Repair of the Peripheral Nerve—Remyelination that Works
Abstract
:1. Introduction
2. Initial Reaction to Injury at the Site of Lesion and in the Distal Nerve Segment
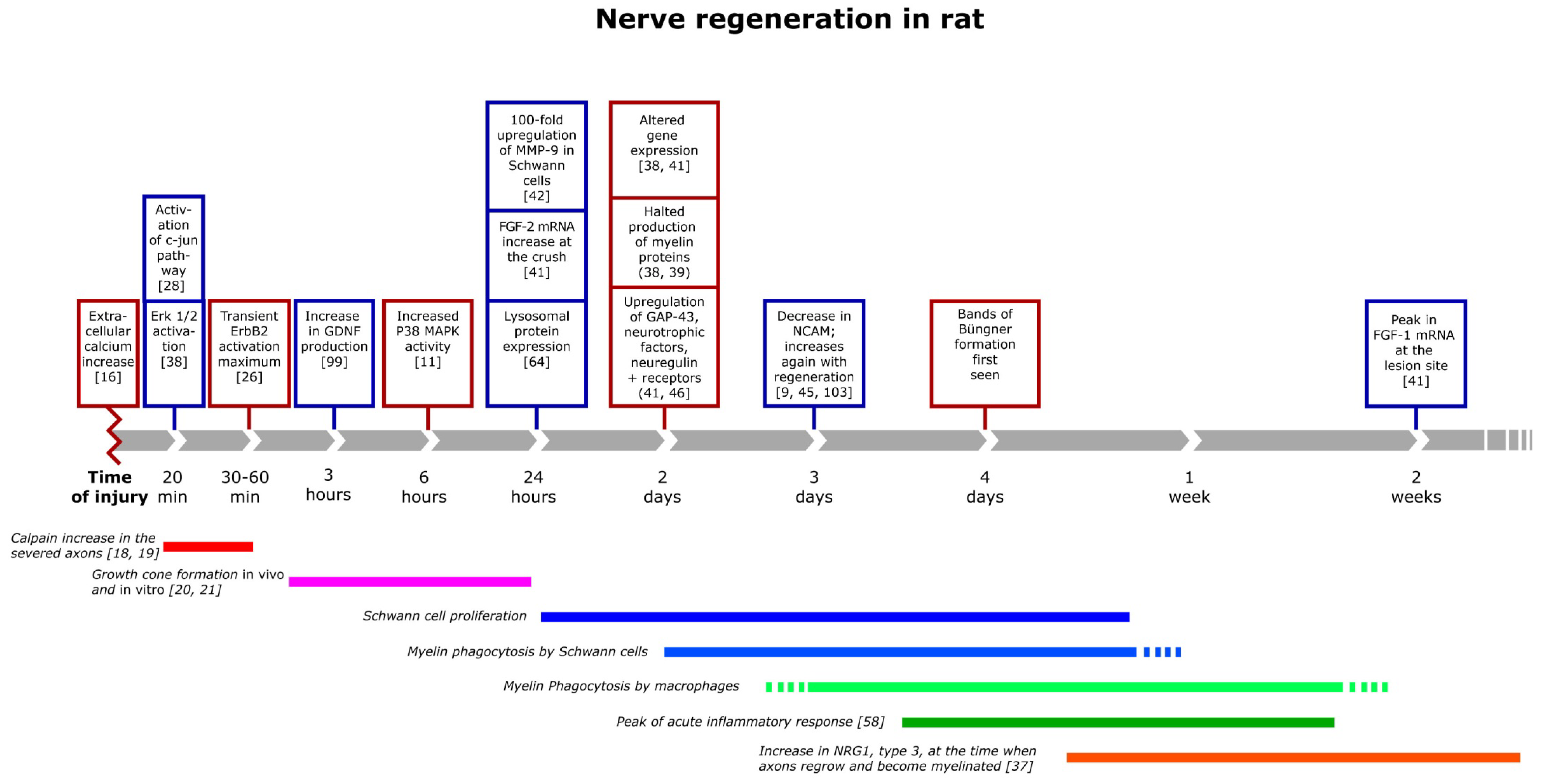
3. The Inflammatory Response and Degradation of Myelin
4. Regeneration and the Relation between the Axon and the Schwann Cells
5. Remyelination of Axons during and after Regeneration
6. Target Innervation
7. Conclusions
Acknowledgements
Dedication
Conflict of Interest
References
- Chemnitz, A.; Andersson, G.; Rosen, B.; Dahlin, L.B.; Bjorkman, A. Poor electroneurography but excellent hand function 31 years after nerve repair in childhood. Neuroreport 2013, 24, 6–9. [Google Scholar] [CrossRef]
- Taylor, K.S.; Anastakis, D.J.; Davis, K.D. Cutting your nerve changes your brain. Brain 2009, 132, 3122–3133. [Google Scholar] [CrossRef]
- Saito, H.; Kanje, M.; Dahlin, L.B. Crossed over repair of the femoral sensory and motor branches influences N-CAM. Neuroreport 2010, 21, 841–845. [Google Scholar] [CrossRef]
- Widerberg, A.; Bergman, S.; Danielsen, N.; Lundborg, G.; Dahlin, L.B. Nerve injury induced by vibration: Prevention of the effect of a conditioning lesion by D600, a Ca2+ channel blocker. Occup. Environ. Med. 1997, 54, 312–315. [Google Scholar] [CrossRef]
- Bruyns, C.N.; Jaquet, J.B.; Schreuders, T.A.; Kalmijn, S.; Kuypers, P.D.; Hovius, S.E. Predictors for return to work in patients with median and ulnar nerve injuries. J. Hand Surg. Am. 2003, 28, 28–34. [Google Scholar] [CrossRef]
- Rosen, B.; Chemnitz, A.; Weibull, A.; Andersson, G.; Dahlin, L.B.; Bjorkman, A. Cerebral changes after injury to the median nerve: A long-term follow up. J. Plast. Surg. Hand Surg. 2012, 46, 106–112. [Google Scholar] [CrossRef]
- Rosberg, H.E.; Carlsson, K.S.; Hojgard, S.; Lindgren, B.; Lundborg, G.; Dahlin, L.B. Injury to the human median and ulnar nerves in the forearm—analysis of costs for treatment and rehabilitation of 69 patients in southern sweden. J. Hand Surg. Br. 2005, 30, 35–39. [Google Scholar]
- Davis, K.D.; Taylor, K.S.; Anastakis, D.J. Nerve injury triggers changes in the brain. Neuroscientist 2011, 17, 407–422. [Google Scholar] [CrossRef]
- Saito, H.; Dahlin, L.B. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008, 9, 88. [Google Scholar] [CrossRef]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.; Joosten, E.A.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Yang, D.P.; Zhang, D.P.; Mak, K.S.; Bonder, D.E.; Pomeroy, S.L.; Kim, H.A. Schwann cell proliferation during wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: Axon-dependent removal of newly generated Schwann cells by apoptosis. Mol. Cell. Neurosci. 2008, 38, 80–88. [Google Scholar] [CrossRef]
- Lucas, J.H.; Gross, G.W.; Emery, D.G.; Gardner, C.R. Neuronal survival or death after dendrite transection close to the perikaryon: Correlation with electrophysiologic, morphologic, and ultrastructural changes. Cent. Nerv. Syst. Trauma 1985, 2, 231–255. [Google Scholar]
- Shi, R.Y.; Lucas, J.H.; Wolf, A.; Gross, G.W. Calcium antagonists fail to protect mammalian spinal neurons after physical injury. J. Neurotrauma 1989, 6, 261–276; discussion 277–278. [Google Scholar]
