Creep Aging Behavior Characterization of 2219 Aluminum Alloy
Abstract
:1. Introduction
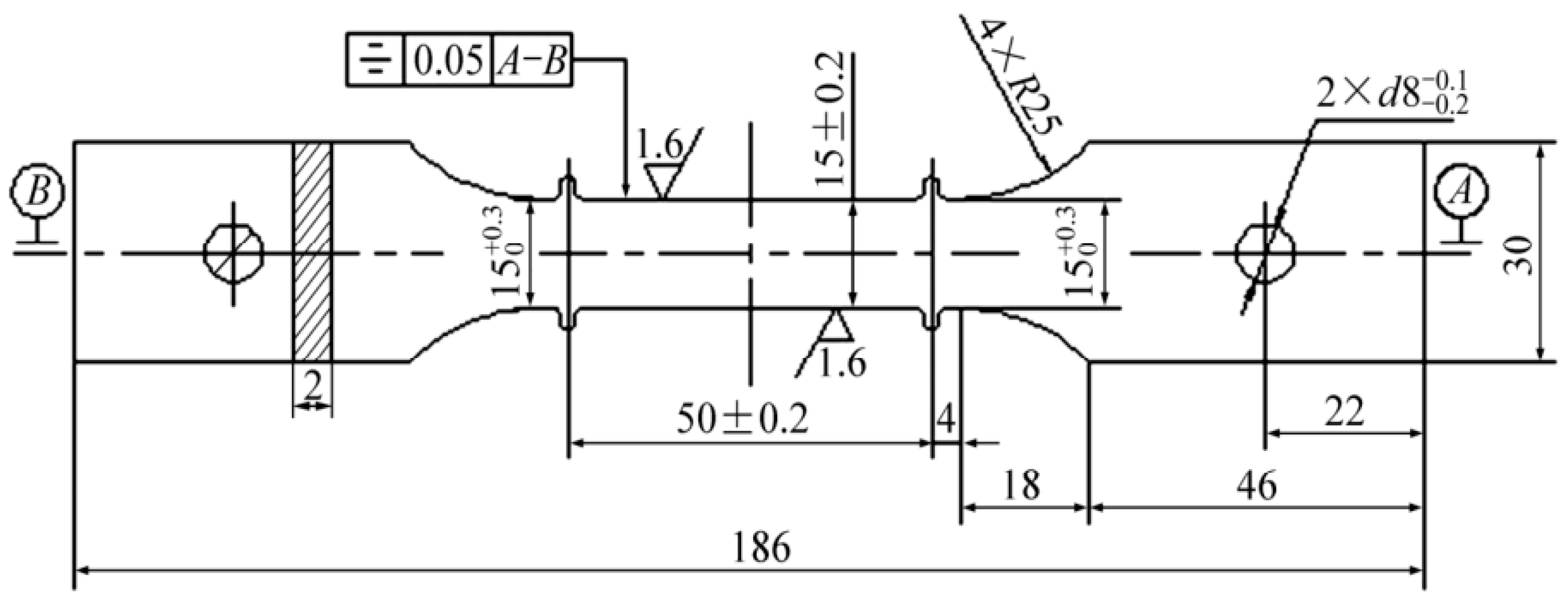
2. Materials and Methods
3. Results and Discussion
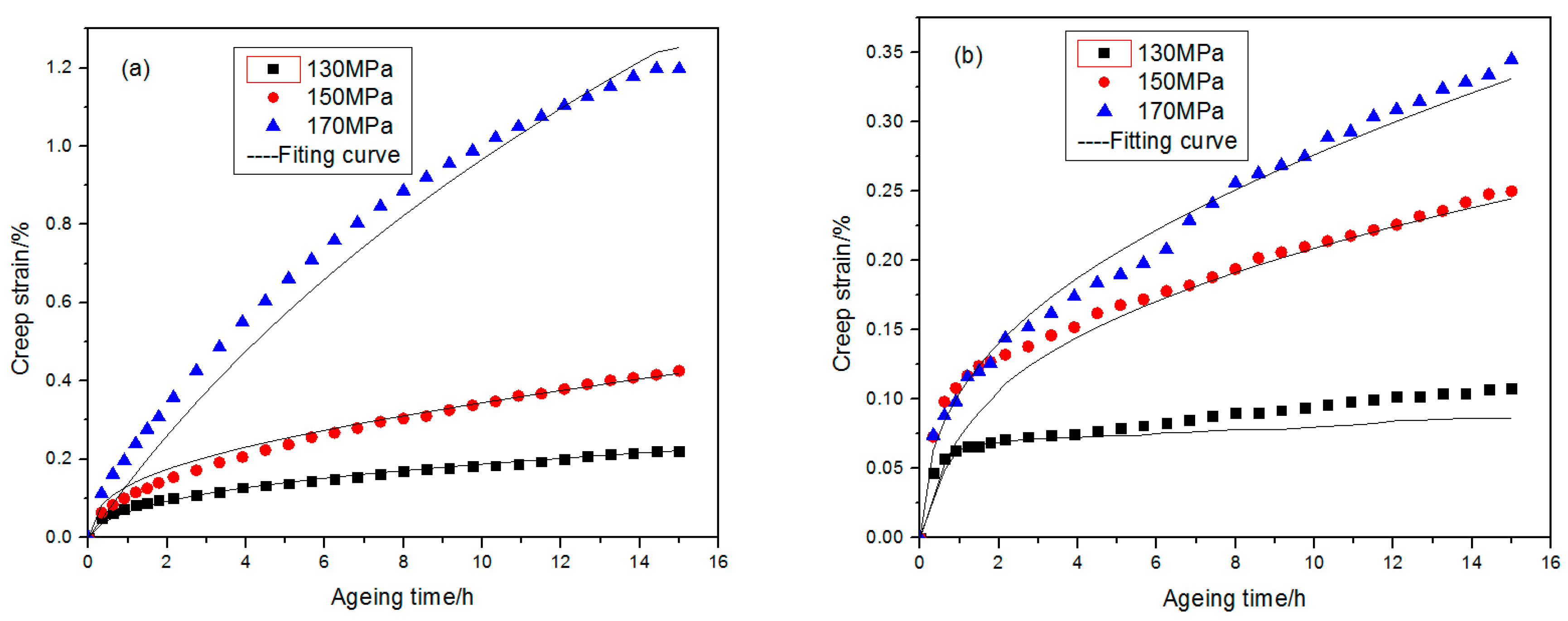
