Magnetically Separable Base Catalysts: Heterogeneous Catalysis vs. Quasi-Homogeneous Catalysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of 1-(2-(Dimethylamino)ethyl)-3-(3-(rimethoxysilyl)propyl)urea
2.2. Synthesis of 1-Butyl-3-(3-trimethoxysilylpropyl)-1H-imidazol-3-ium chloride [34]
2.3. Procedure for Supporting 1-Butyl-3-(3-trimethoxysilylpropyl)-1H-imidazol-3-ium Chloride on Magnetite Nanoparticles [34]
2.4. General Procedure for Functionalization of Magnetite Nanoparticles with Amine Groups
2.5. General Procedure for Preparing Magnetically Separable Silica Sol-Gel Matrices Functionalized with Amine Groups
2.6. General Procedure for the Nitroaldol Reaction Catalyzed by Magnetite Nanoparticles Modified with Amine Groups
2.7. General Procedure for the Nitroaldol Reaction Catalyzed by Magnetically Separable Silica Xerogel Modified with Amine Groups
3. Results and Discussion
3.1. Preparation of Magnetically Separable Quasi-Homogeneous Base Catalysts
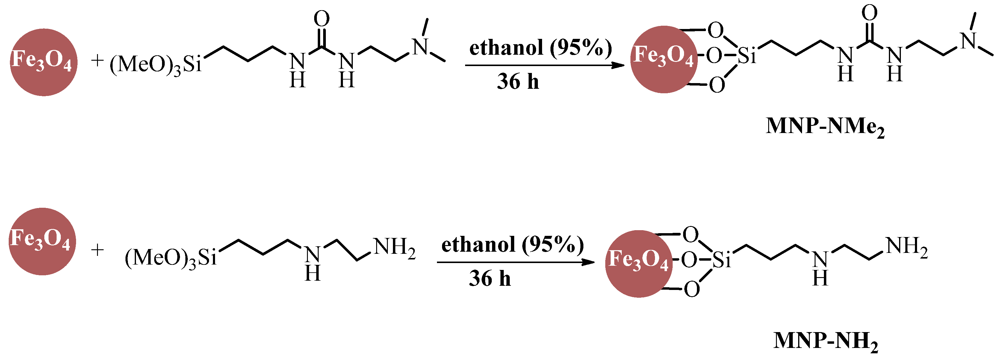
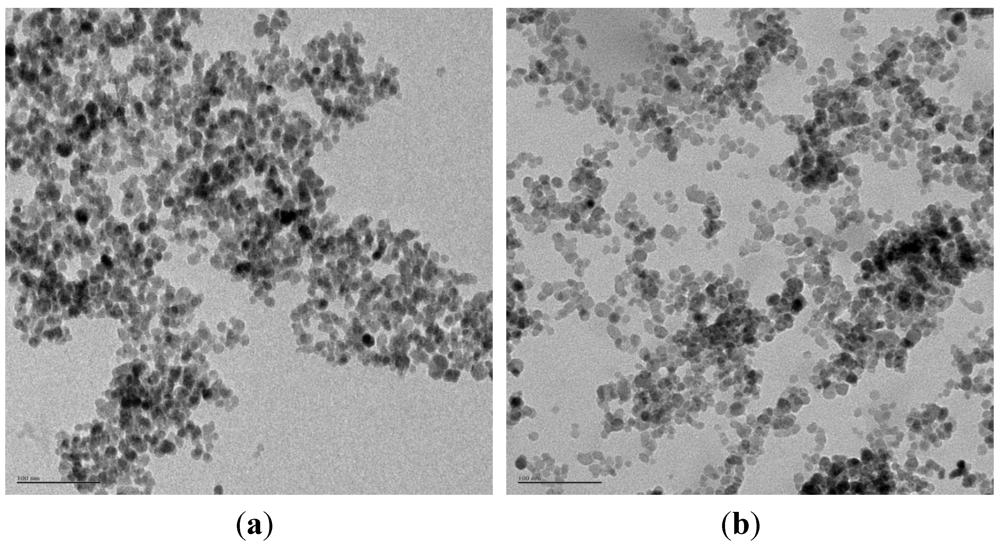
