Computational Study on the Acid Catalyzed Reactions of Fluorine-Containing 2,4-Dialkoxy-3,4-dihydro-2H-pyrans with Aromatic Compounds
Abstract
:1. Introduction


2. Computational Method
3. Results and Discussion
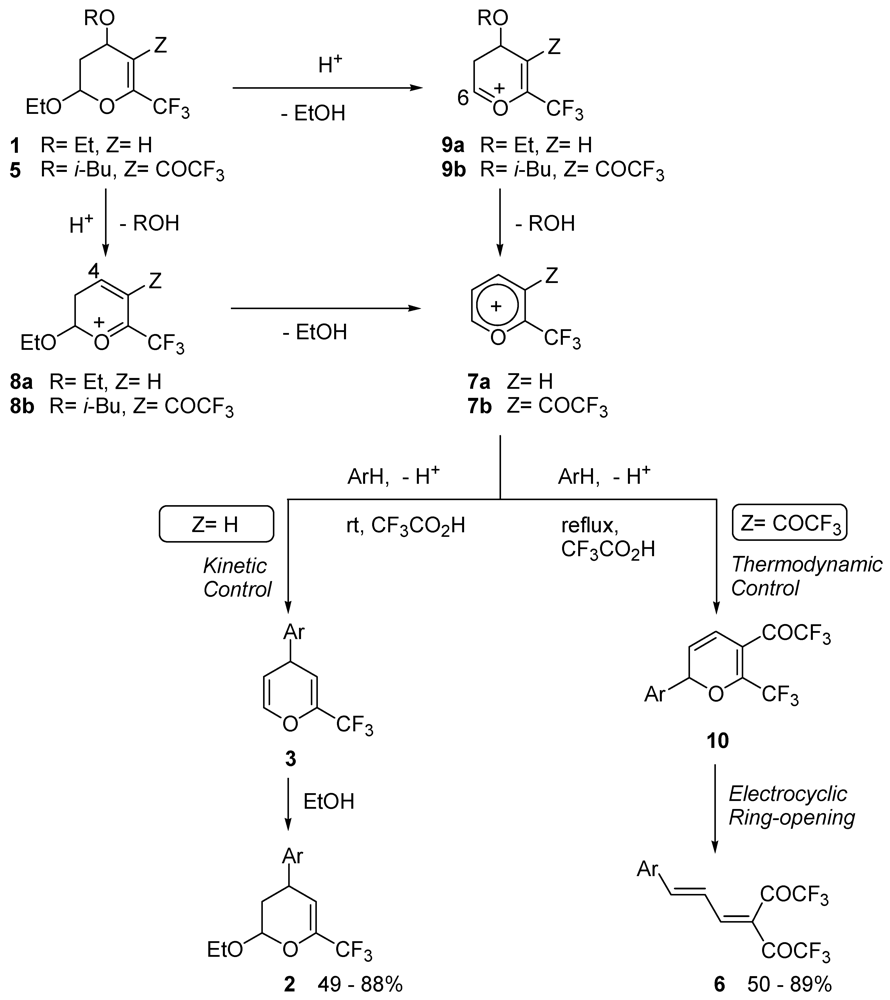
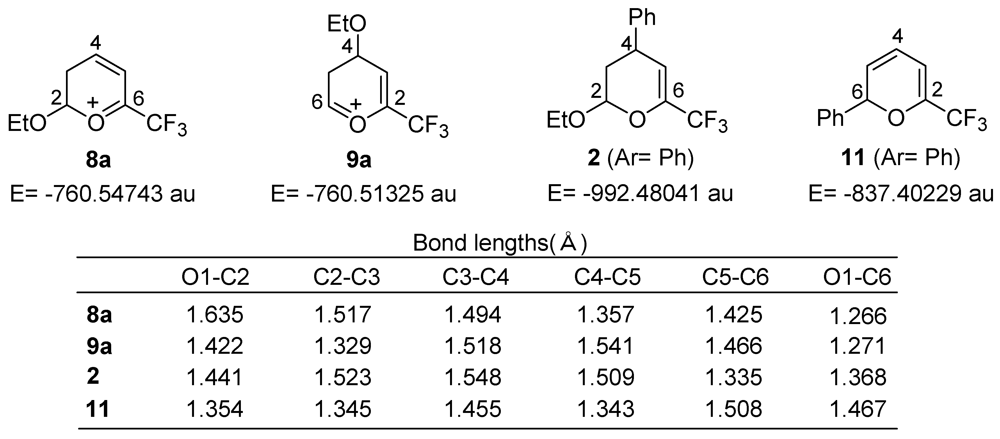



4. Conclusions
References and Notes
- Filler, R.; Kobayashi, Y. Biomedicinal Aspects of Fluorine Chemistry; Kodansha & Elsevier Biomedical: Tokyo, Japan, 1982. [Google Scholar]
- Filler, R. Organofluorine Chemicals and Their Industrial Applications; Ellis Horwood: London, UK, 1979. [Google Scholar]
- Welch, J.T. Advances in the preparation of biologically active organofluorine compounds. Tetrahedron 1987, 43, 3123–3197. [Google Scholar] [CrossRef]
- Filler, R.; Kobayashi, Y.; Yagupolskii, L.M. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Hojo, M.; Masuda, R.; Okada, E. A facile synthesis of 2,4-dialkoxy-, 2-alkoxy-4-phenoxy-, and 2,4-diphenoxy-6-(trifluoromethyl)-3,4-dihydro-2H-pyrans. Hetero Diels-Alder reactions of trans-β-(trifluoroacetyl)vinyl ethers with various vinyl ethers. Synthesis 1989, 215–217. [Google Scholar]
- Ota, N.; Okada, E.; Shibata, D.; Adachi, S.; Saikawa, S. A facile synthesis of 4-aryl-1,1,1-trifluorobut-3-en-2-ones via 4-aryl substituted CF3-containing dihydropyran derivatives: A versatile method for the introduction of fluorine-containing C4- and C6- unit to aromatic compounds. Heterocycles 2010, 80, 515–525. [Google Scholar] [CrossRef]
- Hojo, M.; Masuda, R.; Okada, E. A convenient synthetic route to functionalized 5-(trifluoroacetyl)-3,4-dihydro-2H-pyrans: hetero-Diels-Alder reaction of β,β-bis(trifluoroacetyl)vinyl ethers with electron-rich alkenes. Synthesis 1990, 347–350. [Google Scholar]
- Ota, N.; Okada, E.; Sonoda, A.; Muro, N.; Shibata, D.; Médebielle, M. One step introduction of 4,4-bis(trifluoroacetyl)-1,3-butadiene system to aromatic rings using fluorine-containing 3,4-dihydro-2H-pyrans. A facile synthetic method for 1,1,1,5,5,5-hexafluoro-3-[(E)-3-arylallylidene]pentane-2,4-diones. Heterocycles 2008, 76, 215–219. [Google Scholar] [CrossRef]
