A Facile Method to Prepare Superhydrophobic Coatings for Various Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coatings
2.3. Characterization
3. Results
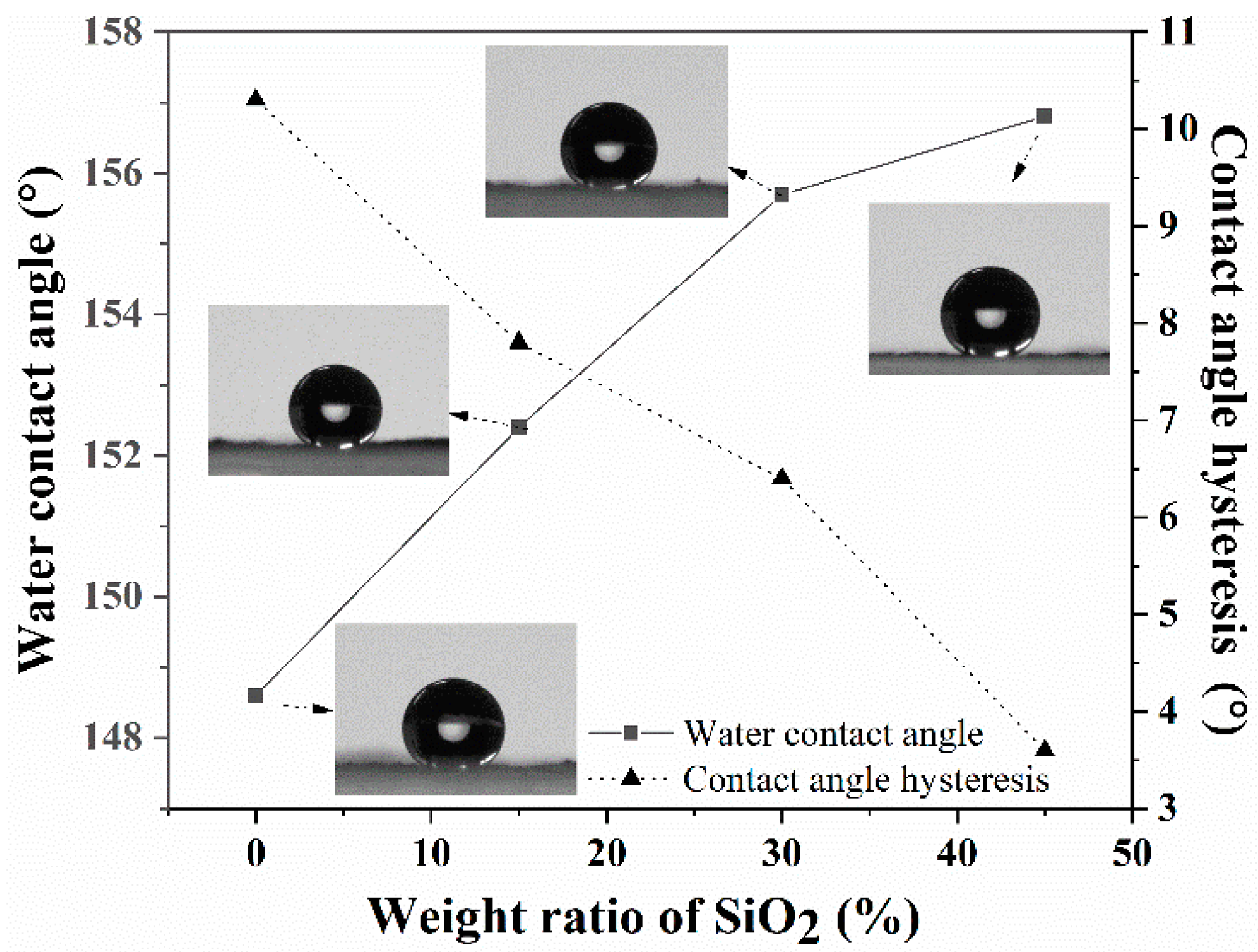
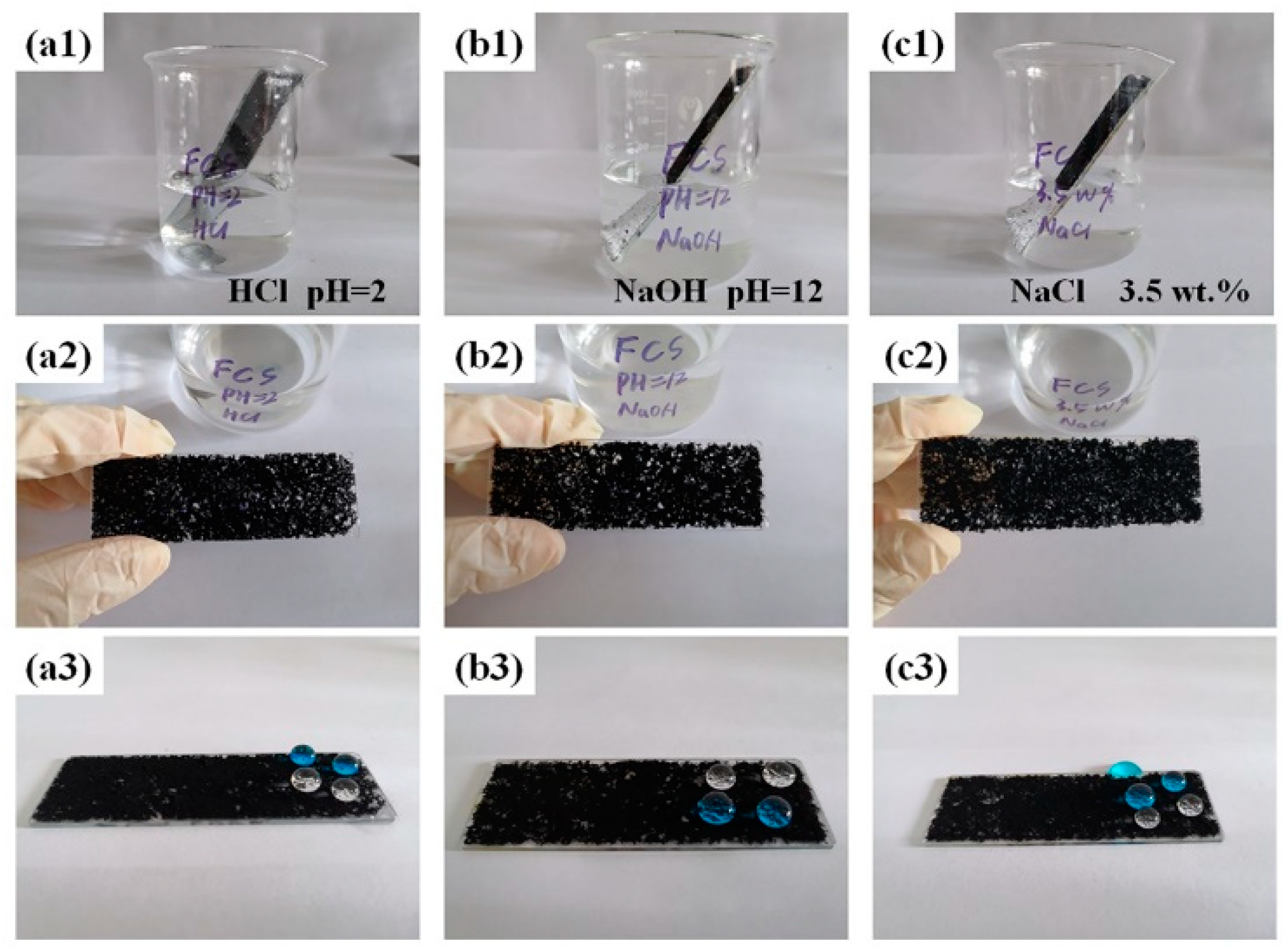
3.1. Preparation and Characterization of the Superhydrophobic Coatings
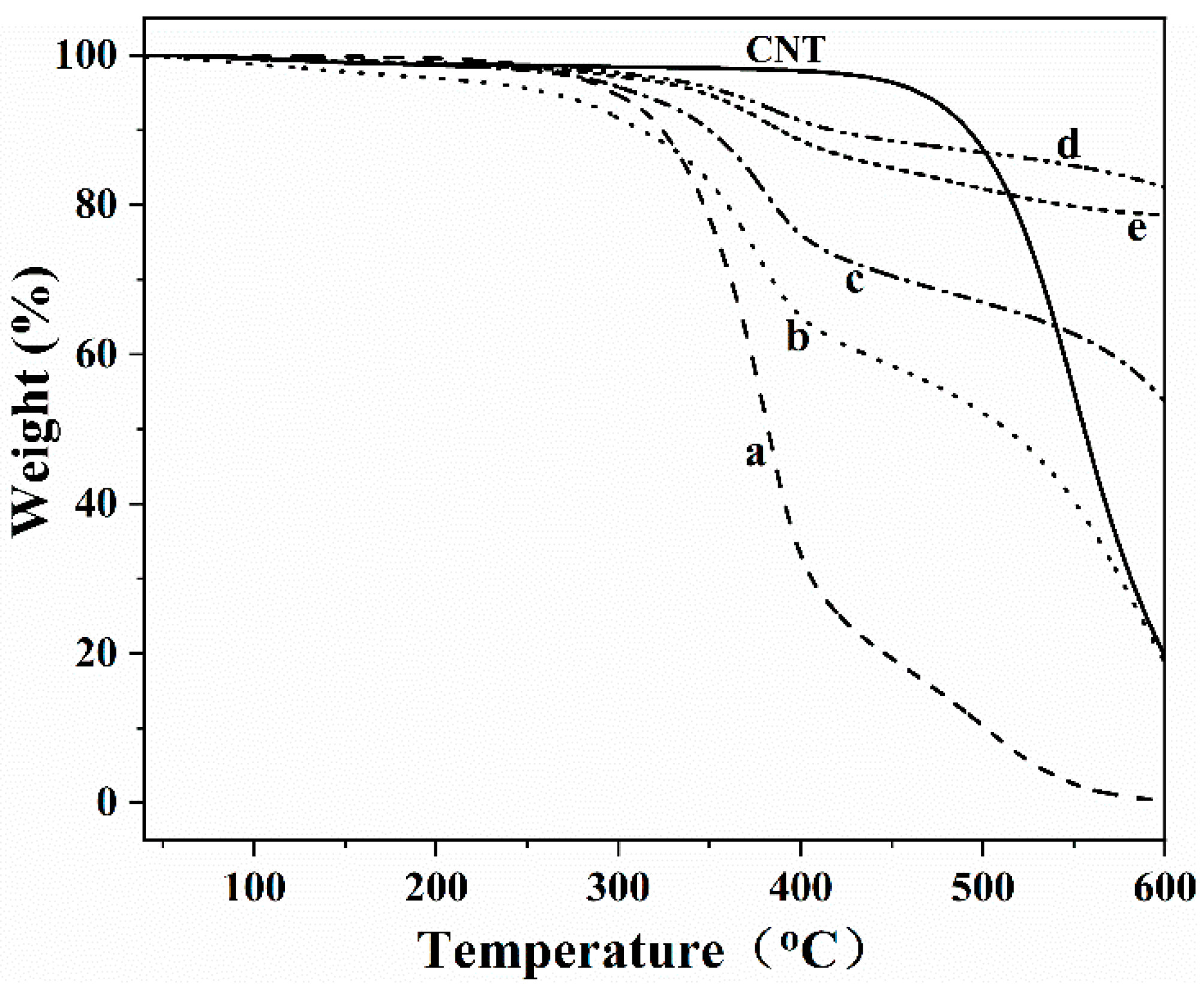
3.2. Thermal Stability Properties of the Superhydrophobic Coatings
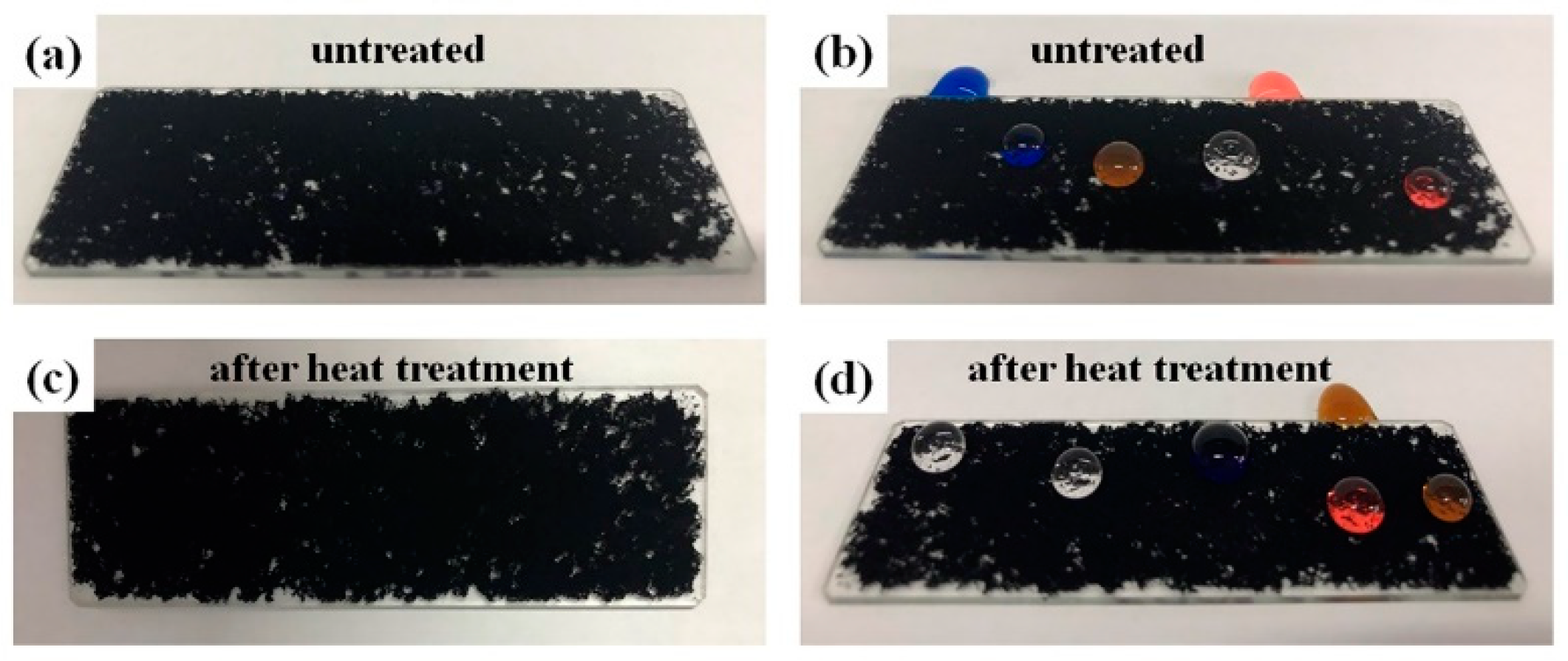
3.3. Mechanical Property of the Superhydrophobic Composite Coatings
3.4. The Self-Cleaning Property of the Superhydrophobic Composite Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wang, F.; Fan, H.; Hong, R.; Li, W. Construction of MOF-based superhydrophobic composite coating with excellent abrasion resistance and durability for self-cleaning, corrosion resistance, anti-icing, and loading-increasing research. Chem. Eng. J. 2021, 408, 127343. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Zhang, X.; Wang, C.; Liu, F.; Yuan, R.; Wang, H. Durable superhydrophobic PVDF/FEVE/GO@TiO2 composite coating with excellent anti-scaling and UV resistance properties. Chem. Eng. J. 2021, 411, 128632. [Google Scholar] [CrossRef]
- Xiao, X.; Xie, W.; Ye, Z. Preparation of corrosion-resisting superhydrophobic surface on aluminium substrate. Surf. Eng. 2019, 35, 411–417. [Google Scholar] [CrossRef]
- Jagdheesh, R.; Kopecek, J.; Brajer, J.; Mocek, T. Superhydrophobic microspiked surface structures by ultrashort laser patterning. Surf. Eng. 2021, 37, 1–11. [Google Scholar] [CrossRef]
