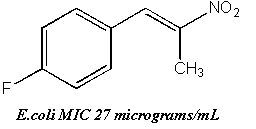
A Study of Fluorinated β-Nitrostyrenes as Antimicrobial Agents
Abstract
:1. Introduction
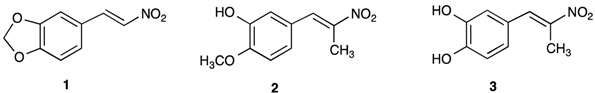
- (a) E.coli was suppressed effectively by chlorine or fluorine C4-aryl-substituents relative to the unsubstituted or the 3-4-methylenedioxy ring compounds.
- (b) S.aureus was suppressed effectively by a wide range of nitropropenyl arenes including β-methyl-β-nitrostyrene, the 4-fluorine and 4-chlorine substituted β-methyl-β-nitrostyrene, imidazolyl, benzothiazole and the 3,4-methylenedioxy derivatives. An important finding was that when two hydroxy groups were present on the ring in the 2- and 4- positions or the 2- or 5- positions, activity against this microorganism noticeably reduced. The fact that this also occurred with substitution by N,N-dimethyl and N,N-diethyl groups indicated that the more polar nature of these substituents was detrimental to activity. This was supported by the KD values of the latter compounds being relatively low compared with the unsubstituted and halogenated-substituted compounds.
- (c) B.subtilis was suppressed by a wide range of compounds in a similar fashion to S.aureus. The 2,4- and 2,5- dihydroxyaryl substituted compounds exhibited poor bacterial inhibition whereas the 3,4-dihydroxy derivative gave high activity.
- (d) C.albicans was suppressed by the 3,4-dichloro, 4-chloro, 4-fluoro, derivatives. However 2,4-dihydroxy, 2,5-dihydroxy and the benzimidazole derivatives were very unreactive.
 |  |  | |
| MIC50 (microgram/mL) | |||
| E. coli | 62.5 | 25 | 12.5 |
| S. aureus | 100 | 250 | 100 |
2. Experimental Section
2.1. Chemicals
2.2. Instrumentation and Synthesis
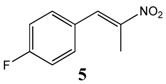
2.2.1. Synthesis of 1-Fluoro-4-(Nitroprop-1-Enyl)Benzene 5
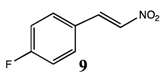
2.2.2. Synthesis of 4-fluoro-β-nitrostyrene 9 [4-fluoro-2-(nitroethen-1-enyl)benzene]
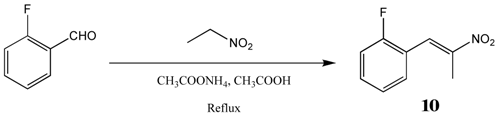
2.2.3. 1-Fluoro-2-(Nitroprop-1-Enyl)Benzene 10
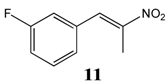
2.2.4. 1-Fluoro-3-(Nitroprop-1-Enyl)Benzene 11
2.2.5. 1,3-Difluoro-4-(Nitroprop-1-Enyl)Benzene 12
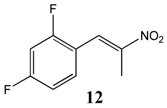
2.2.6. 1-Trifluoromethyl-4-(Nitroprop-1-Enyl)Benzene 13
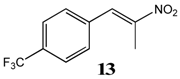
2.2.7. 1-Trifluoromethyl-2-(Nitroprop-1-Enyl)Benzene 14
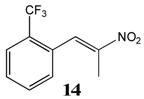
2.2.8. 1-Trifluoromethyl-3-(Nitroprop-1-Enyl)Benzene 15
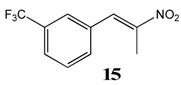
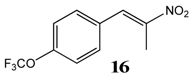
2.2.9. 1-Trifluoromethoxy-4-(Nitroprop-1-Enyl)Benzene 16
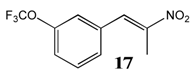
2.2.10. 1-Trifluoromethoxy-3-(Nitroprop-1-Enyl)Benzene 17
2.2.11. Minimum Inhibitory Concentrations
2.2.12. Octanol-Water Partition Coefficients
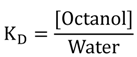
3. Results and Discussion
 | ||||||||||||||
| Strain | 5 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| S. aureus | 2 | 128 | 3 | 8 | 2 | 2 | 16 | 16 | 2 | 16 | 3 | 2 | 256 | 8 |
| B. subtilis | 2 | 256 | 3 | 16 | 2 | 2 | 8 | 8 | 4 | 8 | 2 | 2 | 256 | 16 |
| E. faecalis | 5.5 | 64 | 5 | 16 | 6 | 4 | 32 | 16 | 8 | 16 | 4 | 5 | 128 | 16 |
| E. coli | 27 | 256 | 42 | 256 | 45 | 96 | 512 | 256 | 512 | 256 | 256 | 128 | 256 | 96 |