- Strautman, A.F.; Cork, R.J.; Robinson, K.R. The distribution of free calcium in transected spinal axons and its modulation by applied electrical fields. J. Neurosci. 1990, 10, 3564–3575. [Google Scholar]
- Rehncrona, S.; Mela, L.; Siesjo, B.K. Recovery of brain mitochondrial function in the rat after complete and incomplete cerebral ischemia. Stroke 1979, 10, 437–446. [Google Scholar] [CrossRef]
- Smith, K.J.; Hall, S.M. Peripheral demyelination and remyelination initiated by the calcium-selective ionophore ionomycin: In vivo observations. J. Neurol. Sci. 1988, 83, 37–53. [Google Scholar] [CrossRef]
- Fex Svenningsen, Å.; Kanje, M. Regulation of schwann cell proliferation in cultured segments of the adult rat sciatic nerve. J. Neurosci. Res. 1998, 52, 530–537. [Google Scholar] [CrossRef]
- Touma, E.; Kato, S.; Fukui, K.; Koike, T. Calpain-mediated cleavage of collapsin response mediator protein(CRMP)-2 during neurite degeneration in mice. Eur. J. Neurosci. 2007, 26, 3368–3381. [Google Scholar] [CrossRef]
- Wang, M.S.; Davis, A.A.; Culver, D.G.; Wang, Q.; Powers, J.C.; Glass, J.D. Calpain inhibition protects against taxol-induced sensory neuropathy. Brain 2004, 127, 671–679. [Google Scholar]
- Chierzi, S.; Ratto, G.M.; Verma, P.; Fawcett, J.W. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur. J. Neurosci. 2005, 21, 2051–2062. [Google Scholar] [CrossRef]
- Bradke, F.; Fawcett, J.W.; Spira, M.E. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat. Rev. Neurosci. 2012, 13, 183–193. [Google Scholar]
- Martensson, L.; Gustavsson, P.; Dahlin, L.B.; Kanje, M. Activation of extracellular-signal-regulated kinase-1/2 precedes and is required for injury-induced Schwann cell proliferation. Neuroreport 2007, 18, 957–961. [Google Scholar] [CrossRef]
- Tan, Y.; Rouse, J.; Zhang, A.; Cariati, S.; Cohen, P.; Comb, M.J. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 map kinase and mapkap kinase-2. EMBO J. 1996, 15, 4629–4642. [Google Scholar]
- Sun, C.; Qi, R.; Wang, L.; Yan, J.; Wang, Y. P38 MAPK regulates calcium signal-mediated lipid accumulation through changing VDR expression in primary preadipocytes of mice. Mol. Biol. Rep. 2012, 39, 3179–3184. [Google Scholar] [CrossRef]
- Liang, H.L.; Dhar, S.S.; Wong-Riley, M.T. P38 mitogen-activated protein kinase and calcium channels mediate signaling in depolarization-induced activation of peroxisome proliferator-activated receptor gamma coactivator-1alpha in neurons. J. Neurosci. Res. 2012, 88, 640–649. [Google Scholar]
- Yang, D.P.; Kim, J.; Syed, N.; Tung, Y.J.; Bhaskaran, A.; Mindos, T.; Mirsky, R.; Jessen, K.R.; Maurel, P.; Parkinson, D.B.; et al. P38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J. Neurosci. 2012, 32, 7158–7168. [Google Scholar] [CrossRef]
- Kim, H.A.; Pomeroy, S.L.; Whoriskey, W.; Pawlitzky, I.; Benowitz, L.I.; Sicinski, P.; Stiles, C.D.; Roberts, T.M. A developmentally regulated switch directs regenerative growth of Schwann cells through cyclin d1. Neuron 2000, 26, 405–416. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Bhaskaran, A.; Droggiti, A.; Dickinson, S.; D’Antonio, M.; Mirsky, R.; Jessen, K.R. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell. Biol. 2004, 164, 385–394. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. C-jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef]