- Zanatta, N.; Fernandes, L.S.; Nachtigall, F.M.; Coelho, H.S.; Amaral, S.S.; Flores, A.F.C.; Bonacorso, H.G.; Martins, M.A.P. Highly chemoselective synthesis of 6-alkoxy-1-alkyl(aryl)-3-trifluoroacetyl-1,4,5,6-tetrahydropyridines and 1-alkyl(aryl)-6-amino-3-trifluoroacetyl-1,4,5,6-tetrahydropyridines. Eur. J. Org. Chem. 2009, 1435–1444. [Google Scholar]
- Shimizu, M.; Oishi, A.; Taguchi, Y.; Sano, T.; Gama, Y.; Shibuya, I. Quinoline ring formation by cycloaddition of N-arylketenimines with enol ethers under high pressure. Heterocycles 2001, 55, 1971–1980. [Google Scholar] [CrossRef]
- Caramella, P.; Invernizzi, A.G.; Pastormelo, E.; Quadrelli, P.; Corsaro, A. A pericyclic cascade in the addition of diphenyl nitrile imine to pyridine. Heterocycles 1995, 40, 515–520. [Google Scholar] [CrossRef]
- Oinuma, H.; Dan, S.; Kakisawa, H. Stereoselective syntheses of α-isosparteine. J. Chem. Soc. Perkin Trans. 1990, 2593–2597. [Google Scholar]
- Wendelin, W.; Schramm, H.-W.; Blasi-Rabassa, A. Reactions of guanidine and thiourea with α,β,γ,δ-unsaturated ketones. Monatsh. Chem. 1985, 116, 385–400. [Google Scholar] [CrossRef]
- Mohammed, F.K. Synthesis of some new benzo[b]carbazole-6,11-diones. Egypt. J. Chem. 2006, 49, 139–147. [Google Scholar]
- Rubinov, D.B.; Rubinova, I.L.; Lakhvich, F.A. Synthesis of exo- and endocyclic enamino derivatives of 2-(3-arylprop-2-enoyl)cyclohexane-1,3-diones. Russ. J. Org. Chem. 2011, 47, 319–330. [Google Scholar] [CrossRef]
- Ota, N.; Kamitori, Y.; Nishiguchi, E.; Ishii, M.; Okada, E. A molecular orbital calculation study on the interesting reactivity of fluorine-containing 3,4-dihydro-2H-pyrans with aromatic compounds in the presence of trifluoroacetic acid. Heterocycles 2011, 82, 1337–1343. [Google Scholar]
- Wavefunction, Inc. Irvine, CA, USA. Available online: http://www.wavefun.com (accessed on 20 February 2012).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Clark, M.; Cramer, R.D., III.; van Opdensch, N. Validation of the general purpose Tripos 5.2 force field. J. Comput. Chem. 1989, 10, 982–1012. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- The previously calculated energy value (−837.40329 au: see ref. 16) was used for 3 (Ar= Ph). The energy of ethanol was calculated as −155.06425 au (this work).