| C. albicans | 2 | 32 | 3 | 8 | 3 | 4 | 16 | 8 | 8 | 8 | 3 | 4 | 32 | 6 |
| Meas. KD | 88 | 73 | 207 | 23 | 155 | 68 | 74 | 213 | 66 | 132 | 354 | 247 | 44 | 133 |
| Meas. logP | 1.94 | 1.86 | 2.32 | 1.36 | 2.19 | 1.83 | 1.87 | 2.33 | 1.82 | 2.12 | 2.55 | 2.39 | 1.64 | 2.12 |
| Calc. logP | 2.34 | 2.27 | 2.34 | 2.34 | 3.49 | 3.08 | 3.08 | 3.08 | 3.63 | 3.63 | 1.82 | 1.88 | 2.13 | 2.20 |


4. Conclusions
Acknowledgments
References
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Schales, O.; Graefe, H.A. Arylnitroalkenes: A new group of antibacterial agents. J. Am. Chem. Soc. 1952, 74, 4486–4490. [Google Scholar] [CrossRef]
- Denisenko, P.P.; Sapronov, N.S.; Tarasenko, A.A. Antimicrobial and radioprotective compounds. Patent 20040266844, 30 December 2004. [Google Scholar]
- Milhazes, N.; Calheiros, R.; Marques, M.P.M.; Garrido, J.; Cordeiro, M.N.D.S.; Rodrigues, C.; Quinteira, S.; Novais, C.; Peixe, L.; Borges, F. β-Nitrostyrene derivatives as potential antibacterial agents: A structure-property-activity relationship study. Bioorg. Med. Chem. 2006, 14, 4078–4088. [Google Scholar]
- Bialy, L.; Waldmann, H. Inhibitors of protein tyrosine phosphatases: Next-generation drugs? Angew. Chem. Int. Ed. 2005, 44, 3814–3839. [Google Scholar] [CrossRef]
- Heneberg, P. Use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs. Curr. Med. Chem. 2009, 16, 706–733. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.-Y. PTP1B as a drug target: Recent developments in PTP1B inhibitor discovery. Drug Discov. Today 2007, 12, 373–381. [Google Scholar] [CrossRef]
- Park, J.; Pei, D. Trans-β-nitrostyrene derivatives as slow-binding inhibitors of protein tyrosine phosphatases. Biochemistry 2004, 43, 15014–15021. [Google Scholar] [CrossRef]
- Jung, G.; Fouad, H.; Heusel, G. 2-Nitro-1-phenylethyl: A new protecting and chiroptical reporter group for cysteine peptides. Angew. Chem. Int. Ed. 1975, 14, 817–818. [Google Scholar] [CrossRef]
- Hwu, J.R.; Wong, F.F.; Shiao, M.-J. Reduction of aromatic nitro compounds to aromatic amines by sodium trimethylsilanethiolate. J. Org. Chem. 1992, 57, 5254–5255. [Google Scholar] [CrossRef]
- Berner, O.M.; Tedeschi, L.; Enders, D. Asymmetric Michael additions to nitroalkenes. Eur. J. Org. Chem. 2002, 67, 1877–1894. [Google Scholar]
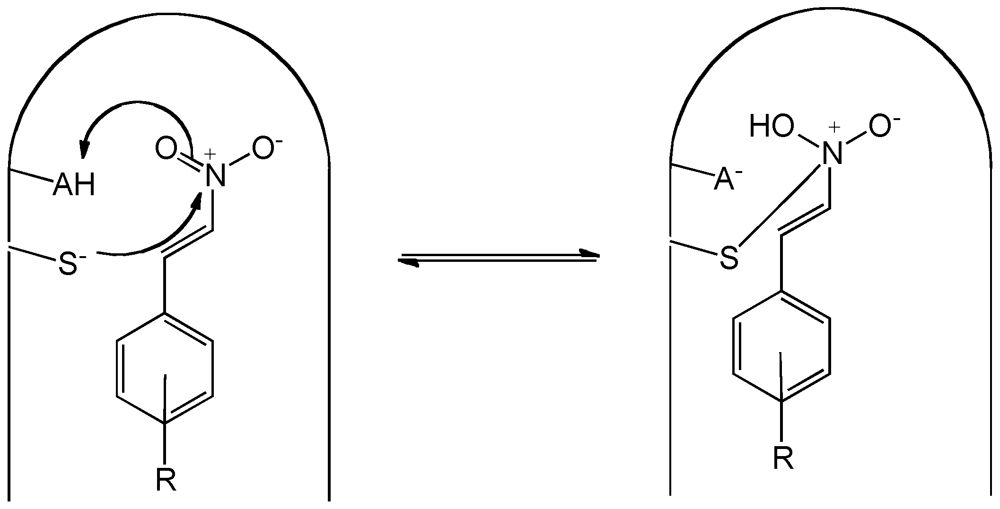
- Baker, L.M.S.; Baker, P.R.S.; Golin-Bisello, F.; Schopfer, F.J.; Fink, M.; Woodcock, S.R.; Branchaud, B.P.; Radi, R.; Freeman, B.A. Nitro-fatty acid reaction with glutathione and cysteine: Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 2007, 282, 31085–31093. [Google Scholar]
- Bernasconi, C.F.; Schuck, D.F. Kinetics of reversible thiolate ion addition to substituted beta -nitrostyrenes in water. Radicaloid transition state or principle of nonperfect synchronization? J. Org. Chem. 1992, 57, 2365–2373. [Google Scholar] [CrossRef]
- Nicoletti, G.; Cornell, H.; Hügel, H.; White, K.S.; Nguyen, T.; Zalizniak, L. Synthesis and biological activity of nitropropenyl arenes. 2012; in preparation. [Google Scholar]
- Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar]
- Smart, B.E. Fluorine substituent effects (on bioactivity). J. Fluorine Chem. 2001, 109, 3–11. [Google Scholar] [CrossRef]
