Synthesis and Characterization of Nanocrystalline Al-20 at. % Cu Powders Produced by Mechanical Alloying
Abstract
:1. Introduction
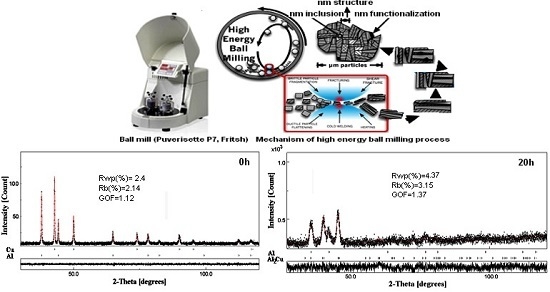
2. Materials and Methods
3. Results and Discussion
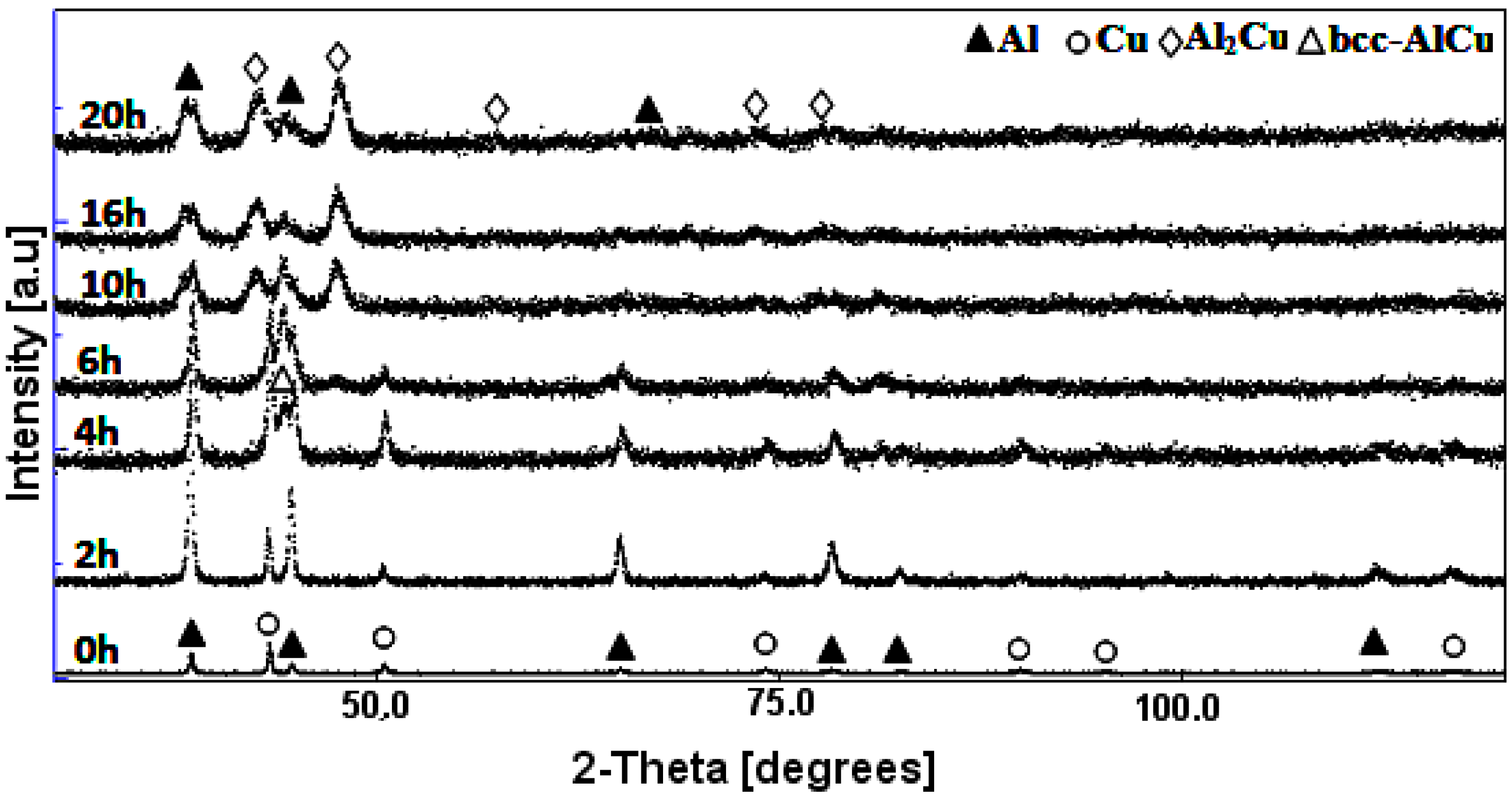
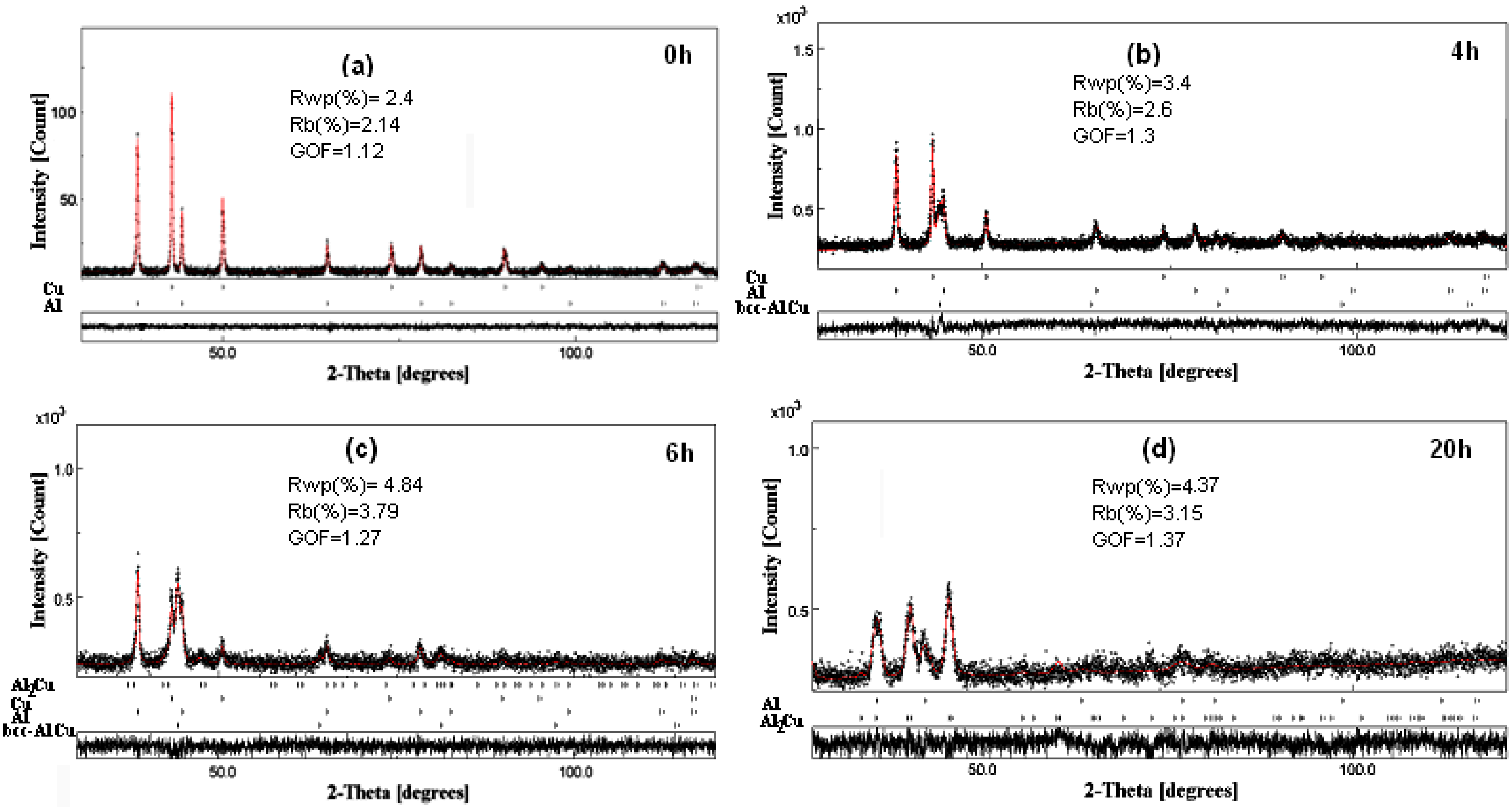
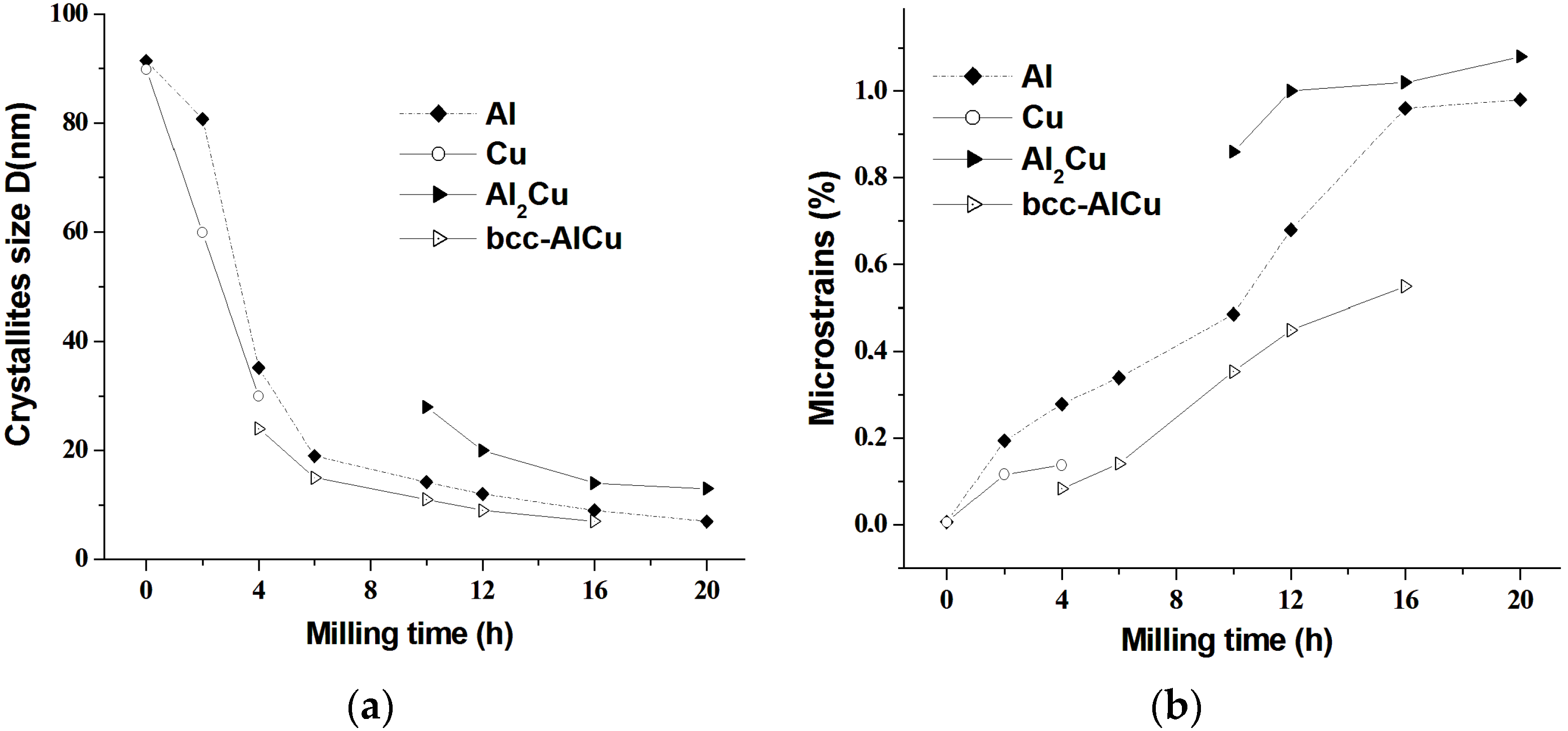
3.1. X-ray Diffraction
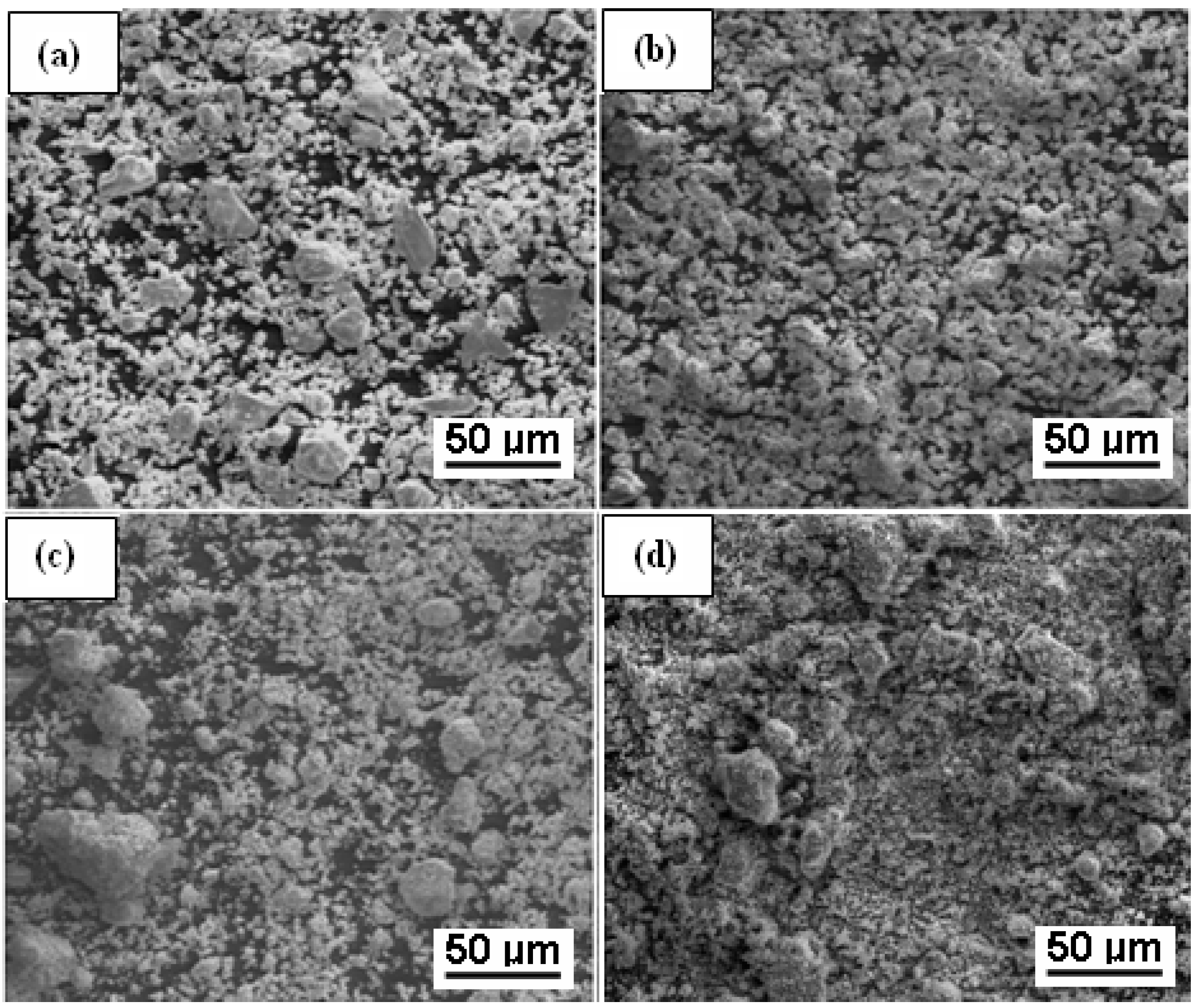
3.2. Scanning Electron Microscopy
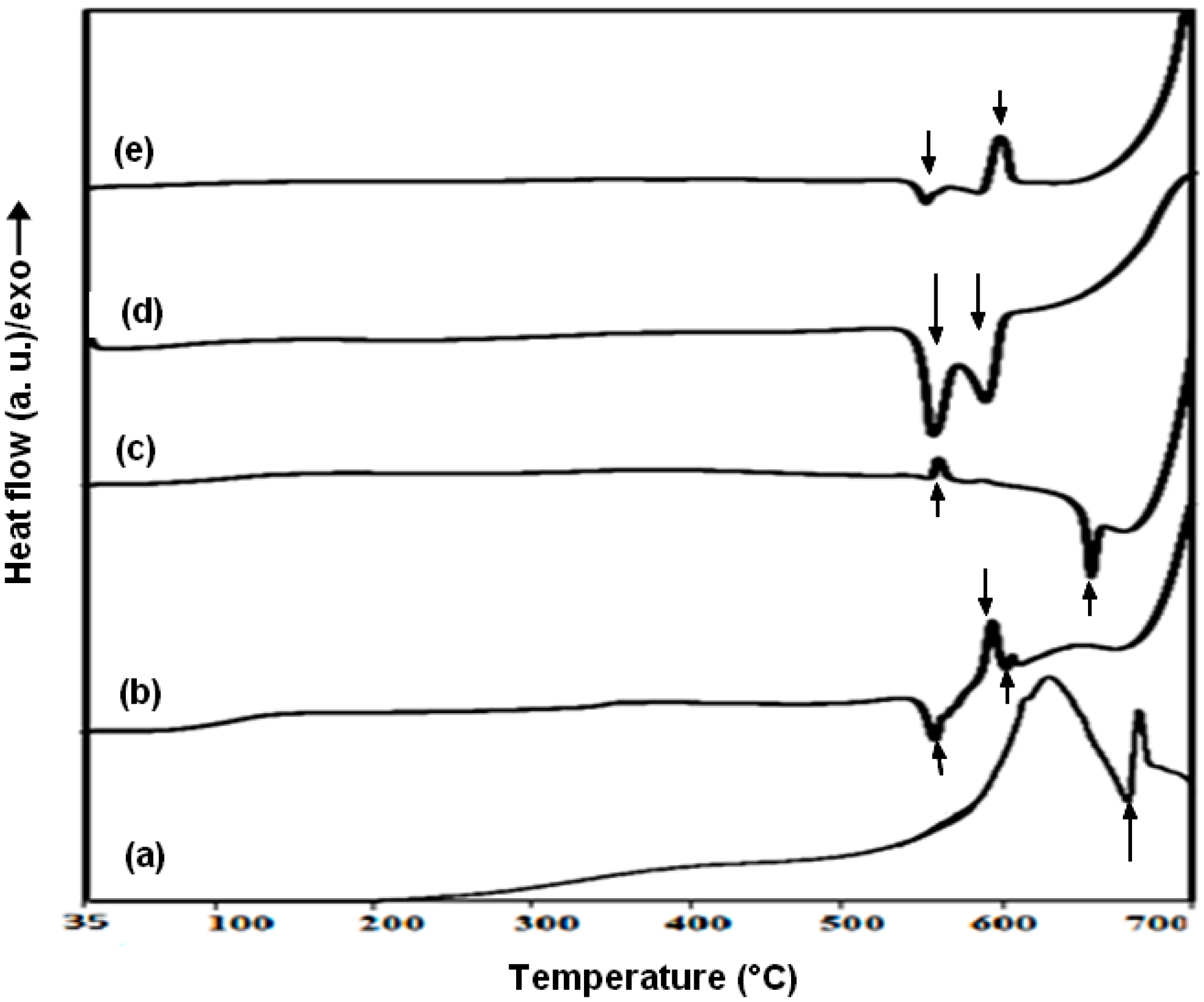
