Oxyfluoride Chemistry of Layered Perovskite Compounds
Abstract
:1. Introduction
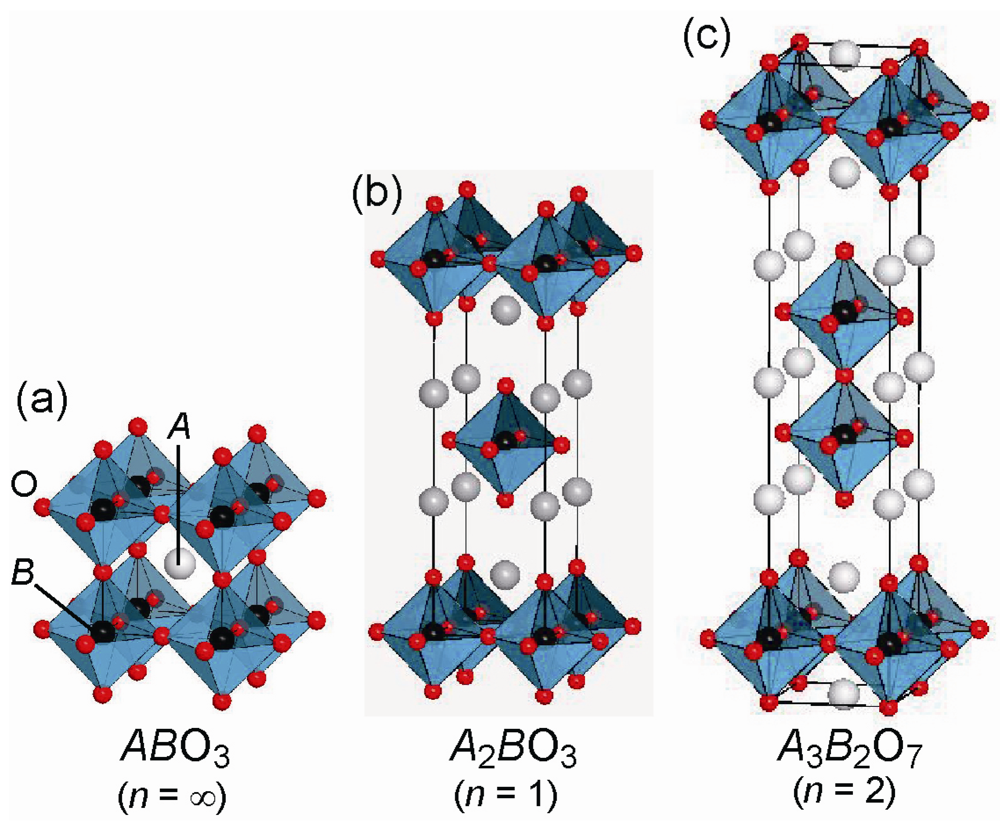
2. Fluorine Occupation Patterns in Layered Oxyfluoride Perovskite
2.1. Regular or Random Anion Occupation Pattern in the Perovskite Blocks

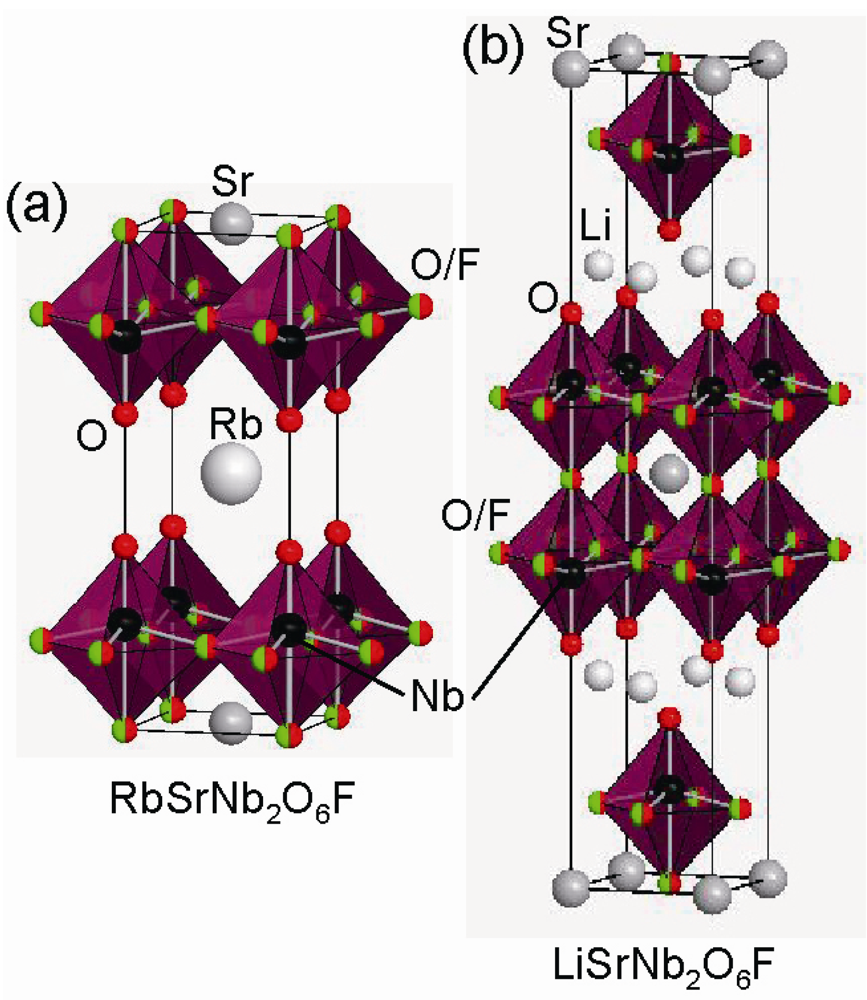
2.2. Fluorine Insertion into Only Interstitial Sites between the Perovskite Blocks
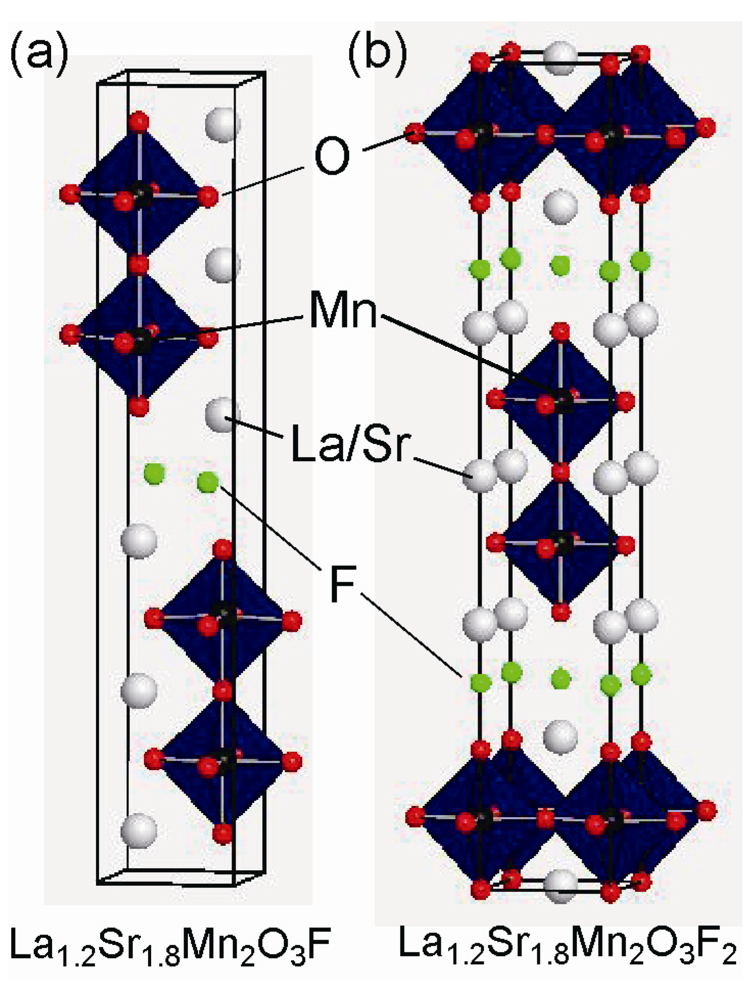
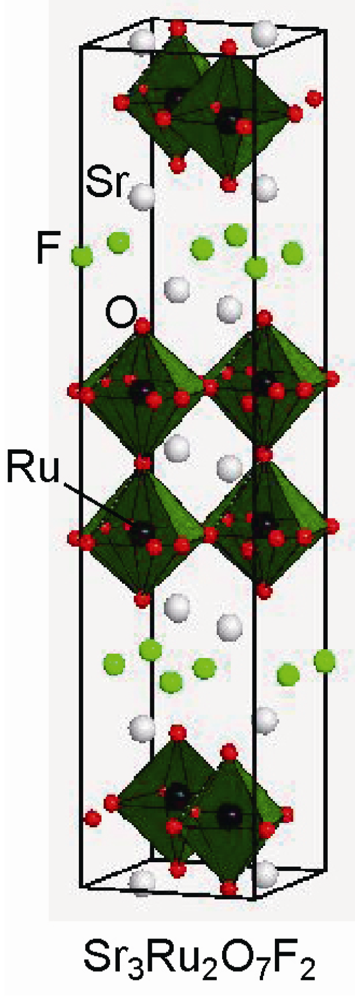

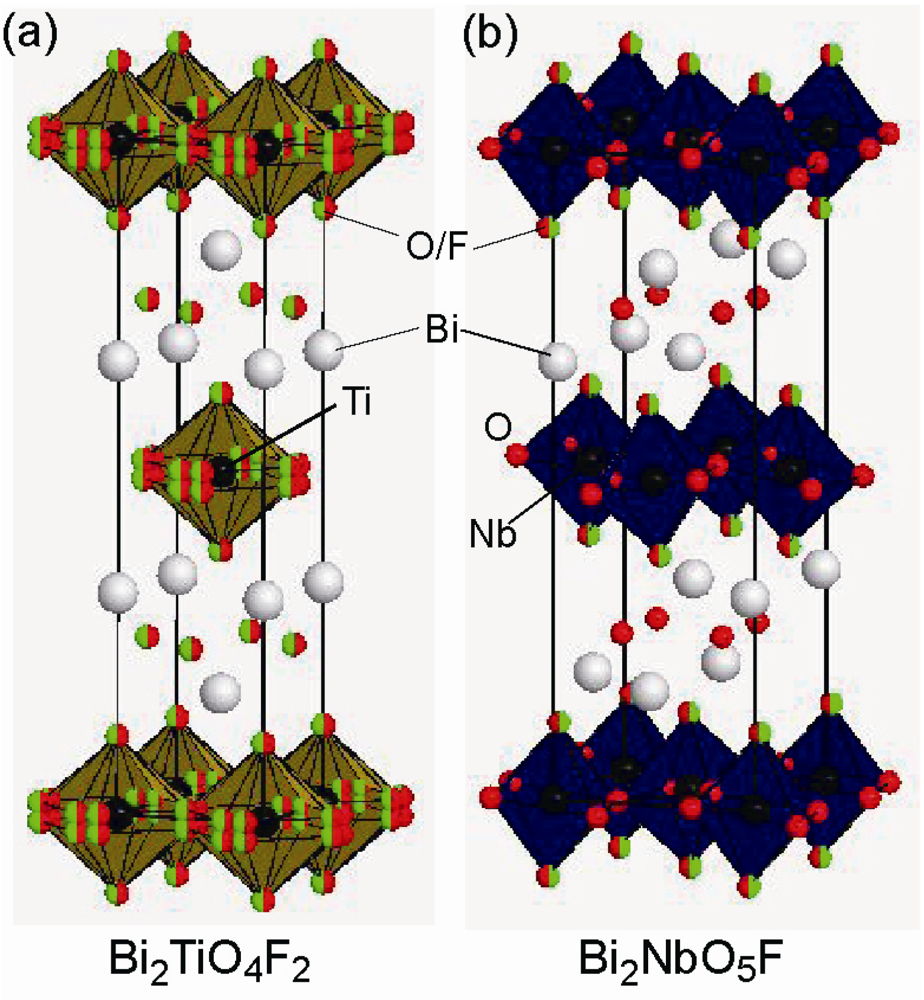
2.3. Fluorine Occupation of Both the Terminal Apical Sites and the Interstitial Sites

3. Recent Results on New Layered Iron and Cobalt Oxyfluoride Compounds
3.1. Unusual O/F Site Disorder in Layered Cobalt Oxyfluoride
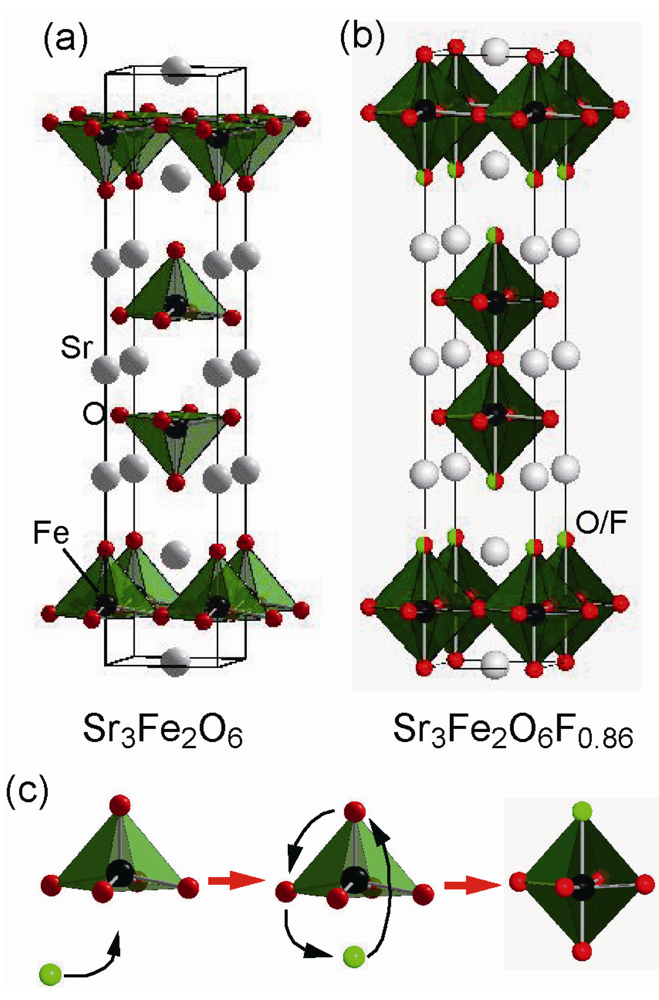
3.2. Highly Fluorinated Iron Oxides
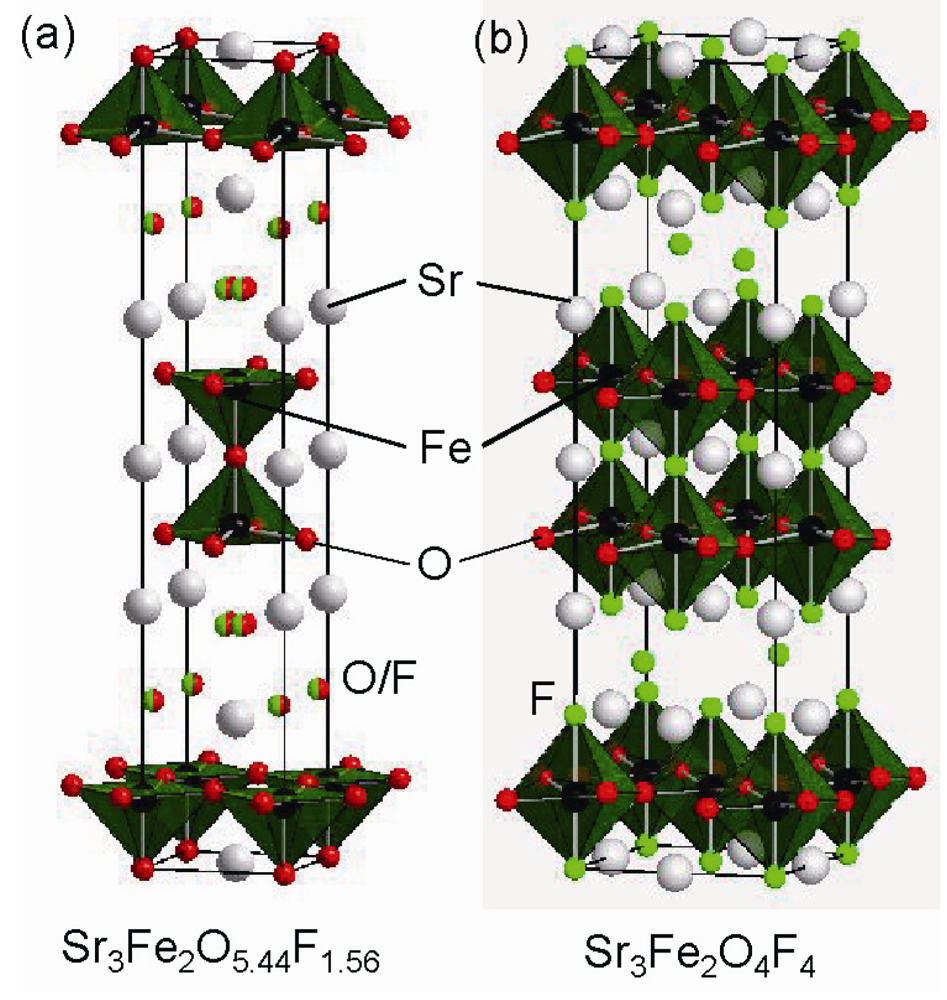
5. Conclusions
Acknowledgments
References
- Bednorz, J.G.; Müller, K.A. Possible high Tc superconductivity in the Ba-La-Cu-O. Z. Phys. B 1986, 64, 189–193. [Google Scholar] [CrossRef]
- Urushibara, A.; Moritomo, Y.; Arima, T.; Asamitsu, A.; kido, G.; Tokura, Y. Insulator-metal transition and giant magnetoresistance in La1−xSrxMnO3. Phys. Rev. B 1995, 51, 14103–14109. [Google Scholar]
- Moritomo, Y.; Asamitsu, A.; Kuwahara, H.; Tokura, Y. Giant magnetoresistance of manganese oxides with a layered perovskite structure. Nature 1996, 380, 141–144. [Google Scholar] [CrossRef]