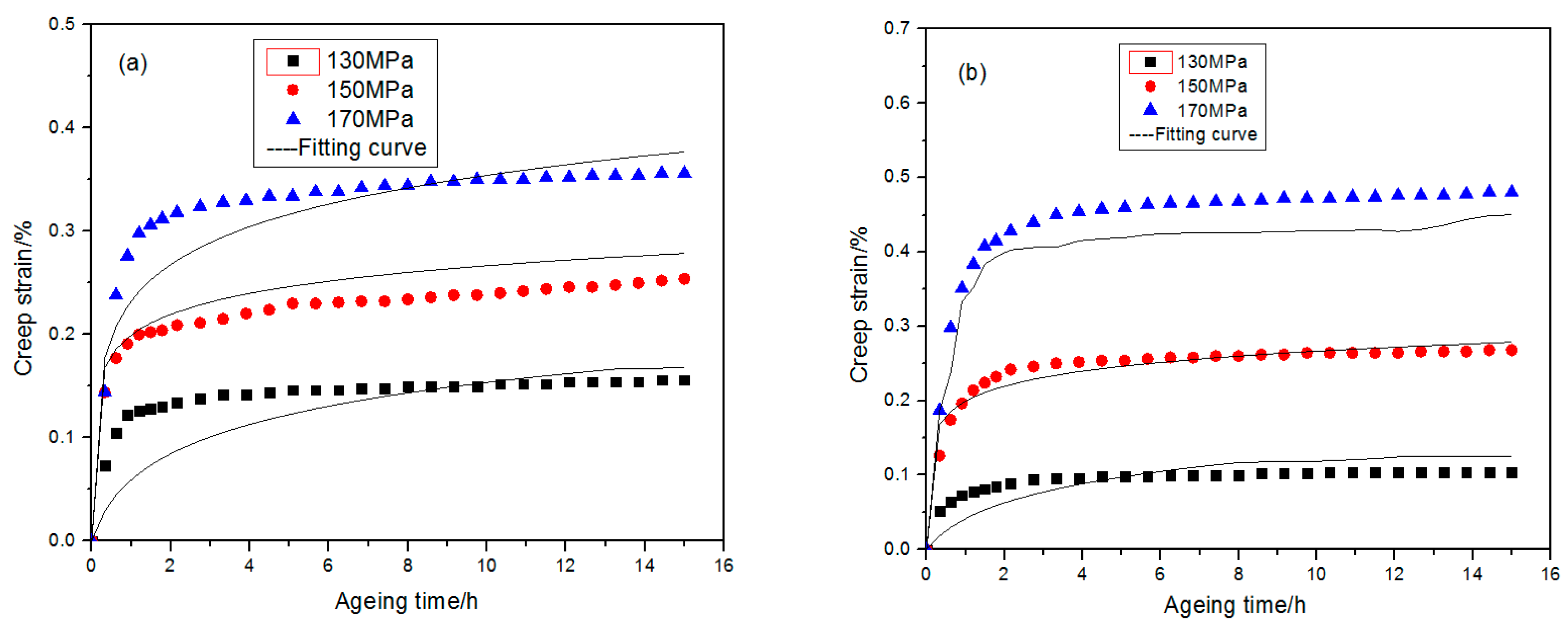
3.1. Alloy Creep Behaviors
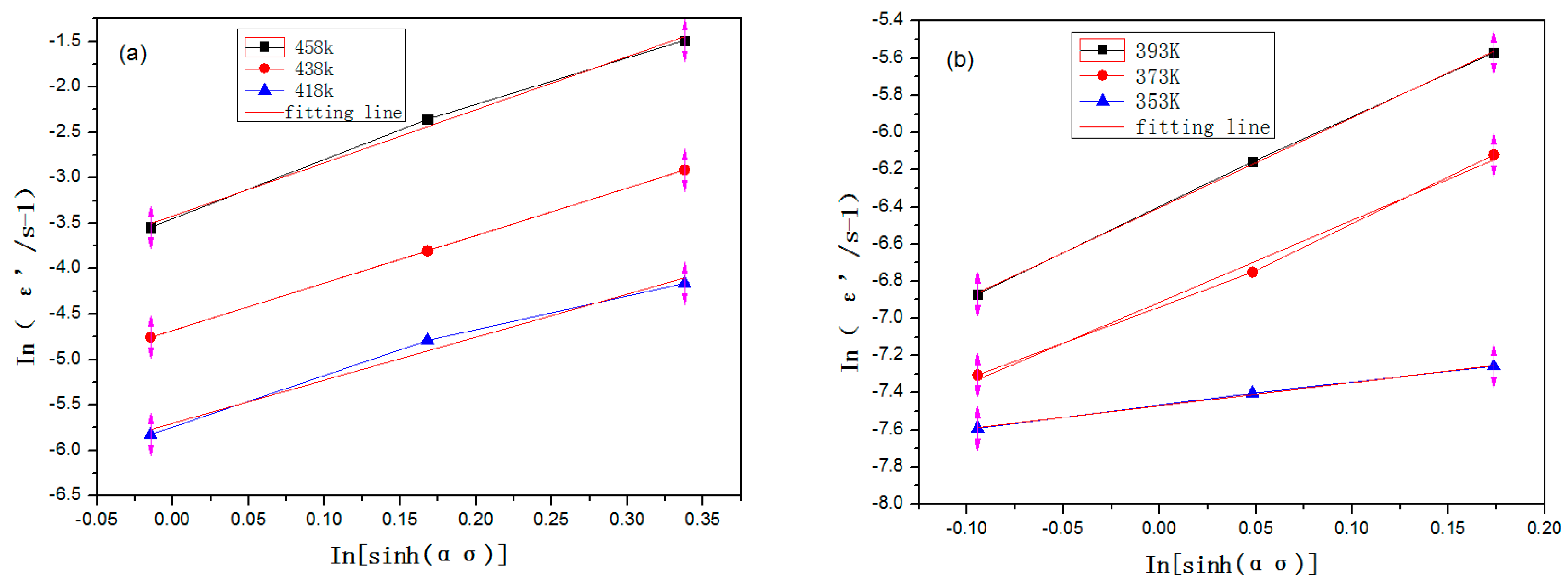
3.2. Computational Analysis of Creep Mechanism and Constitutive Equation Setup
4. Conclusions
- (1)
- This paper carried out creep experiments for 2219 aluminum alloy at different temperature and stress conditions separately. It was found that stress state at a lower temperature is the principal factor affecting the creep aging behaviors of 2219 aluminum alloy.