- Zong, L.; Wu, Y.; Li, X.; Jiang, B. The preparation of superhydrophobic photocatalytic fluorosilicone/SiO2-TiO2 coating and its self-cleaning performance. J. Coat. Technol. Res. 2021, 18, 1245–1259. [Google Scholar] [CrossRef]
- Jafari, R.; Momen, G.; Eslami, E. Fabrication of icephobic aluminium surfaces by atmospheric plasma jet polymerisation. Surf. Eng. 2019, 35, 450–455. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, J.; Guo, Z. A CVD-Assisted Modification Approach for Preparing a Dual Superlyophobic Fabric with In-Air Superhydrophobicity and Underwater Superoleophobicity. Langmuir 2020, 36, 5802–5808. [Google Scholar] [CrossRef]
- Guo, X.-J.; Xue, C.-H.; Sathasivam, S.; Page, K.; He, G.; Guo, J.; Promdet, P.; Heale, F.L.; Carmalt, C.J.; Parkin, I.P. Fabrication of robust superhydrophobic surfaces via aerosol-assisted CVD and thermo-triggered healing of superhydrophobicity by recovery of roughness structures. J. Mater. Chem. A 2019, 7, 17604–17612. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; He, H.; Cao, R.; Miao, D.; Ning, X. Superhydrophobic cotton nonwoven fabrics through atmospheric plasma treatment for applications in self-cleaning and oil-water separation. Cellulose 2019, 26, 7507–7522. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Evaluation of atmospheric-pressure plasma parameters to achieve superhydrophobic and self-cleaning HTV silicone rubber surfaces via a single-step, eco-friendly approach. Surf. Coat. Technol. 2019, 375, 100–111. [Google Scholar] [CrossRef]