- Fontana, X.; Hristova, M.; Da Costa, C.; Patodia, S.; Thei, L.; Makwana, M.; Spencer-Dene, B.; Latouche, M.; Mirsky, R.; Jessen, K.R.; et al. C-jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J. Cell Biol. 2012, 198, 127–141. [Google Scholar] [CrossRef]
- Saito, H.; Kanje, M.; Dahlin, L.B. Delayed nerve repair increases number of caspase 3 stained Schwann cells. Neurosci. Lett. 2009, 456, 30–33. [Google Scholar] [CrossRef]
- Michailov, G.V.; Sereda, M.W.; Brinkmann, B.G.; Fischer, T.M.; Haug, B.; Birchmeier, C.; Role, L.; Lai, C.; Schwab, M.H.; Nave, K.A. Axonal neuregulin-1 regulates myelin sheath thickness. Science 2004, 304, 700–703. [Google Scholar] [CrossRef]
- Taveggia, C.; Zanazzi, G.; Petrylak, A.; Yano, H.; Rosenbluth, J.; Einheber, S.; Xu, X.; Esper, R.M.; Loeb, J.A.; Shrager, P.; et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 2005, 47, 681–694. [Google Scholar] [CrossRef]
- Nave, K.A.; Salzer, J.L. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006, 16, 492–500. [Google Scholar] [CrossRef]
- Birchmeier, C.; Nave, K.A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 2008, 56, 1491–1497. [Google Scholar] [CrossRef]
- Syed, N.; Kim, H.A. Soluble neuregulin and Schwann cell myelination: A therapeutic potential for improving remyelination of adult axons. Mol. Cell. Pharmacol. 2010, 2, 161–167. [Google Scholar]
- Stassart, R.M.; Fledrich, R.; Velanac, V.; Brinkmann, B.G.; Schwab, M.H.; Meijer, D.; Sereda, M.W.; Nave, K.A. A role for Schwann cell-derived neuregulin-1 in remyelination. Nat. Neurosci. 2013, 16, 48–54. [Google Scholar]
- Guertin, A.D.; Zhang, D.P.; Mak, K.S.; Alberta, J.A.; Kim, H.A. Microanatomy of axon/glial signaling during wallerian degeneration. J. Neurosci. 2005, 25, 3478–3487. [Google Scholar] [CrossRef]
- Zanazzi, G.; Einheber, S.; Westreich, R.; Hannocks, M.J.; Bedell-Hogan, D.; Marchionni, M.A.; Salzer, J.L. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J. Cell Biol. 2001, 152, 1289–1299. [Google Scholar] [CrossRef]
- Heermann, S.; Schwab, M.H. Molecular control of Schwann cell migration along peripheral axons: Keep moving! Cell Adh. Migr. 2013, 7, 18–22. [Google Scholar] [CrossRef]
- Meisinger, C.; Grothe, C. Differential regulation of fibroblast growth factor (FGF)-2 and FGF receptor 1 mRNAs and FGF-2 isoforms in spinal ganglia and sciatic nerve after peripheral nerve lesion. J. Neurochem. 1997, 68, 1150–1158. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Angert, M.; Dolkas, J.; Campana, W.M.; Palenscar, K.; Myers, R.R. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol. Cell. Neurosci. 2006, 31, 407–415. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Shubayev, V.I. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ERBB receptor-mediated activation of MEK/ERK pathway. Glia 2009, 57, 1316–1325. [Google Scholar] [CrossRef]