- Tokura, Y.; Nagaosa, N. Orbital physics in transition-metal oxides. Science 2000, 288, 462–468. [Google Scholar] [CrossRef]
- Cava, R.J. Oxide superconductors. J. Am. Ceram. Soc. 2000, 83, 1–28. [Google Scholar]
- Dagotto, E. Correlated electrons in high-temperature superconductors. Rev. Mod. Phys. 1994, 66, 763–841. [Google Scholar] [CrossRef]
- Kasahara, A.; Nukumizu, K.; Hitoki, G.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Photoreaction on LaTiO2N under visible light irradiation. J. Phys. Chem.A 2002, 106, 6750–6753. [Google Scholar]
- Al-Mamouri, M.; Edwards, P.P.; Greaves, C.; Slaski, M. Synthesis and superconducting properties of the strontium copper oxy-fluoride Sr2CuO2F2+δ. Nature 1994, 369, 382–384. [Google Scholar] [CrossRef]
- Slater, P.R.; Hodges, J.P.; Francesconi, M.G.; Edwards, P.P.; Greaves, C.; Gameson, I.; Slaski, M. An improved route to the synthesis of superconducting copper oxyfluorides Sr2−xAxCuO2F2+δ (A = Ca, Ba) using transition metal difluorides as fluorinating reagents. Phys. C 1995, 253, 16–22. [Google Scholar] [CrossRef]
- Ardashnikova, E.I.; Lubarsky, S.V.; Denisenko, D.I.; Shpanchenko, R.V.; Antipov, E.V.; van Tendeloo, G. A new way of synthesis and characterization of superconducting oxyfluoride Sr2Cu(O, F)4+δ. Phys. C 1995, 253, 259–265. [Google Scholar] [CrossRef]
- Slater, P.R. Poly(vinylidene fluoride) as a reagent for the synthesis of K2NiF4-related inorganic oxide fluorides. J. Fluor. Chem. 2002, 117, 43–45. [Google Scholar] [CrossRef]