3.3. Thermal Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bachaga, T.; Daly, R.; Escoda, L.; Suñol, J.J.; Khitouni, M. Amorphization of Al50(Fe2B)30Nb20 mixture by mechanical alloying. J. Metall. Mater. Trans. A 2013, 44, 4718–4724. [Google Scholar] [CrossRef]
- Esparza, R.; Rosas, G.; Ascencio, J.A.; Pérez, R. Effects of minor element additions to the nanocrystalline FeAl intermetallic alloy obtained by mechanical alloying. Mater. Manuf. Process. 2005, 20, 823–832. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Khanduja, D. On the use of ball milling for the production of ceramic powders. Mater. Manuf. Process. 2015, 30, 1370–1376. [Google Scholar] [CrossRef]
- Chittineni, K.; Bhat, D.G. X-ray Diffraction investigation of the formation of nanostructured metastable phases during short-duration mechanical alloying of Cu-Al powder mixtures. Mater. Manuf. Process. 2006, 21, 527–533. [Google Scholar] [CrossRef]
- Chattopadhyay, P.P.; Mann, I. Effect of Partial Substitution of Cu in Al65Cu35 by Transition Metal in Mechanical Alloying of Al65Cu20TM15. Mater. Manuf. Process. 2002, 17, 583–594. [Google Scholar] [CrossRef]