- Chu, J.H.; Sun, G.X.; Tong, L.B.; Jiang, Z.H. Facile one-step hydrothermal fabrication of Allium giganteum-like superhydrophobic coating on Mg alloy with self-cleaning and anti-corrosion properties. Colloids Surf. A 2021, 617, 126370. [Google Scholar] [CrossRef]
- Wang, M.; Peng, M.; Weng, Y.-X.; Li, Y.-D.; Zeng, J.-B. Toward durable and robust superhydrophobic cotton fabric through hydrothermal growth of ZnO for oil/water separation. Cellulose 2019, 26, 8121–8133. [Google Scholar] [CrossRef]
- Cui, M.; Xu, C.; Shen, Y.; Tian, H.; Feng, H.; Li, J. Electrospinning superhydrophobic nanofibrous poly(vinylidene fluoride)/stearic acid coatings with excellent corrosion resistance. Thin Solid Films 2018, 657, 88–94. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.J.; Wright, C.J.; Hilal, N. Robust superhydrophobic electrospun membrane fabricated by combination of electrospinning and electrospraying techniques for air gap membrane distillation. Desalination 2018, 446, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Pei, L.; Ambreen, J.; He, C.; Ngai, T. Facile Preparation of a Fluorine-Free, Robust, Superhydrophobic Coating through Dip Coating Combined with Non-Solvent Induced Phase Separation (Dip-Coating-NIPS) Method. Macromol. Chem. Phys. 2020, 221, 2070017. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Altiparmak, F.; Nguyen, N.; Tuduri, L.; Ouellet-Plamondon, C.M.; Prud’homme, R.E. Robust Superhydrophobic Cotton Fibers Prepared by Simple Dip-Coating Approach Using Chemical and Plasma-Etching Pretreatments. ACS Omega 2019, 4, 7829–7837. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. One-step facile fabrication of sustainable cellulose membrane with superhydrophobicity via a sol-gel strategy for efficient oil/water separation. Surf. Coat. Technol. 2019, 361, 19–26. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Latthe, S.S.; Gao, L.; An, S.; Yoon, S.S.; Liu, B.; Xing, R. Self-cleaning transparent superhydrophobic coatings through simple sol-gel processing of fluoroalkylsilane. Appl. Surf. Sci. 2015, 351, 897–903. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, Y.; Zhang, Y.; Jiang, W.; Wang, T.; Fu, J. Dual-templating synthesis of compressible and superhydrophobic spongy polystyrene for oil capture. Chem. Eng. J. 2018, 354, 245–253. [Google Scholar] [CrossRef]
- Tao, C.; Yan, H.; Yuan, X.; Yao, C.; Yin, Q.; Zhu, J.; Ni, W.; Yan, L.; Zhang, L. Synthesis of shape-controlled hollow silica nanostructures with a simple soft-templating method and their application as superhydrophobic antireflective coatings with ultralow refractive indices. Colloids Surf. A 2016, 501, 17–23. [Google Scholar] [CrossRef]
- Celik, N.; Altindal, S.; Gozutok, Z.; Ruzi, M.; Onses, M.S. Effect of fabric texture on the durability of fluorine-free superhydrophobic coatings. J. Coat. Technol. Res. 2020, 17, 785–796. [Google Scholar] [CrossRef]
- Hu, J.; Fang, Z.; Huang, Y.; Lu, J. Fabrication of superhydrophobic surfaces based on fluorosilane and TiO2/SiO2 nanocomposites. Surf. Eng. 2021, 37, 271–277. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhang, Z.; Kang, Y.; Sun, M.; Ma, L. Preparation of a superhydrophobic TiN/PTFE composite film toward self-cleaning and corrosion protection applications. J. Mater. Sci. 2021, 56, 1413–1425. [Google Scholar] [CrossRef]