- Pinlac, R.A.; Stern, C.L.; Poeppelmeier, K.R. New layered oxide-fluoride perovskites: KNaNbOF5 and KNaMO2F4 (M = Mo6+, W6+). Crystals 2011, 1, 3–14. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Kasper, N.V.; Mantytskaya, O.S.; Shapovalova, E.F. High-pressure synthesis of some perovskite-like compounds with a mixed anion type. Mater. Res. Bull. 1995, 30, 421–425. [Google Scholar] [CrossRef]
- Katsumata, T.; Nakashima, M.; Umemoto, H.; Inaguma, Y. Synthesis of the novel perovskite-type oxyfluoride PbScO2F under high pressure and high temperature. J. Solid State Chem. 2008, 181, 2737–2740. [Google Scholar] [CrossRef]
- Galasso, F.; Darby, W. Preparation, structure, and properties of K2NbO3. J. Phys. Chem. 1962, 66, 1318–1320. [Google Scholar] [CrossRef]
- Galasso, F.; Darby, W. Preparation and properties of Sr2FeO3F. J. Phys. Chem. 1963, 67, 1451–1453. [Google Scholar] [CrossRef]
- Case, G.S.; Hector, A.L.; Levason, W.; Needs, R.L.; Thomas, M.F.; Weller, M.T. Synthesis powder neutron diffraction structures and Mössbauer studies of some complex iron oxyfluorides: Sr3Fe2O6F0.87, Sr2FeO3F and Ba2InFeO5F0.68. J. Mater. Chem. 1999, 9, 2821–2827. [Google Scholar] [CrossRef]
- Needs, R.L.; Weller, M.T.; Scheler, U.; Harris, R.K. Synthesis and structure of Ba2InO3X (X = F, Cl, Br) and Ba2ScO3F; oxide/halide ordering in K2NiF4-type structures. J. Mater. Chem. 1996, 6, 1219–1224. [Google Scholar] [CrossRef]