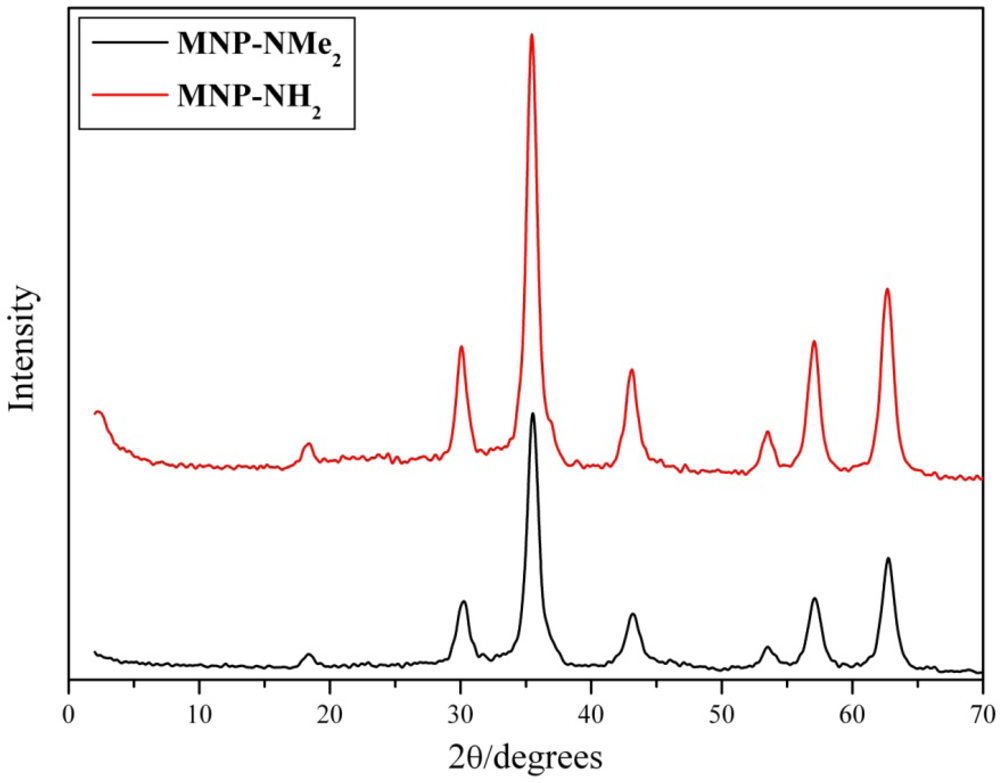
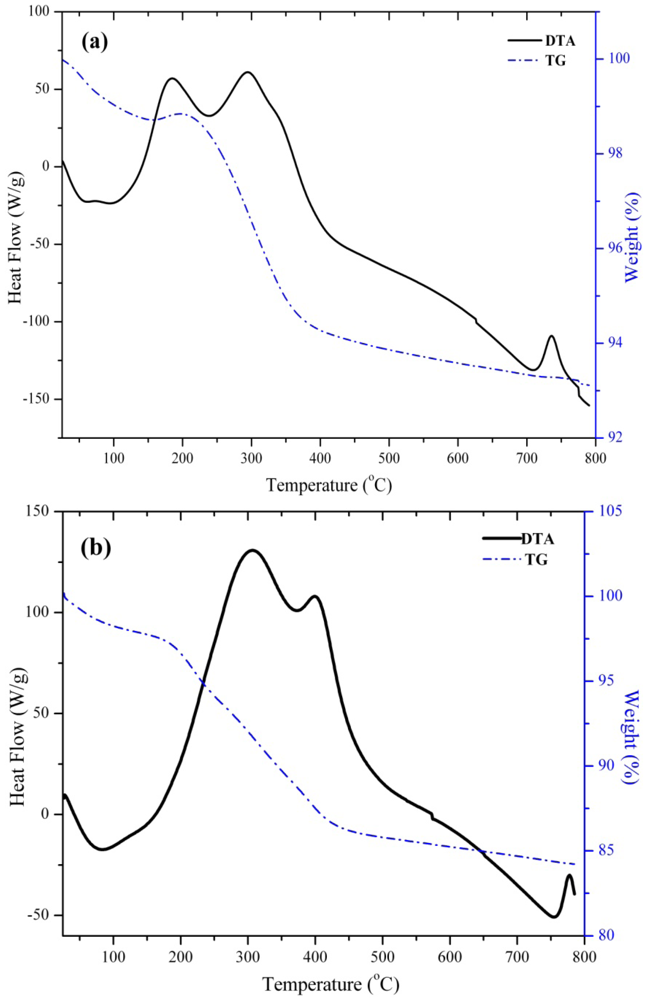
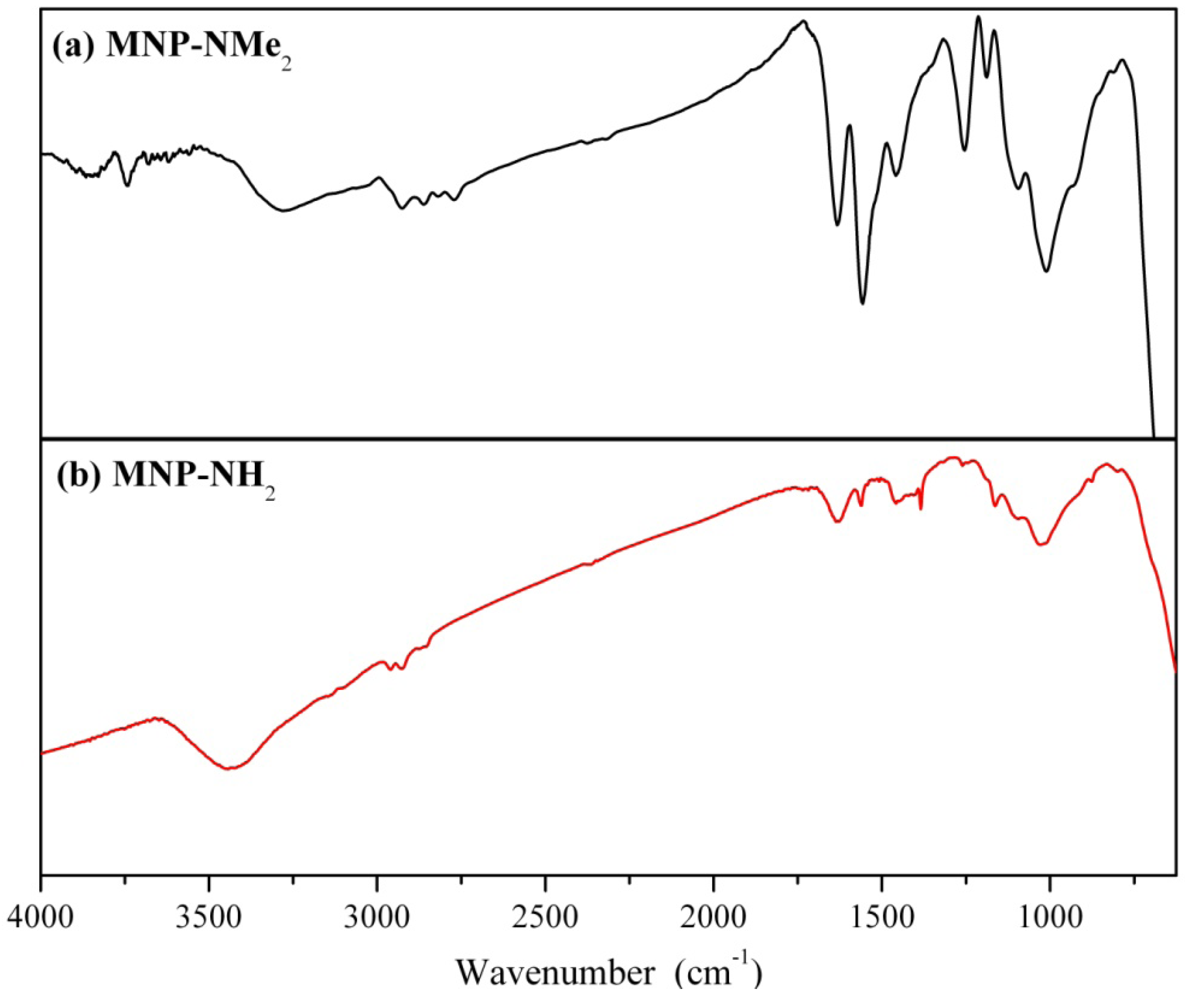
3.2. Preparation of Magnetically Separable Heterogeneous Base Catalysts
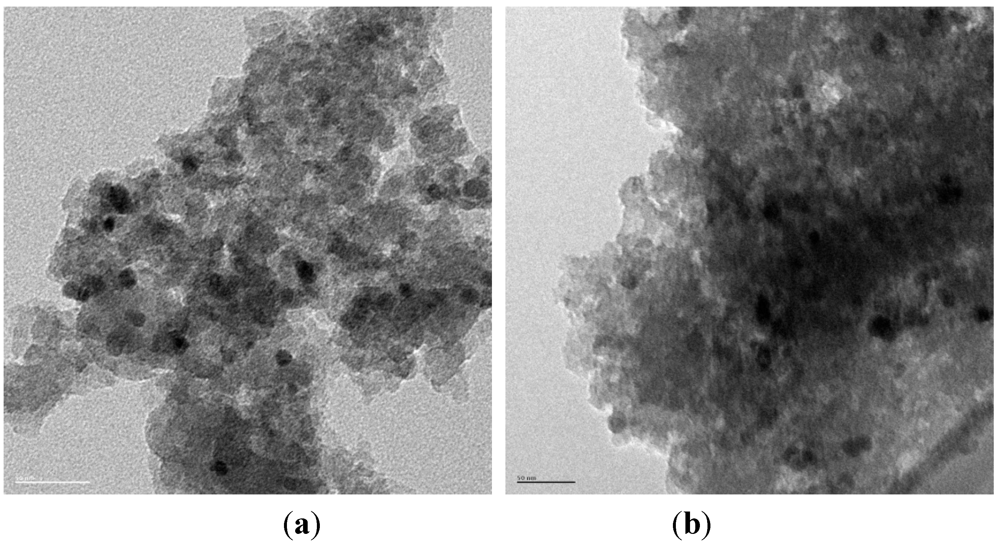
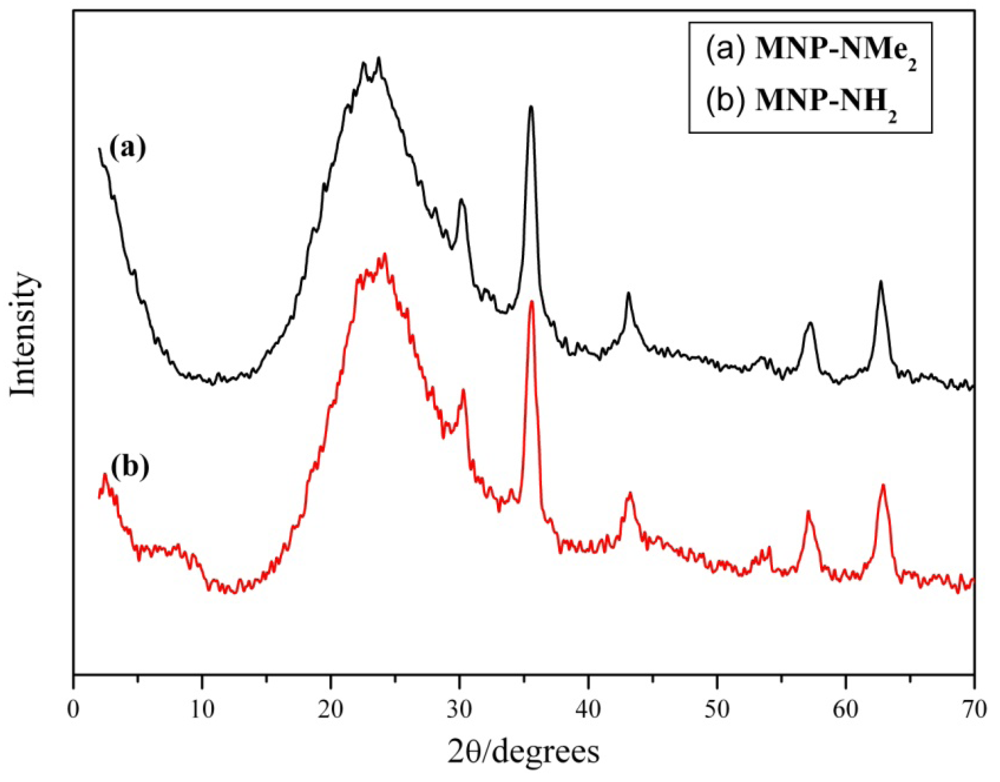
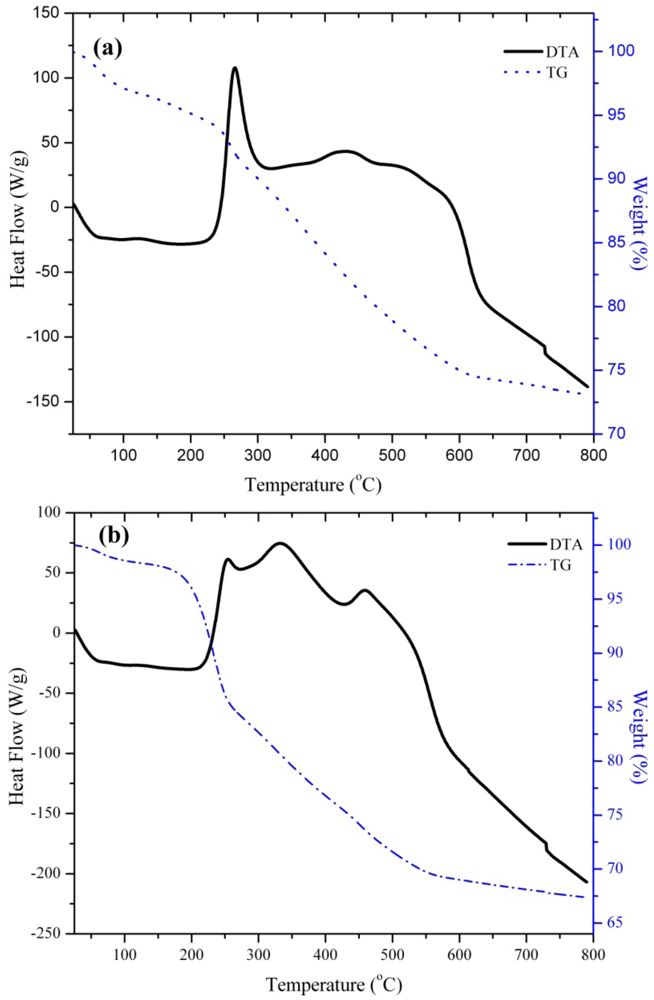
3.3. Catalytic Studies of the Quasi-Homogeneous and Heterogeneous Base Catalysts
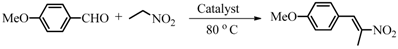
| Entry | Catalyst | Solvent | Yield (%) |
|---|---|---|---|