- Qiu, Y.; Gu, M.L.; Zhang, F.G.; Wei, Z. Influence of Tool Inclination on Micro-Ball-End Milling of Quartz Glass. Mater. Manuf. Process. 2014, 29, 1436–1440. [Google Scholar] [CrossRef]
- Archana, M.S.; Ramakrishna, M.; Ravi, C.; Gundakaram, V.V.; Srikanth, S.S.; Joshi, S.V. Nanocrystalline Phases during Mechanically Activated Processing of an Iron (Fe)-Aluminium(40 at.% Al) Alloy. Mater. Manuf. Process. 2014. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Qingquan, K.; Lian, L.; Liu, Y.; Zhang, J. Fabrication and Characterization of Nanocrystalline Al-Cu Alloy by Spark Plasma Sintering. Mater. Manuf. Process. 2014, 29, 1232–1236. [Google Scholar]
- Eckert, J.; Holzer, J.C.; Krill, C.E.; Johnson, W.L. Mechanically driven alloying and grain size changes in Non crystalline Fe-Cu powders. J. Appl. Phys. 1993, 73, 2794–2802. [Google Scholar] [CrossRef]
- Slimi, M.; Azabou, M.; Escoda, L.; Suñol, J.J.; Khitouni, M. Stacking faults and structural characterization of mechanically alloyed Ni50Cu10(Fe2B)10P30 powders. Eur. Phys. J. Plus 2015, 130, 1–8. [Google Scholar] [CrossRef]
- Murray, J.L. The aluminium-copper system. Int. Met. Rev. 1985, 30, 211–233. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Novelty, OH, USA, 1990. [Google Scholar]
- Nguyen, L.T.; McDonald, D.; Danker, A.R.; Ng, P. Optimization of copper wire bonding on Al-Cu metallization. IEEE Trans. Compon. Packag. Manuf. Technol. A 1995, 18, 423–429. [Google Scholar] [CrossRef]
- Premkumar, J.; Kumar, B.S.; Madhu, M.; Sivakumar, M.; Song, K.Y.J.; Wong, Y.M. Key factors in Cu wire bonding reliability: Remnant aluminum and Cu/Al IMC thickness. In Proceedings of the 10th Electronics Packaging Technology Conference, Singapore, 9–12 December 2008.