- Choy, J.H.; Kim, J.Y.; Kim, S.J.; Sohn, J.S. New Dion-Jacobson-type layered perovskite oxyfluorides, ASrNb2O6F (A = Li, Na, and Rb). Chem. Mater. 2001, 13, 906–912. [Google Scholar] [CrossRef]
- Caruntu, G.; Spinu, L.; Wiley, J.B. New rare-earth double-layered-perovskite oxyfluorides, RbLnTiNbO6F (Ln = La, Pr, Nd). Mater. Res. Bull. 2002, 37, 133–140. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tian, M.; Eguchi, M.; Mallouk, T. Ion-exchangeable, electronically conducting layered perovskite oxyfluorides. J. Am. Chem. Soc. 2009, 131, 9849–9855. [Google Scholar]
- Needs, R.L.; Weller, M.T. Structure of Ba3In2O5F2 by combined powder analysis; oxide/fluoride ordering in a Ruddlesden-Popper phase. J. Chem. Soc. Dalton Trans. 1995, 18, 3015–3017. [Google Scholar]
- Aurivillius, B. The structure of Bi2NbO5F and isomorphous compounds. Ark. Kemi. 1952, 5, 39–47. [Google Scholar]
- Needs, R.L.; Dann, S.E.; Weller, M.T. Cherryman, J.C.; Harris, R.K. The structure and oxide/fluoride ordering of the ferroelectrics Bi2TiO4F2 and Bi2NbO5F. J. Mater. Chem. 2005, 15, 2399–2407. [Google Scholar]
- McCabe, E.E.; Jones, I.P.; Zhang, D.; Hyatt, N.C.; Greaves, C. Crystal structure and electrical characterization of Bi2NbO5F: An Aurivillius oxide fluoride. J. Mater. Chem. 2007, 17, 1193–1200. [Google Scholar] [CrossRef]
