Curr. Opin. Immunol. 2011, 23, 421–429. [Google Scholar] [CrossRef]
- Martinon, F.; Kaldma, K.; Sikut, R.; Culina, S.; Romain, G.; Tuomela, M.; Adojaan, M.; Mannik, A.; Toots, U.; Kivisild, T.; et al. Persistent immune responses induced by a human immunodeficiency virus DNA vaccine delivered in association with electroporation in the skin of nonhuman primates. Hum. Gene Ther. 2009, 20, 1291–1307. [Google Scholar] [CrossRef]
- Song, J.M.; Kim, Y.C.; Lipatov, A.S.; Pearton, M.; Davis, C.T.; Yoo, D.G.; Park, K.M.; Chen, L.M.; Quan, F.S.; Birchall, J.C.; et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin. Vaccine Immunol. 2010, 17, 1381–1389. [Google Scholar] [CrossRef]
- Mathiesen, I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 1999, 6, 508–514. [Google Scholar] [CrossRef]
- Otten, G.; Schaefer, M.; Doe, B.; Liu, H.; Srivastava, I.; zur Megede, J.; O’Hagan, D.; Donnelly, J.; Widera, G.; Rabussay, D.; et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 2004, 22, 2489–2493. [Google Scholar] [CrossRef]
- Otten, G.R.; Schaefer, M.; Doe, B.; Liu, H.; Megede, J.Z.; Donnelly, J.; Rabussay, D.; Barnett, S.; Ulmer, J.B. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine 2006, 24, 4503–4509. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Draghia-Akli, R.; Wang, Q. Plasmid-based gene therapy of diabetes mellitus. Gene Ther. 2007, 14, 553–564. [Google Scholar] [CrossRef]
- Widera, G.; Austin, M.; Rabussay, D.; Goldbeck, C.; Barnett, S.W.; Chen, M.; Leung, L.; Otten, G.R.; Thudium, K.; Selby, M.J.; et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 2000, 164, 4635–4640. [Google Scholar]
- Kopycinski, J.; Cheeseman, H.; Ashraf, A.; Gill, D.; Hayes, P.; Hannaman, D.; Gilmour, J.; Cox, J.H.; Vasan, S. A DNA-based candidate hiv vaccine delivered via in vivo electroporation induces CD4 responses toward the α4β7-binding V2 loop of HIV gp120 in healthy volunteers. Clin. Vaccine Immunol. 2012, 19, 1557–1559. [Google Scholar] [CrossRef]
- Diaz, C.M.; Chiappori, A.; Aurisicchio, L.; Bagchi, A.; Clark, J.; Dubey, S.; Fridman, A.; Fabregas, J.C.; Marshall, J.; Scarselli, E.; et al. Phase 1 studies of the safety and immunogenicity of electroporated HER2/CEA DNA vaccine followed by adenoviral boost immunization in patients with solid tumors. J. Transl. Med. 2013, 11, e62. [Google Scholar] [CrossRef]
- Chudley, L.; McCann, K.; Mander, A.; Tjelle, T.; Campos-Perez, J.; Godeseth, R.; Creak, A.; Dobbyn, J.; Johnson, B.; Bass, P.; et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8+ T-cell responses and increases PSA doubling time. Cancer Immunol. Immunother. 2012, 61, 2161–2170. [Google Scholar] [CrossRef]
- Vasan, S.; Hurley, A.; Schlesinger, S.J.; Hannaman, D.; Gardiner, D.F.; Dugin, D.P.; Boente-Carrera, M.; Vittorino, R.; Caskey, M.; Andersen, J.; et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011, 6, e19252. [Google Scholar] [CrossRef] [Green Version]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E.; et al. Immunotherapy against HPV16/18 generates potent Th1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Hoffmann, G.A.; Hoffman, R.M. Depth-targeted efficient gene delivery and expression in the skin by pulsed electric fields: An approach to gene therapy of skin aging and other diseases. Biochem. Biophys. Res. Commun. 1996, 220, 633–636. [Google Scholar] [CrossRef]
- Heller, L.C.; Jaroszeski, M.J.; Coppola, D.; McCray, A.N.; Hickey, J.; Heller, R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007, 14, 275–280. [Google Scholar] [CrossRef]
- Donate, A.; Coppola, D.; Cruz, Y.; Heller, R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PLoS One 2011, 6, e19181. [Google Scholar]
- Heller, R.; Cruz, Y.; Heller, L.C.; Gilbert, R.A.; Jaroszeski, M.J. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum. Gene Ther. 2010, 21, 357–362. [Google Scholar] [CrossRef]
- Zhang, L.; Nolan, E.; Kreitschitz, S.; Rabussay, D.P. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim. Biophys. Acta 2002, 1572, 1–9. [Google Scholar] [CrossRef]