- Pellegrino, R.G.; Politis, M.J.; Ritchie, J.M.; Spencer, P.S. Events in degenerating cat peripheral nerve: Induction of Schwann cell s phase and its relation to nerve fibre degeneration. J. Neurocytol. 1986, 15, 17–28. [Google Scholar] [CrossRef]
- Clemence, A.; Mirsky, R.; Jessen, K.R. Non-myelin-forming cells proliferate rapidly during wallerian degeneration in the rat sciatic nerve. J. Neurocytol. 1989, 18, 185–192. [Google Scholar] [CrossRef]
- Sjöberg, J. Regeneration of the rat sciatic nerve: The role of non-neuronal cells and insulin-like growth factor I. Doctorial Thesis, Lund University, Lund, Sweden, 1990. [Google Scholar]
- Kleitman, N.; Simon, D.K.; Schachner, M.; Bunge, R.P. Growth of embryonic retinal neurites elicited by contact with Schwann cell surfaces is bloced by antibodies to L1. Exp. Neurol. 1988, 102, 298–306. [Google Scholar] [CrossRef]
- Banner, L.R.; Patterson, P.H. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc. Natl. Acad. Sci. USA 1994, 91, 7109–7113. [Google Scholar] [CrossRef]
- Martini, R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J. Neurocytol. 1994, 23, 1–28. [Google Scholar] [CrossRef]
- Bolin, L.M.; Verity, A.N.; Silver, J.E.; Shooter, E.M.; Abrams, J.S. Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J. Neurochem. 1995, 64, 850–858. [Google Scholar]
- Araki, T.; Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 1996, 17, 353–361. [Google Scholar] [CrossRef]
- Toews, A.D.; Barrett, C.; Morell, P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J. Neurosci. Res. 1998, 53, 260–267. [Google Scholar] [CrossRef]
- Tofaris, G.K.; Patterson, P.H.; Jessen, K.R.; Mirsky, R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 2002, 22, 6696–6703. [Google Scholar]
- Scherer, S.S.; Salzer, J.L. Axon-Schwann cell interactions during peripheral nerve degeneration and regeneration. In Glial Cell Development, 2nd ed.; Richardson, W.D., Jessen, K.R., Eds.; Oxford University Press: London, UK, 2001; pp. 299–330. [Google Scholar]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 2011, 8, 110. [Google Scholar] [CrossRef]
- Weerasuriya, A.; Hockman, C.H. Perineurial permeability to sodium during wallerian degeneration in rat sciatic nerve. Brain Res. 1992, 581, 327–333. [Google Scholar] [CrossRef]
- Mizisin, A.P.; Weerasuriya, A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol. 2011, 121, 291–312. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; da Costa, C.; Miethke, T.; Kirschning, C.J.; Hacker, H.; Wagner, H. Endocytosed hsp60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001, 276, 31332–31339. [Google Scholar]
- Kariko, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA is an endogenous ligand for toll-like receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar]
- Brunn, G.J.; Bungum, M.K.; Johnson, G.B.; Platt, J.L. Conditional signaling by toll-like receptor 4. FASEB J. 2005, 19, 872–874. [Google Scholar]
- Goethals, S.; Ydens, E.; Timmerman, V.; Janssens, S. Toll-like receptor expression in the peripheral nerve. Glia 2010, 58, 1701–1709. [Google Scholar] [CrossRef]