- Hamzah, N.; Leo, C.P.; Ooi, B.S. Superhydrophobic PVDF/TiO2-SiO2 Membrane with Hierarchical Roughness in Membrane Distillation for Water Recovery from Phenolic Rich Solution Containing Surfactant. Chin. J. Polym. Sci. 2019, 37, 609–616. [Google Scholar] [CrossRef]
- Jia, W.; Kharraz, J.A.; Guo, J.; An, A.K. Superhydrophobic (polyvinylidene fluoride-co-hexafluoropropylene)/(polystyrene) composite membrane via a novel hybrid electrospin-electrospray process. J. Membr. Sci. 2020, 611, 118360. [Google Scholar] [CrossRef]
- Shen, Y.; Li, K.; Chen, H.; Wu, Z.; Wang, Z. Superhydrophobic F-SiO2@PDMS composite coatings prepared by a two-step spraying method for the interface erosion mechanism and anti-corrosive applications. Chem. Eng. J. 2021, 413, 127455. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, Y.; Gao, C.; Liu, Y. Hierarchical Cu2O/CuO/TiO2 composite films with high superhydrophobicity and strong adhesion. Surf. Eng. 2021, 37, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, A.; Gu, H.; Qin, G.; Zhang, J.; Xu, J.; Jiang, G.; Liu, W.; Zhang, Z.; Huang, H. Highly interconnected porous PDMS/CNTs sandwich sponges with anti-icing/deicing microstructured surfaces. J. Mater. Sci. 2021, 56, 11723–11735. [Google Scholar] [CrossRef]