| 1 | SG-MNP-NH2 | acetonitrile | 47 |
| 2 | SG-MNP-NH2 | dimethylformamide | 68 |
| 3 | SG-MNP-NH2 | dioxane | 53 |
| 4 | SG-MNP-NH2 | dimethyl sulfoxide | 34 |
| 5 | SG-MNP-NH2 | ethanol (95%) | 48 |
| 6 | SG-MNP-NH2 | ethanol (dry) | 80 |
| 7 | SG-MNP-NH2 | benzene | 87 |
| 8 | SG-MNP-NH2 | heptane | 88 |
| 9 | SG-MNP-NH2 | cyclohexane | 90 |
| 10 | SG-MNP-NH2 | toluene | 85 |
| 11 | SG-MNP-NMe2 | toluene | 72b |
| 12 | SG-MNP-NMe2 | ethanol (dry) | 23b |
| 13 | SG-MNP-NMe2 | dimethyl sulfoxide | - |
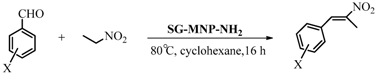
| Entry | Aromatic aldehyde | Yield (%) |
|---|---|---|
| 1 | benzaldehyde | >99 |
| 2 | 3-methoxybenzaldehyde | 95 |
| 3 | 2-methoxybenzaldehyde | 97 |
| 4 | 4-nitrobenzaldehyde | 78 |
| 5 | 3-nitrobenzaldehyde | 69 |
| 7 | 4-chlorobenzaldehyde | 99 |
| 8 | 2-chlorobenzaldehyde | 98 |
| 9 | 4-isopropylbenzaldehyde | 95 |
| 10 | 1-naphthaldehyde | 99 |
4. Conclusions
Conflict of Interest
References and Notes
- Recoverable and Recyclable Catalysts; Benaglia, M. (Ed.) John Wiley & Sons: Chichester, UK, 2009.