- Lee, H.; Jo, E.K.; Choi, S.Y.; Oh, S.B.; Park, K.; Kim, J.S.; Lee, S.J. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: Implication in wallerian degeneration. Biochem. Biophys. Res. Commun. 2006, 350, 742–747. [Google Scholar] [CrossRef]
- Karanth, S.; Yang, G.; Yeh, J.; Richardson, P.M. Nature of signals that initiate the immune response during wallerian degeneration of peripheral nerves. Exp. Neurol. 2006, 202, 161–166. [Google Scholar] [CrossRef]
- Boivin, A.; Pineau, I.; Barrette, B.; Filali, M.; Vallieres, N.; Rivest, S.; Lacroix, S. Toll-like receptor signaling is critical for wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci. 2007, 27, 12565–12576. [Google Scholar] [CrossRef]
- Fernandez-Valle, C.; Bunge, R.P.; Bunge, M.B. Schwann cells degrade myelin and proliferate in the absence of macrophages: Evidence from in vitro studies of wallerian degeneration. J. Neurocytol. 1995, 24, 667–679. [Google Scholar] [CrossRef]
- Band, H.; Bhattacharya, A.; Talwar, G.P. Mechanism of phagocytosis by Schwann cells. J. Neurol. Sci. 1986, 75, 113–119. [Google Scholar] [CrossRef]
- Hirata, K.; Kawabuchi, M. Myelin phagocytosis by macrophages and nonmacrophages during wallerian degeneration. Microsc. Res. Tech. 2002, 57, 541–547. [Google Scholar] [CrossRef]
- Kurek, J.B.; Austin, L.; Cheema, S.S.; Bartlett, P.F.; Murphy, M. Up-regulation of leukaemia inhibitory factor and interleukin-6 in transected sciatic nerve and muscle following denervation. Neuromuscul. Disord. 1996, 6, 105–114. [Google Scholar] [CrossRef]
- Bourde, O.; Kiefer, R.; Toyka, K.V.; Hartung, H.P. Quantification of interleukin-6 mRNA in wallerian degeneration by competitive reverse transcription polymerase chain reaction. J. Neuroimmunol. 1996, 69, 135–140. [Google Scholar]
- Shamash, S.; Reichert, F.; Rotshenker, S. The cytokine network of wallerian degeneration: Tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. J. Neurosci. 2002, 22, 3052–3060. [Google Scholar]
- Murphy, P.G.; Grondin, J.; Altares, M.; Richardson, P.M. Induction of interleukin-6 in axotomized sensory neurons. J. Neurosci. 1995, 15, 5130–5138. [Google Scholar]
- Verge, V.M.; Gratto, K.A.; Karchewski, L.A.; Richardson, P.M. Neurotrophins and nerve injury in the adult. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 423–430. [Google Scholar] [CrossRef]
- Lindholm, D.; Heumann, R.; Meyer, M.; Thoenen, H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 1987, 330, 658–659. [Google Scholar] [CrossRef]
- Martini, R.; Fischer, S.; Lopez-Vales, R.; David, S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 2008, 56, 1566–1577. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Bitsch, A.; Stadelmann, C.; Siebert, H.; Bruck, W. Macrophages are eliminated from the injured peripheral nerve via local apoptosis and circulation to regional lymph nodes and the spleen. J. Neurosci. 2001, 21, 3401–3408. [Google Scholar]
- Moalem, G.; Xu, K.; Yu, L. T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 2004, 129, 767–777. [Google Scholar] [CrossRef]
