Neural Correlates of Processing Passive Sentences
Abstract
:1. Introduction
- a.
- The boy is hugging the girl.
- b.
- Whoi is the boy hugging ti?
- c.
- The girli was hugged ti by the boy.
2. Results and Discussion
2.1. Behavioral Results
2.2. fMRI Results
| Contrast | Region | Peak Coordinates | k | t | p | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Passive > Active | RH IFG (pars opercularis, pars triangularis) | 56 | 24 | 28 | 497 | 6.09 | 0.005 |
| LH IFG (pars opercularis, pars triangularis) | −36 | 4 | 32 | 349 | 4.52 | 0.019 | |
| LH middle occipital gyrus, pMTG | −46 | −76 | 4 | 265 | 5.21 | 0.048 | |
| Bilateral SMA | −2 | 22 | 54 | 217 | 4.77 | 0.084 | |
| L SPL | −40 | −46 | 52 | 136 | 3.60 | 0.227 | |
| L precentral gyrus | −38 | −2 | 54 | 117 | 4.2 | 0.289 | |
| Active > Passive Age | None | ||||||
| (Passive > Active, Active > Passive) | None | ||||||
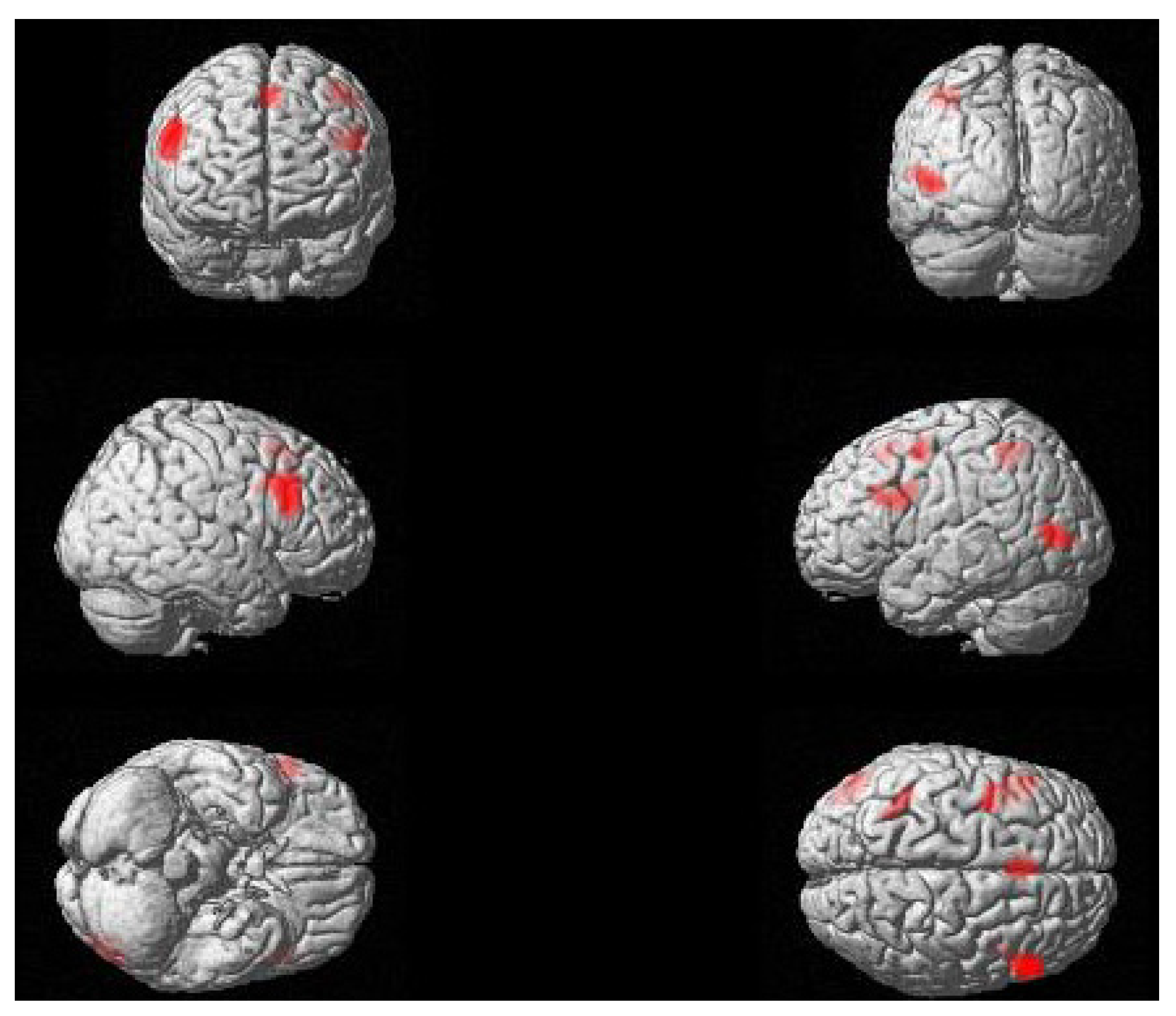
2.3. Discussion
2.3.1. Roles of the Left and Right IFG in Passive Sentence Comprehension
2.3.2. The Role of Left Posterior Temporal Cortex in Passive Sentence Comprehension
3. Experimental Section
3.1. Participants
3.2. Materials
3.3. Procedures
3.4. Data Acquisition
3.5. Data Analysis
3.5.1. Behavioral Data
3.5.2. Neuroimaging Data
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Chomsky, N. Lectures on Government and Binding; Foris: Dordrecht, The Netherlands, 1981. [Google Scholar]
- Dickey, M.W.; Choy, J.J.; Thompson, C.K. Real-time comprehension of wh-movement in aphasia: Evidence from eyetracking while listening. Brain Lang. 2007, 100, 1–22. [Google Scholar] [CrossRef]
- Dickey, M.W.; Thompson, C.K. Automatic processing of wh- and NP-movement in agrammatic aphasia: Evidence from eyetracking. J. Neurolinguist. 2009, 22, 563–583. [Google Scholar] [CrossRef]
- Love, T.; Swinney, D.; Walenski, M.; Zurif, E. How left inferior frontal cortex participates in syntactic processing: Evidence from aphasia. Brain Lang. 2008, 107, 203–219. [Google Scholar] [CrossRef]
- Nicol, J.; Fodor, J.D.; Swinney, D. Using Cross-Modal lexical decision tasks to investigate sentence processing. J. Exp. Psychol. Learn. 1994, 20, 1229–1238. [Google Scholar] [CrossRef]
- Sussman, R.S.; Sedivy, J.C. The time-course of processing syntactic dependencies: Evidence from eye movements. Lang. Cogn. Process. 2003, 18, 143–161. [Google Scholar] [CrossRef]
- Zurif, E.; Swinney, D.; Prather, P.; Solomon, J.; Bushell, C. An on-line analysis of syntactic processing in Broca’s and Wernicke’s aphasia. Brain Lang. 1993, 45, 448–464. [Google Scholar] [CrossRef]
- Fiebach, C.J.; Schlesewsky, M.; Friederici, A.D. Separating syntactic memory costs and syntactic integration during parsing: The processing of German WH-questions. J. Mem. Lang. 2002, 47, 250–272. [Google Scholar] [CrossRef]
- Pollard, C.; Sag, I.A. Head-Driven Phrase Structure Grammar; The University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Bresnan, J. Lexical-Functional Syntax; Blackwell: Oxford, UK, 2000. [Google Scholar]