- (2)
- At different aging temperatures, 2219 aluminum alloy displayed different creep mechanisms. When T ≥ 418 K, stress exponents n = 4~6 and fell in the category of the dislocation climb mechanism; when 373 K ≤ T < 418 K, n = 4~6 and fell in the category of the dislocation glide mechanism; when the temperature was around 353 K, n ≈ 1 and fell in the category of the grain boundary sliding mechanism. When the temperature was lower than 353 K, the alloy can be basically considered with no creep.
- (3)
- By processing and analysis of the experimental data, the stress exponent and activation energy under different conditions were calculated. Coupled with revision of the classical creep equation, a creep constitutive model applicable to different temperatures and stress conditions was established, which proved itself with great consistence with the experimental data.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sallah, M.; Peddieson, J., Jr.; Foroudastan, S. A mathematical model of autoclave age forming. J. Mater. Process Technol. 1991, 28, 211–219. [Google Scholar] [CrossRef]
- Zeng, Y.-S.; Huang, X.; Huang, S. The research situation and the developing tendency of creep age forming technology. J. Plast. Eng. 2008, 15, 1–8. [Google Scholar]
- Kowalewski, Z.L.; Hayhurst, D.R.; Dyson, B.F. Meachanisms-based creep constitutive equations for aluminum alloy. J. Strain Anal. 1994, 29, 309–316. [Google Scholar] [CrossRef]
- Ho, K.C.; Lin, J.; Dean, T.A. Constitutive modelling of primary creep for age forming an aluminium alloy. J. Mater. Process Technol. 2004, 153, 122–127. [Google Scholar] [CrossRef]
- Ho, K.C.; Lin, J.; Dean, T.A. Modelling of springback in creep forming thick aluminum sheets. Int. J. Plast. 2004, 20, 733–751. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Yang, J.; Zhang, X. Experimental studies and constitutive modeling for creep aging of 2124 aluminum alloy. Acta Metall. Sin. 2013, 49, 379–384. [Google Scholar] [CrossRef]
- Guan, C.; He, W.; Zhao, Z.; Wang, G. Creep characteristics of 2024 alloy at cryogenic temperatures. J. Aeronaut. Mater. 2014, 34, 93–96. [Google Scholar] [CrossRef]
- An, L.; Cai, Y.; Liu, W.; Yuan, S.; Zhu, S.; Meng, F. Effect of pre-deformation on microstructure and mechanical properties of 2219 aluminum alloy sheet by thermomechanical treatment. Trans. Nonferr. Met. Soc. China 2012, 22, s370–s375. [Google Scholar] [CrossRef]
- Zhan, L.; Li, J.; Huang, M. Creep aging behavior and constitutive equation of 2524 aluminum alloy. Mater. Mech. Eng. 2013, 37, 92–96. [Google Scholar]
- Wang, J. A Study on Creep Behavior of Titanium Alloy; Harbin Institute Technology: Harbin, China, 2008; pp. 39–41. [Google Scholar]
- Somekawa, H.; Hirai, K.; Watanabe, H.; Takigawa, Y.; Higashi, K. Dislocation creep behavior in Mg-Al-Zn alloys. Mater. Sci. Eng. 2005, A407, 53–61. [Google Scholar] [CrossRef]