- London, C.A.; Abbas, A.K.; Kelso, A. Helper T cell subsets: Heterogeneity, functions and development. Vet. Immunol. Immunopathol. 1998, 63, 37–44. [Google Scholar] [CrossRef]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A central role for the erk-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Chang, H.M.; Shyu, M.K.; Tseng, G.F.; Liu, C.H.; Chang, H.S.; Lan, C.T.; Hsu, W.M.; Liao, W.C. Neuregulin facilitates nerve regeneration by speeding Schwann cell migration via ERBB2/3-dependent fak pathway. PLoS One 2013, 8, e53444. [Google Scholar]
- Kvist, M.; Sondell, M.; Kanje, M.; Dahlin, L.B. Regeneration in, and properties of, extracted peripheral nerve allografts and xenografts. J. Plast. Surg. Hand Surg. 2011, 45, 122–128. [Google Scholar] [CrossRef]
- Stirling, D.P.; Stys, P.K. Mechanisms of axonal injury: Internodal nanocomplexes and calcium deregulation. Trends Mol. Med. 2010, 16, 160–170. [Google Scholar] [CrossRef]
- Mudo, G.; Persson, H.; Timmusk, T.; Funakoshi, H.; Bindoni, M.; Belluardo, N. Increased expression of trkB and trkC messenger RNAs in the rat forebrain after focal mechanical injury. Neuroscience 1993, 57, 901–912. [Google Scholar] [CrossRef]
- Taniuchi, M.; Clark, H.B.; Johnson, E.M., Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc. Natl. Acad. Sci. USA 1986, 83, 4094–4098. [Google Scholar] [CrossRef]
- Taniuchi, M.; Clark, H.B.; Schweitzer, J.B.; Johnson, E.M., Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: Ultrastructural location, suppression by axonal contact, and binding properties. J. Neurosci. 1988, 8, 664–681. [Google Scholar]
- Raivich, G.; Hellweg, R.; Kreutzberg, G.W. NGF receptor-mediated reduction in axonal NGF uptake and retrograde transport following sciatic nerve injury and during regeneration. Neuron 1991, 7, 151–164. [Google Scholar] [CrossRef]
- Rende, M.; Muir, D.; Ruoslahti, E.; Hagg, T.; Varon, S.; Manthorpe, M. Immunolocalization of ciliary neuronotrophic factor in adult rat sciatic nerve. Glia 1992, 5, 25–32. [Google Scholar] [CrossRef]
- Meyer, M.; Matsuoka, I.; Wetmore, C.; Olson, L.; Thoenen, H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: Different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J. Cell Biol. 1992, 119, 45–54. [Google Scholar] [CrossRef]
- Springer, J.E.; Mu, X.; Bergmann, L.W.; Trojanowski, J.Q. Expression of GDNF mRNA in rat and human nervous tissue. Exp. Neurol. 1994, 127, 167–170. [Google Scholar] [CrossRef]
- Kanje, M.; Skottner, A.; Sjoberg, J.; Lundborg, G. Insulin-like growth factor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 1989, 486, 396–398. [Google Scholar] [CrossRef]
- Fernyhough, P.; Willars, G.B.; Lindsay, R.M.; Tomlinson, D.R. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993, 607, 117–124. [Google Scholar] [CrossRef]
- Zhuang, H.X.; Snyder, C.K.; Pu, S.F.; Ishii, D.N. Insulin-like growth factors reverse or arrest diabetic neuropathy: Effects on hyperalgesia and impaired nerve regeneration in rats. Exp. Neurol. 1996, 140, 198–205. [Google Scholar] [CrossRef]
- Newman, J.P.; Verity, A.N.; Hawatmeh, S.; Fee, W.E., Jr.; Terris, D.J. Ciliary neurotrophic factors enhances peripheral nerve regeneration. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 399–403. [Google Scholar] [CrossRef]