- Lu, Z.; Lindner, E.; Mayer, H.A. Applications of sol-gel-processed interphase catalysts. Chem. Rev. 2002, 102, 3543–3578. [Google Scholar] [CrossRef]
- Marr, A.C.; Marr, P.C. Entrapping homogeneous catalysts by sol-gel methods: The bottom-up synthesis of catalysts that recycle and cascade. Dalton Trans. 2011, 40, 20–26. [Google Scholar] [CrossRef]
- Zamboulis, A.; Moitra, N.; Moreau, J.J.E.; Cattoën, X.; Wong Chi Man, M. Hybrid materials: Versatile matrices for supporting homogeneous catalysts. J. Mater. Chem. 2010, 20, 9322–9338. [Google Scholar] [CrossRef]
- Ciriminna, R.; Demma Carà, P.; Sciortino, M.; Pagliaro, M. Catalysis with doped sol-gel silicates. Adv. Synth. Catal. 2011, 353, 677–687. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pagliaro, M. Recent uses of sol-gel doped catalysts in the fine chemicals and pharmaceutical industry. Org. Process Res. Dev. 2006, 10, 320–326. [Google Scholar]
- Schätz, A.; Reiser, O.; Stark, W.J. Nanoparticles as semi-heterogeneous catalyst supports. Chem. Eur. J. 2010, 16, 8950–8967. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S.G. Surface modified superparamagnetic nanoparticles for drug delivery: Interaction studies with human fibroblasts in culture. J. Mater. Sci. Mater. Med. 2004, 15, 493–496. [Google Scholar] [CrossRef]
- Neuberger, T.; Schoepf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Perez, J.M.; Simeone, F.J.; Saeki, Y.; Josephson, L.; Weissleder, R. Viral-induced self-assembly of magnetic nanoparticles allows the detection of viral particles in biological media. J. Am. Chem. Soc. 2003, 125, 10192–10193. [Google Scholar]
- Graham, D.L.; Ferreira, H.A.; Freitas, P.P. Magnetoresistive-based biosensors and biochips. Trends Biotechnol. 2004, 22, 455–462. [Google Scholar] [CrossRef]
- Wang, D.; He, J.; Rosenzweig, N.; Rosenzweig, Z. Nano Lett. 2004, 4, 409–413.
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar]
- Hiergeist, R.; Andra, W.; Buske, N.; Hergt, R.; Hilger, I.; Richter, U.; Kaiser, W. Application of magnetite ferrofluids for hyperthermia. J. Magn. Magn. Mater. 1999, 201, 420–422. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fahling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.M. Magnetically recoverable nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef]
- Shyles, S.; Schünemann, V.; Thiel, W.R. Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar]
- Zhu, Y.; Stubbs, L.P.; Ho, F.; Liu, R.; Ship, C.P.; Maguire, J.A.; Hosmane, N.S. Magnetic nanocomposites: A new perspective in catalysis. ChemCatChem 2010, 2, 365–374. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Kawamura, M.; Sato, K. Magnetically separable phase-transfer catalysts. Chem. Commun. 2006, 4718–4719. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Lee, H.; Jin, S.; Hyeon, T.; Kim, B.M. Filtration-free recyclable catalytic asymmetric dihydroxylation using a ligand immobilized on magnetic mesocellular mesoporous silica. Adv. Synth. Catal. 2006, 348, 41–46. [Google Scholar] [CrossRef]
- Luo, S.; Zheng, X.; Xu, H.; Mi, X.; Zhang, L.; Cheng, J.P. Magnetic nanoparticle-supported Morita-Baylis-Hillman catalysts. Adv. Synth. Catal. 2007, 349, 2431–2434. [Google Scholar] [CrossRef]
- Dálaigh, C.Ó.; Corr, S.A.; Gun'ko, Y.; Connon, S.J. A magnetic-nanoparticle-supported 4-N,N-dialkylaminopyridine catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Angew. Chem. Int. Ed. 2007, 46, 4329–4332. [Google Scholar]
- Luo, S.; Zheng, X.; Cheng, J.-P. Asymmetric bifunctional primary aminocatalysis on magnetic nanoparticles. Chem. Commun. 2008, 5719–5721. [Google Scholar]
- Schätz, A.; Grass, R.N.; Stark, W.J.J.; Reiser, O. TEMPO supported on magnetic C/Co-nanoparticles: A highly active and recyclable organocatalyst. Chem. Eur. J. 2008, 14, 8262–8266. [Google Scholar]