- Li, Y.G. Experimental Study on Creep Aging Behavior and Constitutive Modeling of 2124 Aluminum Alloy. Master’s Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Mckamey, C.G.; Maziasz, P.J.; Jones, J.W. Effect of addition of molybdenum or niobium on creep rupture properties of Fe3Al. J. Mater. Res. 1997, 7, 2089–2106. [Google Scholar]
- Mary, A.W. Properties of Materials; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Yang, S.; Li, J.; Wei, S.; Xu, L.; Zhang, G.; Zhang, E. Pyroplastic deformation behavior of pure molybdenum plate slab and constitutive equation. Trans. Nonferr. Met. Soc. China 2011, 21, 2126–2131. [Google Scholar]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics; Pergamon Press: Oxford, UK, 1982; pp. 102–112. [Google Scholar]
- Li, B.; Lin, J.; Yao, X. Characteristics of optimization of creep constitutive equations. Chin. J. Mech. Eng. 2003, 39, 37–39. [Google Scholar] [CrossRef]
- Zhan, L.; Li, Y.; Huang, M.; Zhang, M. Constitutive equation describing creep ageing of 2124 aluminum alloy. J. South China Univ. Technol. 2012, 40, 107–111. [Google Scholar]
- Shi, X.H.; Liang, Y.C.; Lee, H.P.; Lu, C.; Wang, L.M. An improved GA and a novel PSO-GA-based hybrid algorithm. Inform. Process. Lett. 2005, 9, 255–261. [Google Scholar] [CrossRef]



| Chemical Composition | Cu | Mg | Mn | Si | Fe | Ni | Zr | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| Mass fraction | 5.24 | 0.028 | 0.27 | 0.042 | 0.13 | 0.03 | 0.14 | 0.065 | Bal |
| Temperatures | n1 | α | β |
|---|---|---|---|
| 458 K | 7.76 | 0.00699 | 0.0543 |
| 438 K | 6.84 | 0.00676 | 0.0543 |
| 418 K | 6.22 | 0.00671 | 0.0416 |
| 393 K | 4.93 | 0.00704 | 0.0350 |
| 373 K | 4.44 | 0.00673 | 0.0296 |
| 353 K | 1.32 | 0.00695 | 0.00834 |
| Aging Temperatures | Stress Exponent n |
|---|---|
| 458 K | 5.91 |
| 438 K | 5.17 |
| 418 K | 4.74 |
| 393 K | 3.81 |
| 373 K | 3.35 |
| 353 K | 1.23 |
| Material Constants | A | B | σ0 | m0 | h | H* | m1 | R2 |
|---|---|---|---|---|---|---|---|---|
| Number | 4 × 103 | 0.14998 | 128.1 | 8.214 | 239.76 | 0.3971 | 0.004223 | 0.9727 |
| Material Constants | A | B | σ0 | m0 | h | H* | m1 | R2 |
|---|---|---|---|---|---|---|---|---|
| Number | 8 × 103 | 0.01968 | 129.4 | 9.96 | 491.82 | 0.8499 | 0.164473 | 0.9028 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhan, L.; Li, W. Creep Aging Behavior Characterization of 2219 Aluminum Alloy. Metals 2016, 6, 146. https://doi.org/10.3390/met6070146
Liu L, Zhan L, Li W. Creep Aging Behavior Characterization of 2219 Aluminum Alloy. Metals. 2016; 6(7):146. https://doi.org/10.3390/met6070146
Chicago/Turabian StyleLiu, Lingfeng, Lihua Zhan, and Wenke Li. 2016. "Creep Aging Behavior Characterization of 2219 Aluminum Alloy" Metals 6, no. 7: 146. https://doi.org/10.3390/met6070146
APA StyleLiu, L., Zhan, L., & Li, W. (2016). Creep Aging Behavior Characterization of 2219 Aluminum Alloy. Metals, 6(7), 146. https://doi.org/10.3390/met6070146