- Osterhout, L.; Swinney, D.A. On the temporal course of gap-filling during comprehension of verbal passives. J. Psycholinguist. Res. 1993, 22, 273–286. [Google Scholar]
- Burkhardt, P.; Piñango, M.M.; Wong, K. The role of the anterior left hemisphere in real-time sentence comprehension: Evidence from split intransitivity. Brain Lang. 2003, 86, 9–22. [Google Scholar] [CrossRef]
- Friedmann, N.; Taranto, G.; Shapiro, L.P.; Swinney, D. The leaf fell (the leaf): The online processing of unaccusatives. Linguist. Inq. 2008, 39, 355–377. [Google Scholar] [CrossRef]
- Koring, L.; Mak, P.; Reuland, E. The time course of argument reactivation revealed: Using the visual world paradigm. Cognition 2012, 123, 361–379. [Google Scholar] [CrossRef]
- Kamide, Y.; Scheepers, C.; Altmann, G.T. Integration of syntactic and semantic information in predictive processing: Cross-linguistic evidence from German and English. J. Psycholinguist. Res. 2003, 32, 37–55. [Google Scholar] [CrossRef]
- Olson, D.R.; Filby, N. On the comprehension of passive and active sentences. Cogn. Psychol. 1972, 3, 361–381. [Google Scholar] [CrossRef]
- Knoeferle, P. Comparing the Time-course of Processing Initially Ambiguous and Unambiguous German SVO/OVS Sentences in Depicted Events. In Eye Movement Research: A Window on Mind and Brain; van Gompel, R., Fischer, M., Murray, W., Hill, R., Eds.; Elsevier: Oxford, UK, 2007; pp. 517–531. [Google Scholar]
- Knoeferle, P.; Crocker, M.W.; Scheepers, C.; Pickering, M.J. The influence of the immediate visual context on incremental thematic role-assignment: Evidence from eye-movements in depicted events. Cognition 2005, 95, 95–127. [Google Scholar] [CrossRef]
- Meyer, A.M.; Mack, J.E.; Thompson, C.K. Tracking passive sentence comprehension in agrammatic aphasia. J. Neurolinguist. 2012, 25, 31–43. [Google Scholar] [CrossRef]
- Stromswold, K.; Eisenband, J.; Norland, E.; Ratzan, J. Tracking the Acquisition and Processing of English Passives: Using Acoustic cues to Disambiguate Actives and Passives. In Paper Presented at the CUNY Conference on Sentence Processing, New York, NY, USA, 21–23 March 2002.
- Hanne, S.; Sekerina, I.A.; Vasishth, S.; Burchert, F.; de Bleser, R. Chance in agrammatic sentence comprehension: What does it really mean? Evidence from eye movements of German agrammatic aphasic patients. Aphasiology 2011, 25, 221–244. [Google Scholar] [CrossRef]
- Brookshire, R.H.; Nicholas, L.E. Verification of active and passive sentences by aphasic and nonaphasic subjects. J. Speech Hear. Res. 1980, 23, 878–893. [Google Scholar]
- Ferreira, F. The misinterpretation of noncanonical sentences. Cogn. Psychol. 2003, 47, 164–203. [Google Scholar] [CrossRef]
- Caplan, D.; Alpert, N.; Waters, G. Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J. Cogn. Neurosci. 1998, 10, 541–552. [Google Scholar] [CrossRef]
- Friederici, A.D.; Fiebach, C.J.; Schlesewsky, M.; Bornkessel, I.D.; von Cramon, D.Y. Processing linguistic complexity and grammaticality in the left frontal cortex. Cereb. Cortex 2006, 16, 1709–1717. [Google Scholar]