- Gleeson, O.; Tekoriute, R.; Gun'ko, Y.K.; Connon, S.J. The first magnetic nanoparticle-supported chiral DMAP analogue: Highly enantioselective acylation and excellent recyclability. Chem. Eur. J. 2009, 15, 5669–5673. [Google Scholar] [CrossRef]
- Wang, B.G.; Ma, B.C.; Wang, Q.; Wang, W. Superparamagnetic nanoparticle-supported (S)-diphenyl-prolinol trimethylsilyl ether as a recyclable catalyst for asymmetric Michael addition in water. Adv. Synth. Catal. 2010, 352, 2923–2928. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Magnetic nanoparticle-supported glutathione: A conceptually sustainable organocatalyst. Chem. Commun. 2009, 1837–1839. [Google Scholar]
- Polshettiwar, V.; Varma, R.S. Nano-organocatalyst: Magnetically retrievable ferrite-anchored glutathione for microwave-assisted Paal-Knorr reaction, aza-Michael addition, and pyrazole synthesis. Tetrahedron 2010, 66, 1091–1097. [Google Scholar] [CrossRef]
- Riente, P.; Mendoza, C.; Pericás, M.A. Functionalization of Fe3O4 magnetic nanoparticles for organocatalytic Michael reactions. J. Mater. Chem. 2011, 21, 7350–7355. [Google Scholar]
- Abu-Reziq, R.; Alper, H.; Wang, D.; Post, M.L. Metal supported on dendronized magnetic nanoparticles: Highly selective hydroformylation catalysts. J. Am. Chem. Soc. 2006, 128, 5279–5282. [Google Scholar]
- Abu-Reziq, R.; Wang, D.; Post, M.L.; Alper, H. Platinum nanoparticles supported on ionic liquid-modified magnetic nanoparticles: Selective hydrogenation catalysts. Adv. Synth. Catal. 2007, 349, 2145–2150. [Google Scholar] [CrossRef]
- Abu-Reziq, R.; Wang, D.; Post, M.L.; Alper, H. Separable catalysts in one-pot syntheses for greener chemistry. Chem. Mater. 2008, 20, 2544–2550. [Google Scholar]
- Jones, G. The Knoevenagel condensation. Org. React. 1967, 15, 204–599. [Google Scholar]
- Barret, A.G.M.; Graboski, G.G. Conjugated nitroalkenes: Versatile intermediates in organic synthesis. Chem. Rev. 1986, 86, 751–762. [Google Scholar]
- Ballini, R.; Bosica, G.; Fiorini, D.; Palmieri, A. One-pot synthesis of 1,3-dinitroalkanes under heterogeneous catalysis. Synthesis 2004, 1938–1940. [Google Scholar]
- Kantam, M.L.; Choudary, B.M.; Reddy, C.V.; Rao, K.K.; Figueras, F. Aldol and Knoevenagel condensations catalysed by modified Mg-Al hydrotalcite: A solid base as catalyst useful in synthetic organic chemistry. Chem Commun. 1998, 1033–1034. [Google Scholar]
- Bulbule, V.J.; Deshpande, V.H.; Velu, S.; Sudalai, A.; Sivasankar, S.; Sathe, V.T. Heterogeneous Henry reaction of aldehydes: Diastereoselective synthesis of nitroalcohol derivatives over Mg-Al hydrotalcites. Tetrahedron 1999, 55, 9325–9332. [Google Scholar] [CrossRef]
- Akutu, K.; Kabashima, H.; Seki, T.; Hattori, H. Nitroaldol reaction over solid base catalysts. Appl. Catal.A 2003, 247, 65–74. [Google Scholar] [CrossRef]
- Khan, F.A.; Dash, J.; Satapathy, R.; Upadhyay, S.K. Hydrotalcite catalysis in ionic liquid medium: A recyclable reaction system for heterogeneous Knoevenagel and nitroaldol condensation. Tetraheron Lett. 2004, 45, 3055–3058. [Google Scholar]
- Choudary, B.M.; Kantam, M.L.; Reddy, C.V.; Rao, K.K.; Figueras, F. Henry reactions catalysed by modified Mg-Al hydrotalcite: An efficient reusable solid base for selective synthesis of beta-nitroalkanols. Green Chem. 1999, 187–189. [Google Scholar]
- Saito, T.; Goto, H.; Honda, K.; Fujii, T.T. Acid-base catalysts derived from weakly acidic ion-exchange resin:Efficiency in the Knoevenagel condensation. Tetrahedron Lett. 1992, 33, 7535–7538. [Google Scholar] [CrossRef]
- Hein, R.W.; Astle, M.J.; Shelton, J.R. Ion-exchange Resin Catalysis of the Knoevenagel Condensation of Ketones. J. Org. Chem. 1961, 26, 4874–4878. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Forconi, P. Nitroaldol (Henry) reaction catalyzed by Amberlyst A-21 as a far superior heterogeneous catalyst. Tetrahedron 1996, 52, 1677–1684. [Google Scholar] [CrossRef]