- Giménez, D.; Andreu, C.; del Olmo, M.; Varea, T.; Diaz, D.; Asensio, G. The introduction of fluorine atoms or trifluoromethyl groups in short cationic peptides enhances their antimicrobial activity. Bioorg. Med. Chem. 2006, 14, 6971–6978. [Google Scholar]
- Cherian, J.; Choi, I.; Nayyar, A.; Manjunatha, U.H.; Mukherjee, T.; Lee, Y.S.; Boshoff, H.I.; Singh, R.; Ha, Y.H.; Goodwin, M.; et al. Structure-activity relationships of antitubercular nitroimidazoles. 3. Exploration of the linker and lipohilic tail of ((S)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazin-6-yl)-(4-trifluoromethoxybenzyl)amine (6-amino PA-824). J. Med. Chem. 2011, 54, 5639–5659. [Google Scholar]
- Boonyawan, D.; Sarapirom, S.; Tunma, S.; Chaiwong, C.; Rachtanapun, P.; Auras, R. Characterization and antimicrobial properties of fluorine-rich carbon films deposited on poly(lactic acid). Surf. Coat. Technol. 2011, 205, S552–S557. [Google Scholar] [CrossRef]
- Privett, B.J.; Youn, J.; Hong, SA.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar]
- Gadakh, A.V.; Pandit, C.; Rindhe, S.S.; Karale, B.K. Synthesis and antimicrobial activity of novel fluorine containing 4-(substituted-2-hydroxybenzoyl)-H-pyrazoles and pyrazolylbenzo[d]oxazoles. Bioorg. Med. Chem. Lett. 2010, 20, 5572–5576. [Google Scholar]
- Luzzio, F.A. The Henry Reaction: Recent examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Werbel, L.M.; Cook, P.D.; Elslager, E.F.; Hung, J.H.; Johnson, J.L.; Kesten, S.J.; McNamara, D.J.; Ortwine, D.F.; Worth, D.F. Antimalarial drugs. 60. Synthesis, antimalarial activity, and quantitative structure-activity relationships of tebuquine and a series of related 5-[(7-chloro-4-quinolinyl)amino]-3-[(alkylamino)methyl][1,1'-biphenyl]-2-ols and N-oxides. J. Med. Chem. 1986, 29, 924–939. [Google Scholar] [CrossRef]
- Cote, A.; Lindsay, V.N.G.; Charette, A.B. Application of the chiralbis(phosphine) monoxide ligand to catalytic enantioselective addition of dialkylzinc reagents to beta -nitroalkenes. Org. Lett. 2007, 9, 85–87. [Google Scholar] [CrossRef]
- Bergner, I.; Opatz, T. Modular one-pot synthesis of tetrasubstitutedpyrroles from alpha-(alkylideneamino)nitriles. J. Org. Chem. 2007, 72, 7083–7090. [Google Scholar] [CrossRef]
- NCCLS, Reference Method for Broth Dilution Susceptibility Testing of Yeasts, 2nd ed; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002; Volume 22, 15, M27-A2.
- NCCLS. National Committee for Clinical Laboratory Standards, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Document M7-A6; NCCLS: Wayne, PA, USA, 2003; Volume 22.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lo, K.; Cornell, H.; Nicoletti, G.; Jackson, N.; Hügel, H. A Study of Fluorinated β-Nitrostyrenes as Antimicrobial Agents. Appl. Sci. 2012, 2, 114-128. https://doi.org/10.3390/app2010114
Lo K, Cornell H, Nicoletti G, Jackson N, Hügel H. A Study of Fluorinated β-Nitrostyrenes as Antimicrobial Agents. Applied Sciences. 2012; 2(1):114-128. https://doi.org/10.3390/app2010114
Chicago/Turabian StyleLo, King, Hugh Cornell, Gina Nicoletti, Neale Jackson, and Helmut Hügel. 2012. "A Study of Fluorinated β-Nitrostyrenes as Antimicrobial Agents" Applied Sciences 2, no. 1: 114-128. https://doi.org/10.3390/app2010114