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Chen, Z. A re-examination of the mechanism of thermosonic copper ball bonding on aluminium metallization pads. Scr. Mater. 2009, 61, 165–168. [Google Scholar] [CrossRef]
- Li, F.; Ishihara, K.N.; Singu, P.H. The Formation of Metastable Phases by Mechanical Alloying in the Aluminum and Copper System. Metall. Trans. A 1991, 22, 2849–2854. [Google Scholar] [CrossRef]
- Chattopadhyay, P.P. Ph.D. Thesis, Indian Institute of Technology, Kharagpur, India, 2000.
- Lutterotti, L. MAUD, Int. Union of Crystallography CPD Newsletter (IUCr), No. 24. 2000. [Google Scholar]
- Onuki, J.; Koizumi, M. Investigation of the Reliability of Copper Ball Bonds to Aluminum Electrodes. IEEE Trans. Compon. Hybrids Manuf. Technol. 1987, 12, 550–555. [Google Scholar] [CrossRef]
- Sui, M.L.; Lu, K. Variation in lattice parameters with grain size of a nanophase Ni-3P compound. Mater. Sci. Eng. A 1994, 541, 179–180. [Google Scholar]
- Eckert, J.; Holzer, J.C.; Johnson, W.L. Thermal stability and grain growth behavior of mechanically alloyed nanocrystalline Fe-Cu alloys. J. Appl. Phys. 1993, 73, 131–141. [Google Scholar] [CrossRef]
- Mohamed, F.A. A dislocation model for the minimum grain size obtainable by milling. Acta Mater. 2003, 51, 4107–4119. [Google Scholar] [CrossRef]
- Kalita, M.P.C.; Perumal, A.; Srinivasan, A. Structure and magnetic properties of nanocrystalline Fe75Si25 powders prepared by mechanical alloying. J. Magn. Magn. Mater. 2000, 320, 2780–2783. [Google Scholar] [CrossRef]
- Das, S.; Peperezko, J.; Wu, R.; Wilde, G. Under cooling and glass formation in Al-based alloys. Mater. Sci. Eng. A 2001, 159, 304–306. [Google Scholar]
- Ying, D.Y.; Zhang, D.L. Solid-state reactions between Cu and Al during mechanical alloying and heat treatment. J. Alloy. Compd. 2000, 311, 275–282. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhlouf, M.B.; Bachaga, T.; Sunol, J.J.; Dammak, M.; Khitouni, M. Synthesis and Characterization of Nanocrystalline Al-20 at. % Cu Powders Produced by Mechanical Alloying. Metals 2016, 6, 145. https://doi.org/10.3390/met6070145
Makhlouf MB, Bachaga T, Sunol JJ, Dammak M, Khitouni M. Synthesis and Characterization of Nanocrystalline Al-20 at. % Cu Powders Produced by Mechanical Alloying. Metals. 2016; 6(7):145. https://doi.org/10.3390/met6070145
Chicago/Turabian StyleMakhlouf, Molka Ben, Tarek Bachaga, Joan Josep Sunol, Mohamed Dammak, and Mohamed Khitouni. 2016. "Synthesis and Characterization of Nanocrystalline Al-20 at. % Cu Powders Produced by Mechanical Alloying" Metals 6, no. 7: 145. https://doi.org/10.3390/met6070145
APA StyleMakhlouf, M. B., Bachaga, T., Sunol, J. J., Dammak, M., & Khitouni, M. (2016). Synthesis and Characterization of Nanocrystalline Al-20 at. % Cu Powders Produced by Mechanical Alloying. Metals, 6(7), 145. https://doi.org/10.3390/met6070145