- Angeletti, E.; Canepa, C.; Martinetti, G.; Venturello, P. Amino-groups immobilized on silica-gel—An efficient reusable heterogeneous catalyst for the Knoevenagel condensation. J. Chem. Soc., Perkin Trans. 1989, 1, 105–107. [Google Scholar]
- Ballini, R.; Bosica, G.; Livi, D.; Palmieri, A.; Maggi, R.; Sartori, G. Use of heterogeneous catalyst KG-60-NEt2 in Michael and Henry reactions involving nitroalkanes. Tetrahedron Lett. 2003, 44, 2271–2273. [Google Scholar]
- Corma, A.; Fornes, V.; Martin-Aranda, R.M.; Garcia, H.; Primo, J. Zeolite as base catalysts— Condensation of aldehydes with derivatives of malonic esters. Appl. Catal. 1990, 59, 237–248. [Google Scholar] [CrossRef]
- Kubota, Y.; Nishizaki, Y.; Ikeya, H.; Saeki, M.; Hida, T.; Kawazu, S.; Yoshida, M.; Fujii, H.; Sugi, Y. Organic-silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Micropor. Mesopor. Mater. 2004, 70, 135–149. [Google Scholar] [CrossRef]
- Jaenicke, S.; Chuah, G.K.; Lin, X.H.; Hu, X.C. Organic-inorganic hybrid catalysts for acid- and base-catalyzed reactions. Micropor. Mesopor. Mater. 2000, 35-36, 143–153. [Google Scholar] [CrossRef]
- Inaki, Y.; Kajita, Y.; Yoshida, H.; Ito, K.; Hattori, T. New basic mesoporous silica catalyst obtained by ammonia grafting. Chem. Commun. 2001, 2358–2359. [Google Scholar]
- Huh, S.; Chen, H.-T.; Wiench, J.W.; Pruski, M.; Lin, V.S.-Y. Controlling the selectivity of competitive nitroaldol condensation by using a bifunctionalized mesoporous silica nanosphere-based catalytic system. J. Am.Chem. Soc. 2004, 126, 1010–1011. [Google Scholar]
- Rodriguez, I.; Iborra, S.; Corma, A.; Rey, F.; Jordá, J.L. MCM-41-Quaternary organic tetraalkylammonium hydroxide composites as strong and stable Bronsted base catalysts. Chem. Commun. 1999, 593–594. [Google Scholar]
- Phan, N.T.S.; Jones, C.W. Highly accessible catalytic sites on recyclable organosilane-functionalized magnetic nanoparticles: An alternative to functionalized porous silica catalysts. J. Mol. Catal. A: Chem. 2006, 253, 123–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Xia, C. Basic ionic liquids supported on hydroxyapatite-encapsulated γ-Fe2O3 nanocrystallites: An efficient magnetic and recyclable heterogeneous catalyst for aqueous Knoevenagel condensation. J. Mol. Catal. A: Chem. 2009, 306, 107–112. [Google Scholar] [CrossRef]
- Gill, C.G.; Long, W.; Jones, C.W. Magnetic nanoparticle polymer brush catalysts: Alternative hybrid organic/inorganic structures to obtain high, local catalyst loadings for use in organic transformations. Catal. Lett. 2009, 131, 425–431. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, J.; Cui, F.; Zhu, Y.; Hua, Z.; Shi, J. Superparamagnetic mesoporous Mg-Fe bi-metal oxides as efficient magnetic solid-base catalysts for Knoevenagel condensations. Dalton Trans. 2010, 39, 11132–11135. [Google Scholar]
- Senapati, K.K.; Borgohain, C.; Phukan, P. Synthesis of highly stable CoFe2O4 nanoparticles and their use as magnetically separable catalyst for Knoevenagel reaction in aqueous medium. J. Mol. Catal. A: Chem. 2011, 339, 24–31. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abu-Reziq, R.; Alper, H. Magnetically Separable Base Catalysts: Heterogeneous Catalysis vs. Quasi-Homogeneous Catalysis. Appl. Sci. 2012, 2, 260-276. https://doi.org/10.3390/app2020260
Abu-Reziq R, Alper H. Magnetically Separable Base Catalysts: Heterogeneous Catalysis vs. Quasi-Homogeneous Catalysis. Applied Sciences. 2012; 2(2):260-276. https://doi.org/10.3390/app2020260
Chicago/Turabian StyleAbu-Reziq, Raed, and Howard Alper. 2012. "Magnetically Separable Base Catalysts: Heterogeneous Catalysis vs. Quasi-Homogeneous Catalysis" Applied Sciences 2, no. 2: 260-276. https://doi.org/10.3390/app2020260