- Fex Svenningsen, A.; Kanje, M. Insulin and the insulin-like growth factors I and II are mitogenic to cultured rat sciatic nerve segments and stimulate [3H]thymidine incorporation through their respective receptors. Glia 1996, 18, 68–72. [Google Scholar] [CrossRef]
- Wilhelm, J.C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, K.S.; Bassell, G.J.; English, A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp. Neurol. 2003, 183, 610–619. [Google Scholar] [CrossRef]
- Audisio, C.; Mantovani, C.; Raimondo, S.; Geuna, S.; Perroteau, I.; Terenghi, G. Neuregulin1 administration increases axonal elongation in dissociated primary sensory neuron cultures. Exp. Cell Res. 2012, 318, 570–577. [Google Scholar] [CrossRef]
- Xu, P.; Rosen, K.M.; Hedstrom, K.; Rey, O.; Guha, S.; Hart, C.; Corfas, G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in Schwann cells through purinergic signaling and the PKC-PKD pathway. Glia 2013, 6, 1029–1040. [Google Scholar]
- Thornton, M.R.; Shawcross, S.G.; Mantovani, C.; Kingham, P.J.; Birchall, M.A.; Terenghi, G. Neurotrophins 3 and 4 differentially regulate NCAM, L1 and N-cadherin expression during peripheral nerve regeneration. Biotechnol. Appl. Biochem. 2008, 49, 165–174. [Google Scholar] [CrossRef]
- Corell, M.; Wicher, G.; Limbach, C.; Kilimann, M.W.; Colman, D.R.; Fex Svenningsen, A. Spatiotemporal distribution and function of N-cadherin in postnatal Schwann cells: A matter of adhesion? J. Neurosci. Res. 2010, 88, 2338–2349. [Google Scholar]
- Anderson, A.A.; Kendal, C.E.; Garcia-Maya, M.; Kenny, A.V.; Morris-Triggs, S.A.; Wu, T.; Reynolds, R.; Hohenester, E.; Saffell, J.L. A peptide from the first fibronectin domain of NCAM acts as an inverse agonist and stimulates FGF receptor activation, neurite outgrowth and survival. J. Neurochem. 2005, 95, 570–583. [Google Scholar] [CrossRef]
- Sherman, D.L.; Brophy, P.J. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 2005, 6, 683–690. [Google Scholar] [CrossRef]
- Bermingham-McDonogh, O.; Xu, Y.T.; Marchionni, M.A.; Scherer, S.S. Neuregulin expression inPNS neurons: Isoforms and regulation by target interactions. Mol. Cell. Neurosci. 1997, 10, 184–195. [Google Scholar] [CrossRef]
- Carroll, S.L.; Miller, M.L.; Frohnert, P.W.; Kim, S.S.; Corbett, J.A. Expression of neuregulins and their putative receptors, ERBB2 and ERBB3, is induced during wallerian degeneration. J. Neurosci. 1997, 17, 1642–1659. [Google Scholar]
- Fricker, F.R.; Bennett, D.L. The role of neuregulin-1 in the response to nerve injury. Future Neurol. 2011, 6, 809–822. [Google Scholar] [CrossRef]
- Oka, N.; Kawasaki, T.; Matsui, M.; Tachibana, H.; Sugita, M.; Akiguchi, I. Neuregulin is associated with nerve regeneration in axonal neuropathies. Neuroreport 2000, 11, 3673–3676. [Google Scholar] [CrossRef]
- Syed, N.; Reddy, K.; Yang, D.P.; Taveggia, C.; Salzer, J.L.; Maurel, P.; Kim, H.A. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 2010, 30, 6122–6131. [Google Scholar] [CrossRef]
- Hansson, H.A.; Rozell, B.; Skottner, A. Rapid axoplasmic transport of insulin-like growth factor I in the sciatic nerve of adult rats. Cell Tissue Res. 1987, 247, 241–247. [Google Scholar]