- Greaves, C.; Kissick, J. L.; Francesconi, M.G.; Aikens, L.D. Gillie, L.J. Synthetic strategies for new inorganic oxide fluorides and oxide sulfates. J. Mater. Chem. 1999, 9, 111–116. [Google Scholar] [CrossRef]
- Aikens, L.D.; Gillie, L.J.; Li, R.K.; Greaves, C. Staged fluorine insertion into manganese oxides with Ruddlesden-Popper structures: LaSrMnO4F and La1.2Sr1.8MnO2O7F. J. Mater. Chem. 2002, 12, 264–267. [Google Scholar] [CrossRef]
- Sivakumar, T.; Wiley, J.B. Topotactic route for new layered perovskite oxides containing fluorine: Ln1.2Sr1.8Mn2O7F2 (Ln = Pr, Nd, Sm, Eu, and Gd). Mater. Res. Bull. 2009, 44, 74–77. [Google Scholar] [CrossRef]
- Aikens, L.D.; Li, R.K.; Greaves, C. The synthesis and structure of a new oxide fluoride, LaSrMnO4F, with stated fluorine insertion. Chem. Commun. 2000, 2149–2130. [Google Scholar]
- Li, R.K.; Greaves, V. Double-layered ruthenate Sr3Ru2O7F2 formed by fluorine insertion into Sr3Ru2O7. Phys. Rev. B 2000, 62, 3811–13815. [Google Scholar]
- Baikie, T.; Dixon, E.L.; Rooms, J.F.; Young, N.A.; Francesconi, M.G. Ba2−xSrxPdO2F2 (0 ≤ x ≤ 1.5): The first palladium-oxide-fluoride. Chem. Commun. 2003, 1580–1581. [Google Scholar]
- Baikie, T.; Islam, M.S.; Francesconi, M.G. Defects in the new oxide-fluoride Ba2PdO2F2: The search for fluoride needles in an oxide haystack. J. Mater. Chem. 2005, 15, 119–123. [Google Scholar] [CrossRef]