- The previously calculated energy value (−605.49511 au: see ref. 16) was used for 7a.
- The frontier electron densities (LUMO) at C-4 and C-6 of 7a were calculated as 0.582 and 0.341, respectively: see ref. 16.
- The energy deference between 3 and 11 was estimated to be less than 1 kcal/mol: see ref. 16.
- Our calculations for vibrational frequencies of TS11 showed only one imaginary frequency at −416.3 cm−1 having the vibrational mode corresponding to the bond formation and cleavage between C6 and O1.
- The previously calculated energy value (−1055.82147 au: see ref. 16) was used for 7b. The energy of isobutanol was calculated as −233.66241 au (this work).
- It was predicted that the reaction of pyrylium (7b) with aromatic compounds occurs at C-4 under kinetically controlled conditions and that proceeds at C-6 under thermodynamically controlled conditions: see ref. 16. In addition, the steric hindrance due to trifluoroacetyl group at C-5 would prevent the attack to C-4 on 7b.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ota, N.; Kamitori, Y.; Shirai, R.; Hatakenaka, M.; Okada, E. Computational Study on the Acid Catalyzed Reactions of Fluorine-Containing 2,4-Dialkoxy-3,4-dihydro-2H-pyrans with Aromatic Compounds. Appl. Sci. 2012, 2, 129-138. https://doi.org/10.3390/app2010129
Ota N, Kamitori Y, Shirai R, Hatakenaka M, Okada E. Computational Study on the Acid Catalyzed Reactions of Fluorine-Containing 2,4-Dialkoxy-3,4-dihydro-2H-pyrans with Aromatic Compounds. Applied Sciences. 2012; 2(1):129-138. https://doi.org/10.3390/app2010129
Chicago/Turabian StyleOta, Norio, Yasuhiro Kamitori, Ryusuke Shirai, Mizuki Hatakenaka, and Etsuji Okada. 2012. "Computational Study on the Acid Catalyzed Reactions of Fluorine-Containing 2,4-Dialkoxy-3,4-dihydro-2H-pyrans with Aromatic Compounds" Applied Sciences 2, no. 1: 129-138. https://doi.org/10.3390/app2010129