- Hirotani, M.; Makuuchi, M.; Rüschemeyer, S.A.; Friederici, A.D. Who was the agent? The neural correlates of reanalysis processes during sentence comprehension. Hum. Brain Mapp. 2011, 32, 1775–1787. [Google Scholar] [CrossRef]
- Makuuchi, M.; Bahlmann, J.; Anwander, A.; Friederici, A.D. Segregating the core computational faculty of human language from working memory. Proc. Natl. Acad. Sci. USA 2009, 106, 8362–8367. [Google Scholar] [CrossRef]
- Santi, A.; Grodzinsky, Y. Broca’s area and sentence comprehension: A relationship parasitic on dependency, displacement or predictability? Neuropsychologia 2012, 50, 821–832. [Google Scholar]
- Friederici, A.D. The brain basis of language processing: From structure to function. Physiol. Rev. 2011, 91, 1357–1392. [Google Scholar] [CrossRef]
- Ben-Shachar, M.; Hendler, T.; Kahn, I.; Ben-Bashat, D.; Grodzinsky, Y. The neural reality of syntactic transformations: Evidence from functional magnetic resonance imaging. Psychol. Sci. 2003, 14, 433–440. [Google Scholar] [CrossRef]
- Ben-Shachar, M.; Palti, D.; Grodzinsky, Y. Neural correlates of syntactic movement: Converging evidence from two fMRI experiments. Neuroimage 2004, 21, 1320–1336. [Google Scholar] [CrossRef]
- Grodzinsky, Y. The neurology of syntax: Language use without Broca’s area. Behav. Brain Sci. 2000, 23, 1–21. [Google Scholar] [CrossRef]
- Santi, A.; Grodzinsky, Y. Working memory and syntax interact in Broca’s area. Neuroimage 2007, 37, 8–17. [Google Scholar] [CrossRef]
- Shetreet, E.; Friedmann, N.; Hadar, U. The neural correlates of linguistic distinctions: Unaccusative and unergative verbs. J. Cogn. Neurosci. 2010, 22, 2306–2315. [Google Scholar] [CrossRef]
- Kinno, R.; Kawamura, M.; Shioda, S.; Sakai, K.L. Neural correlates of noncanonical syntactic processing revealed by a picture-sentence matching task. Hum. Brain Mapp. 2008, 29, 1015–1027. [Google Scholar] [CrossRef]
- Ye, Z.; Zhou, X. Conflict control during sentence comprehension: fMRI evidence. Neuroimage 2009, 48, 280–290. [Google Scholar] [CrossRef]
- Yokoyama, S.; Miyamoto, T.; Riera, J.; Kim, J.; Akitsuki, Y.; Iwata, K.; Yoshimoto, K.; Horie, K.; Sato, S.; Kawashima, R. Cortical mechanisms involved in the processing of verbs: An fMRI study. J. Cogn. Neurosci. 2006, 18, 1304–1313. [Google Scholar] [CrossRef]
- Yokoyama, S.; Okamoto, H.; Miyamoto, T.; Yoshimoto, K.; Kim, J.; Iwata, K.; Hyeonjeong, J.; Uchida, S.; Ikuta, N.; Sassa, Y.; et al. Cortical activation in the processing of passive sentences in L1 and L2: An fMRI study. Neuroimage 2006, 30, 570–579. [Google Scholar] [CrossRef]
- Yokoyama, S.; Watanabe, J.; Iwata, K.; Ikuta, N.; Haji, T.; Usui, N.; Taira, M.; Miyamoto, T.; Nakamura, W.; Sato, S.; et al. Is Broca’s area involved in the processing of passive sentences? An event-related fMRI study. Neuropsychologia 2007, 45, 989–996. [Google Scholar] [CrossRef]