- Greaves, C.; Fracesconi, M.G. Fluorine insertion in inorganic materials. Curr. Opni. Solid State Mater. Sci. 1998, 3, 132–136. [Google Scholar] [CrossRef]
- McCabe, E.E.; Greaves, C. Fluorine insertion reactions into preformed metal oxides. J. Fluor. Chem. 2007, 128, 448–458. [Google Scholar] [CrossRef]
- Sanjaya Ranmohotti, K.G.; Josepha, E.; Choi, J.; Zhang, J.; Wiley, J.B. Topochemical manipulation of perovskites: Low-temperature reaction strategies for directing structure and properties. Adv. Mater. 2011, 23, 442–460. [Google Scholar]
- Kissick, J.L.; Greaves, C.; Edwards, P.P.; Cherkashenko, V.M.; Kurmaev, E.Z.; Bartkowski, S.; Neumann, M. Synthesis, structure, and XPS characterization of the stoichiometric phase Sr2CuO2F2. Phys. Rev. B 1997, 56, 2831–2835. [Google Scholar]
- Slater, P.R.; Gover, R.K.B. Synthesis and structure of the new oxide fluoride Ba2ZrO3F2∙xH2O. J. Mater. Chem. 2001, 11, 2035–2038. [Google Scholar] [CrossRef]
- Slater, P.R.; Gover, R.K.B. Synthesis and structure of the new oxide fluoride Sr2TiO3F2 from the low temperature fluorination of Sr2TiO4: An example of a staged fluorine substitution/insertion reaction. J. Mater. Chem. 2002, 12, 291–294. [Google Scholar] [CrossRef]
- Headspith, D.A.; Sullivan, E.; Greaves, C.; Francesconi, M.G. Synthesis and characterization of the quaternary nitride-fluoride Ce2MnN3F2−δ. Dalton Trans. 2009, 9273–9279. [Google Scholar]