- Ding, Y.-R.; Xue, C.-H.; Guo, X.-J.; Wang, X.; Jia, S.-T.; An, Q.-F. Fabrication of TPE/CNTs film at air/water interface for flexible and superhydrophobic wearable sensors. Chem. Eng. J. 2021, 409, 128199. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Sun, S.; Zhang, H.; Zhou, R.; Li, Y.; Liang, Y.; Wang, J. Preparation of a temperature-sensitive superhydrophobic self-cleaning SiO2-TiO2@PDMS coating with photocatalytic activity. Surf. Coat. Technol. 2021, 408, 126853. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Huang, Y.; Lv, Y.; Kong, M.; Li, G. Durable fluorinated-SiO2/epoxy superhydrophobic coatings on polycarbonate with strong interfacial adhesion enhanced by solvent-induced crystallization. Prog. Org. Coat. 2021, 150, 106002. [Google Scholar] [CrossRef]
- Liang, Z.; Geng, M.; Dong, B.; Zhao, L.; Wang, S. Transparent and robust SiO2/PDMS composite coatings with self-cleaning. Surf. Eng. 2020, 36, 643–650. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Grant Chen, X. Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties. Appl. Surf. Sci. 2015, 356, 1012–1024. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, S.; Lv, Z.; Fan, L.; Huang, Y.; Liu, X. A Facile Method to Prepare Superhydrophobic Coatings for Various Substrates. Appl. Sci. 2022, 12, 1240. https://doi.org/10.3390/app12031240
Zhang Y, Zhou S, Lv Z, Fan L, Huang Y, Liu X. A Facile Method to Prepare Superhydrophobic Coatings for Various Substrates. Applied Sciences. 2022; 12(3):1240. https://doi.org/10.3390/app12031240
Chicago/Turabian StyleZhang, Yuxuan, Shuwen Zhou, Zaosheng Lv, Lixia Fan, Yanfen Huang, and Xuegang Liu. 2022. "A Facile Method to Prepare Superhydrophobic Coatings for Various Substrates" Applied Sciences 12, no. 3: 1240. https://doi.org/10.3390/app12031240