- Wartenburger, I.; Heekeren, H.R.; Burchert, F.; de Bleser, R.; Villringer, A. Grammaticality judgments on sentences with and without movement of phrasal constituents: An event-related fMRI study. J. Neurolinguist. 2003, 16, 301–314. [Google Scholar] [CrossRef]
- Hoshi, H. Theta-role assignment, passivization, and excorporation. J. East Asian Ling. 1994, 3, 147–178. [Google Scholar] [CrossRef]
- Meltzer-Asscher, A.; Schuchard, J.; den Ouden, D.B.; Thompson, C.K. The neural substrates of complex argument structure representations: Processing “alternating transitivity” verbs. Lang. Cogn. Process. 2012. [Google Scholar] [CrossRef]
- Thompson, C.K.; Bonakdarpour, B.; Fix, S.; Blumenfeld, H.K.; Parrish, T.B.; Gitelman, D.R.; Mesulam, M.-M. Neural correlates of verb argument structure processing. J. Cogn. Neurosci. 2007, 19, 1753–1767. [Google Scholar] [CrossRef]
- Thompson, C.K.; Bonakdarpour, B.; Fix, S. Neural mechanisms of verb argument structure processing in agrammatic aphasic and healthy age-matched listeners. J. Cogn. Neurosci. 2010, 22, 1993–2011. [Google Scholar] [CrossRef]
- Bornkessel, I.; Zysset, S.; Friederici, A.D.; von Cramon, D.Y.; Schlesewsky, M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage 2005, 26, 221–233. [Google Scholar] [CrossRef]
- Friederici, A.D.; Rüschemeyer, S.A.; Hahne, A.; Fiebach, C.J. The role of left inferior frontal and superior temporal cortex in sentence comprehension: Localizing syntactic and semantic processes. Cereb. Cortex 2003, 13, 170–177. [Google Scholar] [CrossRef]
- Thompson, C.K.; Meltzer-Asscher, A. Neurocognitive Mechanisms of Verb Argument Structure Processing. In Structuring the Argument; Bachrach, A., Roy, I., Stockall, L., Eds.; John Benjamins: Amsterdam, The Netherlands, 2013; in press. [Google Scholar]
- Bastiaanse, R.; Edwards, S. Word order and finiteness in Dutch and English Broca’s and Wernicke’s aphasia. Brain Lang. 2004, 89, 91–107. [Google Scholar] [CrossRef]
- Grodzinsky, Y.; Piñango, M.M.; Zurif, E.; Drai, D. The critical role of group studies in neuropsychology: Comprehension regularities in Broca’s aphasia. Brain Lang. 1999, 67, 134–147. [Google Scholar] [CrossRef]
- Linebarger, M.C.; Schwartz, M.F.; Saffran, E.M. Sensitivity to grammatical structure in so-called agrammatic aphasics. Cognition 1983, 13, 361–392. [Google Scholar] [CrossRef]
- Luzzatti, C.; Toraldo, A.; Guasti, M.T.; Ghirardi, G.; Lorenzi, L.; Guarnaschelli, C. Comprehension of reversible active and passive sentences in agrammatism. Aphasiology 2001, 15, 419–441. [Google Scholar] [CrossRef]
- Kielar, A.; Meltzer-Asscher, A.; Thompson, C.K. Electrophysiological responses to argument structure violations in healthy adults and individuals with agrammatic aphasia. Neuropsychologia 2012, 50, 3320–3337. [Google Scholar] [CrossRef]
- Thompson, C.K.; den Ouden, D.B.; Bonakdarpour, B.; Garibaldi, K.; Parrish, T.B. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia 2010, 48, 3211–3227. [Google Scholar] [CrossRef]
- Caplan, D.; Hildebrandt, N.; Makris, N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain 1996, 119, 933–949. [Google Scholar] [CrossRef]