- Cheng, H.L.; Randolph, A.; Yee, D.; Delafontaine, P.; Tennekoon, G.; Feldman, E.L. Characterization of insulin-like growth factor-I and its receptor and binding proteins in transected nerves and cultured Schwann cells. J. Neurochem. 1996, 66, 525–536. [Google Scholar]
- Syroid, D.E.; Zorick, T.S.; Arbet-Engels, C.; Kilpatrick, T.J.; Eckhart, W.; Lemke, G. A role for insulin-like growth factor-I in the regulation of Schwann cell survival. J. Neurosci. 1999, 19, 2059–2068. [Google Scholar]
- Cheng, H.L.; Russell, J.W.; Feldman, E.L. IGF-I promotes peripheral nervous system myelination. Ann. N. Y. Acad. Sci. 1999, 883, 124–130. [Google Scholar] [CrossRef]
- Kim, Y.; Remacle, A.G.; Chernov, A.V.; Liu, H.; Shubayev, I.; Lai, C.; Dolkas, J.; Shiryaev, S.A.; Golubkov, V.S.; Mizisin, A.P.; et al. The MMP-9/TIMP-1 axis controls the status of differentiation and function of myelin-forming Schwann cells in nerve regeneration. PLoS One 2012, 7, e33664. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Kohne, A.; Bernal, F.; Jangouk, P.; Meyer Zu Horste, G.; Dehmel, T.; Hartung, H.P.; Previtali, S.C.; Kieseier, B.C. Matrix metalloproteinase-2 is involved in myelination of dorsal root ganglia neurons. Glia 2009, 57, 479–489. [Google Scholar] [CrossRef]
- Massing, M.W.; Robinson, G.A.; Marx, C.E.; Alzate, O.; Madison, R.D. Applications of proteomics to nerve regeneration research. In Neuroproteomics; Alzante, O., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Weis, J.; Schroder, J.M. Differential effects of nerve, muscle, and fat tissue on regenerating nerve fibers in vivo. Muscle Nerve 1989, 12, 723–734. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Irintchev, A.; Streppel, M.; Lenzen, M.; Grosheva, M.; Wewetzer, K.; Neiss, W.F.; Angelov, D.N. Factors limiting motor recovery after facial nerve transection in the rat: Combined structural and functional analyses. Eur. J. Neurosci. 2005, 21, 391–402. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Lin, W.M.; Chiang, H.; Chang, L.Y.; Wu, C.T.; Pu, C.M.; Wu, J.T.; Hsieh, S.T. Patterns of target tissue reinnervation and trophic factor expression after nerve grafting. Plast. Reconstr. Surg. 2013, 131, 989–1000. [Google Scholar] [CrossRef]
- Campenot, R.B. Development of sympathetic neurons in compartmentalized cultures. II Local control of neurite growth by nerve growth factor. Dev. Biol. 1982, 93, 1–12. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Svennigsen, Å.F.; Dahlin, L.B. Repair of the Peripheral Nerve—Remyelination that Works. Brain Sci. 2013, 3, 1182-1197. https://doi.org/10.3390/brainsci3031182
Svennigsen ÅF, Dahlin LB. Repair of the Peripheral Nerve—Remyelination that Works. Brain Sciences. 2013; 3(3):1182-1197. https://doi.org/10.3390/brainsci3031182
Chicago/Turabian StyleSvennigsen, Åsa Fex, and Lars B. Dahlin. 2013. "Repair of the Peripheral Nerve—Remyelination that Works" Brain Sciences 3, no. 3: 1182-1197. https://doi.org/10.3390/brainsci3031182
APA StyleSvennigsen, Å. F., & Dahlin, L. B. (2013). Repair of the Peripheral Nerve—Remyelination that Works. Brain Sciences, 3(3), 1182-1197. https://doi.org/10.3390/brainsci3031182