- Broderick, K.E.; Kardos, T.; McCoy, J.R.; Fons, M.P.; Kemmerrer, S.; Sardesai, N.Y. Piezoelectric permeabilization of mammalian dermal tissue for in vivo DNA delivery leads to enhanced protein expression and increased immunogenicity. Hum. Vaccin. 2011, 7, 22–28. [Google Scholar] [CrossRef]
- Connolly, R.J.; Chapman, T.; Hoff, A.M.; Kutzler, M.A.; Jaroszeski, M.J.; Ugen, K.E. Non-contacthelium-based plasma for delivery of DNA vaccines. Enhancement of humoral and cellular immune responses. Hum. Vaccin. Immunother. 2012, 8, 1729–1733. [Google Scholar] [CrossRef]
- Connolly, R.J.; Rey, J.I.; Lambert, V.M.; Wegerif, G.; Jaroszeski, M.J.; Ugen, K.E. Enhancement of antigen specific humoral immune responses after delivery of a DNA plasmid based vaccine through a contact-independent helium plasma. Vaccine 2011, 29, 6781–6784. [Google Scholar] [CrossRef]
- Roos, A.K.; Eriksson, F.; Timmons, J.A.; Gerhardt, J.; Nyman, U.; Gudmundsdotter, L.; Brave, A.; Wahren, B.; Pisa, P. Skin electroporation: Effects on transgene expression, DNA persistence and local tissue environment. PLoS One 2009, 4, e7226. [Google Scholar] [CrossRef]
- Roos, A.K.; Moreno, S.; Leder, C.; Pavlenko, M.; King, A.; Pisa, P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 2006, 13, 320–327. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Billington, M.; Deitz, S.; Colby, E.; Rhinehart, H.; Wu, Y.; Blackwelder, W.; Edelman, R.; Lee, A.; King, A. Safety and tolerability of the Easy Vax clinical epidermal electroporation system in healthy adults. Mol. Ther. 2012, 20, 214–220. [Google Scholar] [CrossRef]
- Hirao, L.A.; Wu, L.; Khan, A.S.; Satishchandran, A.; Draghia-Akli, R.; Weiner, D.B. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008, 26, 440–448. [Google Scholar] [CrossRef]
- Laddy, D.J.; Yan, J.; Khan, A.S.; Andersen, H.; Cohn, A.; Greenhouse, J.; Lewis, M.; Manischewitz, J.; King, L.R.; Golding, H.; et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J. Virol. 2009, 83, 4624–4630. [Google Scholar] [CrossRef]
- Hirao, L.A.; Draghia-Akli, R.; Prigge, J.T.; Yang, M.; Satishchandran, A.; Wu, L.; Hammarlund, E.; Khan, A.S.; Babas, T.; Rhodes, L.; et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2010, 203, 95–102. [Google Scholar]
- Diehl, M.C.; Lee, J.C.; Daniels, S.E.; Tebas, P.; Khan, A.; Giffear, M.; Sardesai, N.Y.; Bagarazzi, M.L. Tolerability of intramuscular and intradermal delivery by cellectra® adaptive constant current electroporation device in healthy volunteers. Hum. Vaccin. Immunother. 2013, 9, 1–6. [Google Scholar] [CrossRef]
- Broderick, K.E.; Shen, X.; Soderholm, J.; Lin, F.; McCoy, J.; Khan, A.S.; Yan, J.; Morrow, M.P.; Patel, A.; Kobinger, G.P.; et al. Prototype development and preclinical immunogenicity analysis of a novel minimally invasive electroporation device. Gene Ther. 2011, 18, 258–265. [Google Scholar] [CrossRef]
- Lin, F.; Shen, X.; Kichaev, G.; Mendoza, J.M.; Yang, M.; Armendi, P.; Yan, J.; Kobinger, G.P.; Bello, A.; Khan, A.S.; et al. Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device. Hum. Gene Ther. Methods 2012, 23, 157–168. [Google Scholar] [CrossRef]
- Gronevik, E.; von Steyern, F.V.; Kalhovde, J.M.; Tjelle, T.E.; Mathiesen, I. Gene expression and immune response kinetics using electroporation-mediated DNA delivery to muscle. J. Gene Med. 2005, 7, 218–227. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mendoza, J.M.; Amante, D.H.; Kichaev, G.; Knott, C.L.; Kiosses, W.B.; Smith, T.R.F.; Sardesai, N.Y.; Broderick, K.E. Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation. Vaccines 2013, 1, 384-397. https://doi.org/10.3390/vaccines1030384
Mendoza JM, Amante DH, Kichaev G, Knott CL, Kiosses WB, Smith TRF, Sardesai NY, Broderick KE. Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation. Vaccines. 2013; 1(3):384-397. https://doi.org/10.3390/vaccines1030384
Chicago/Turabian StyleMendoza, Janess M., Dinah H. Amante, Gleb Kichaev, Christine L. Knott, William B. Kiosses, Trevor R. F. Smith, Niranjan Y. Sardesai, and Kate E. Broderick. 2013. "Elucidating the Kinetics of Expression and Immune Cell Infiltration Resulting from Plasmid Gene Delivery Enhanced by Surface Dermal Electroporation" Vaccines 1, no. 3: 384-397. https://doi.org/10.3390/vaccines1030384