- Caplan, D.; Waters, G.; Kennedy, D.; Alpert, N.; Makris, N.; Dede, G.; Michaud, J.; Reddy, A. A study of syntactic processing in aphasia II: Neurological aspects. Brain Lang. 2007, 101, 151–177. [Google Scholar] [CrossRef]
- Dronkers, N.F.; Wilkins, D.P.; van Valin, R.D.; Redfern, B.B.; Jaeger, J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004, 92, 145–177. [Google Scholar] [CrossRef]
- Rogalski, E.; Cobia, D.; Harrison, T.M.; Wienecke, C.; Thompson, C.K.; Weintraub, S.; Mesulam, M.-M. Anatomy of language impairments in primary progressive aphasia. J. Neurosci. 2011, 31, 3344–3350. [Google Scholar] [CrossRef]
- Wilson, S.M.; Dronkers, N.F.; Ogar, J.M.; Jang, J.; Growdon, M.E.; Agosta, F.; Henry, M.L.; Miller, B.-.L.; Gorno-Tempini, M.L. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J. Neurosci. 2010, 15, 16845–16854. [Google Scholar]
- Thothathiri, M.; Kimberg, D.Y.; Schwartz, M.F. The neural basis of reversible sentence comprehension: Evidence from voxel-based lesion symptom mapping in aphasia. J. Cogn. Neurosci. 2012, 24, 212–222. [Google Scholar] [CrossRef]
- Just, M.A.; Carpenter, P.A.; Keller, T.A.; Eddy, W.F.; Thulborn, K.R. Brain activation modulated by sentence comprehension. Science 1996, 274, 114–116. [Google Scholar] [CrossRef]
- Caplan, D.; Chen, E.; Waters, G. Task-dependent and task-independent neurovascular responses to syntactic processing. Cortex 2008, 44, 257–275. [Google Scholar] [CrossRef]
- Meltzer, J.A.; McArdle, J.J.; Schafer, R.J.; Braun, A.R. Neural aspects of sentence comprehension: Syntactic complexity, reversibility, and reanalysis. Cereb. Cortex 2010, 20, 1853–1864. [Google Scholar] [CrossRef]
- Bornkessel-Schlesewsky, I.; Schlesewsky, M.; von Cramon, D.Y. Word order and Broca’s region: Evidence for a supra-syntactic perspective. Brain Lang. 2009, 111, 125–139. [Google Scholar] [CrossRef]
- Bornkessel-Schlesewsky, I.; Grewe, T.; Schlesewsky, M. Prominence vs. aboutness in sequencing: A functional distinction within the left inferior frontal gyrus. Brain Lang. 2012, 120, 96–107. [Google Scholar]
- Bornkessel-Schlesewsky, I.; Schlesewsky, M. The role of prominence information in the real-time comprehension of transitive constructions: A cross-linguistic approach. Lang. Linguist. Compass 2009, 3, 19–58. [Google Scholar] [CrossRef]
- Grewe, T.; Bornkessel, I.; Zysset, S.; Wiese, R.; von Cramon, D.Y.; Schlesewsky, M. The emergence of the unmarked: A new perspective on the language-specific function of Broca’s area. Hum. Brain Mapp. 2005, 26, 178–190. [Google Scholar] [CrossRef]
- Grewe, T.; Bornkessel, I.; Zysset, S.; Wiese, R.; von Cramon, D.Y.; Schlesewsky, M. Linguistic prominence and Broca’s area: The influence of animacy as a linearization principle. Neuroimage 2006, 32, 1395–1402. [Google Scholar] [CrossRef]
- Fiebach, C.J.; Schlesewsky, M.; Lohmann, G.; von Cramon, D.Y.; Friederici, A.D. Revisiting the role of Broca’s area in sentence processing: Syntactic integration versus syntactic working memory. Hum. Brain Mapp. 2005, 24, 79–91. [Google Scholar] [CrossRef]