- El Shinawi, H; Marco, J.F.; Berry, F.J.; Greaves, C. LaSrCoFeO5, LaSrCoFeO5F and LaSrCoFeO5.5: New La-Sr-Co-Fe perovskites. J. Mater. Chem. 2010, 20, 3253–3259. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Li, J.J.; Yamaura, K.; Matsushita, Y.; Katsuya, Y.; Tanaka, M.; Shirako, Y.; Akaogi, M.; Takayama-Muromachi, E. New layered cobalt oxyfluoride, Sr2CoO3F. Chem. Commun. 2011, 47, 3263–3265. [Google Scholar]
- Wang, X.L.; Takayama-Muromachi, E. Magnetic and transport properties of the layered perovskite system Sr2−yYyCoO4 (0 ≤ y ≤ 1). Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Belik, A.A.; Iikubo, S.; Kodama, K.; Igawa, N.; Shamoto, S.; Niitaka, S.; Azuma, M.; Shimakawa, Y.; Takano, M.; Izumi, F.; et al. Neutron powder diffraction study on the crystal and magnetic structures of BiCoO3. Chem. Mater. 2006, 18, 798–803. [Google Scholar] [CrossRef]
- Loureiro, S.M.; Felser, C.; Huang, Q.; Cava, R.J. Refinement of the crystal structures of strontium cobalt oxychlorides by neutron powder diffraction. Chem. Mater. 2000, 12, 3181. [Google Scholar]
- Shpanchenko, R.V.; Rozova, M.G.; Abakumov, A.M.; Ardashnikova, E.I.; Kovba, M.L.; Putilin, S.N.; Antipov, E.V.; Lebedev, O.I.; Tendeloo, G.V. Inducing superconductivity and structural transformations by fluorination of reduced YBCO. Phys. C 1997, 280, 272–280. [Google Scholar] [CrossRef]