- Christensen, K.R. Interfaces, syntactic movement, and neural activation: A new perspective on the implementation of language in the brain. J. Neurolinguist. 2008, 21, 71–103. [Google Scholar] [CrossRef]
- Moro, A.; Tettamanti, M.; Perani, D.; Donati, C.; Cappa, S.F.; Fazio, F. Syntax and the brain: Disentangling grammar by selective anomalies. Neuroimage 2001, 13, 110–118. [Google Scholar]
- Tettamanti, M.; Rotondi, I.; Perani, D.; Scotti, G.; Fazio, F.; Cappa, S.F.; Moro, A. Syntax without language: Neurobiological evidence for cross-domain syntactic computations. Cortex 2009, 45, 825–838. [Google Scholar] [CrossRef]
- Davis, S.W.; Kragel, J.E.; Madden, D.J.; Cabeza, R. The architecture of cross-hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb. Cortex 2012, 22, 232–242. [Google Scholar] [CrossRef]
- Park, D.; Reuter-Lorenz, P. The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef]
- Tyler, L.K.; Shafto, M.A.; Randall, B.; Wright, P.; Marslen-Wilson, W.D.; Stamatakis, E.A. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cereb. Cortex 2010, 20, 352–364. [Google Scholar] [CrossRef]
- Caplan, D. Task effects on BOLD signal correlates of implicit syntactic processing. Lang. Cogn. Process. 2010, 25, 866–901. [Google Scholar] [CrossRef]
- Cooke, A.; Zurif, E.B.; DeVita, C.; Alsop, D.; Koenig, P.; Detre, J.; Gee, J.; Pinãngo, M.; Balogh, J.; Grossman, M. Neural basis for sentence comprehension: Grammatical and short-term memory components. Hum. Brain Mapp. 2002, 15, 80–94. [Google Scholar] [CrossRef]
- Davies, M. The Corpus of Contemporary American English, 2008. Available online: http://corpus.byu.edu/coca/ (accessed on 1 August 2011).
- Jaeger, T.F. Categorical data analysis: Away from ANOVAs (transformation or not) and towards logit mixed models. J. Mem. Lang. 2008, 59, 434–446. [Google Scholar] [CrossRef]
- Baayen, R.H.; Davidson, D.J.; Bates, D.M. Mixed-effects modeling with crossed random effects for subjects and items. J. Mem. Lang. 2008, 49, 390–412. [Google Scholar] [CrossRef]
- Thompson, C.K.; Kielar, A. Neural bases of sentence processing: Evidence from neurolinguistic and neuroimaging studies. In The Oxford Handbook of Language Production; Goldrick, M., Ferreira, V., Miozzo, M., Eds.; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mack, J.E.; Meltzer-Asscher, A.; Barbieri, E.; Thompson, C.K. Neural Correlates of Processing Passive Sentences. Brain Sci. 2013, 3, 1198-1214. https://doi.org/10.3390/brainsci3031198
Mack JE, Meltzer-Asscher A, Barbieri E, Thompson CK. Neural Correlates of Processing Passive Sentences. Brain Sciences. 2013; 3(3):1198-1214. https://doi.org/10.3390/brainsci3031198
Chicago/Turabian StyleMack, Jennifer E., Aya Meltzer-Asscher, Elena Barbieri, and Cynthia K. Thompson. 2013. "Neural Correlates of Processing Passive Sentences" Brain Sciences 3, no. 3: 1198-1214. https://doi.org/10.3390/brainsci3031198