- Berry, F.J.; Ren, X.; Hea, R.; Slater, P.; Thomas, M.F. Fluorination of perovskite-related SrFeO3−δ. Solid State Commun. 2005, 134, 621–624. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Yamaura, K.; Hayashi, N.; Kodama, K.; Igawa, N.; Matsushita, Y.; Katsuya, Y.; Shirako, Y.; Akaogi, M.; Takayama-Muromachi, E. Topotactic synthesis and crystal structure of a highly fluorinated Ruddlesden–Popper-type Iron oxide, Sr3Fe2O5+xF2–x (x ≈ 0.44). Chem. Mater. 2011, 23, 3652–3658. [Google Scholar] [CrossRef]
- Hancock, C.A.; Herranz, T.; Marco, J.F.; Berry, F.J.; Slater, P.R. Low temperature fluorination of Sr3Fe2O7−δ with polyvinylidine fluoride: An X-ray powder diffraction and Mössbauer spectroscopy study. J. Solid State Chem. 2012, 186, 195–203. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsujimoto, Y.; Yamaura, K.; Takayama-Muromachi, E. Oxyfluoride Chemistry of Layered Perovskite Compounds. Appl. Sci. 2012, 2, 206-219. https://doi.org/10.3390/app2010206
Tsujimoto Y, Yamaura K, Takayama-Muromachi E. Oxyfluoride Chemistry of Layered Perovskite Compounds. Applied Sciences. 2012; 2(1):206-219. https://doi.org/10.3390/app2010206
Chicago/Turabian StyleTsujimoto, Yoshihiro, Kazunari Yamaura, and Eiji Takayama-Muromachi. 2012. "Oxyfluoride Chemistry of Layered Perovskite Compounds" Applied Sciences 2, no. 1: 206-219. https://doi.org/10.3390/app2010206
APA StyleTsujimoto, Y., Yamaura, K., & Takayama-Muromachi, E. (2012). Oxyfluoride Chemistry of Layered Perovskite Compounds. Applied Sciences, 2(1), 206-219. https://doi.org/10.3390/app2010206



