Examination of Solubility Models for the Determination of Transition Metals within Liquid Alkali Metals
Abstract
:1. Introduction
2. Solubility Equations
2.1. Enthalpy Calculation
2.2. Entropy Calculation
3. Results
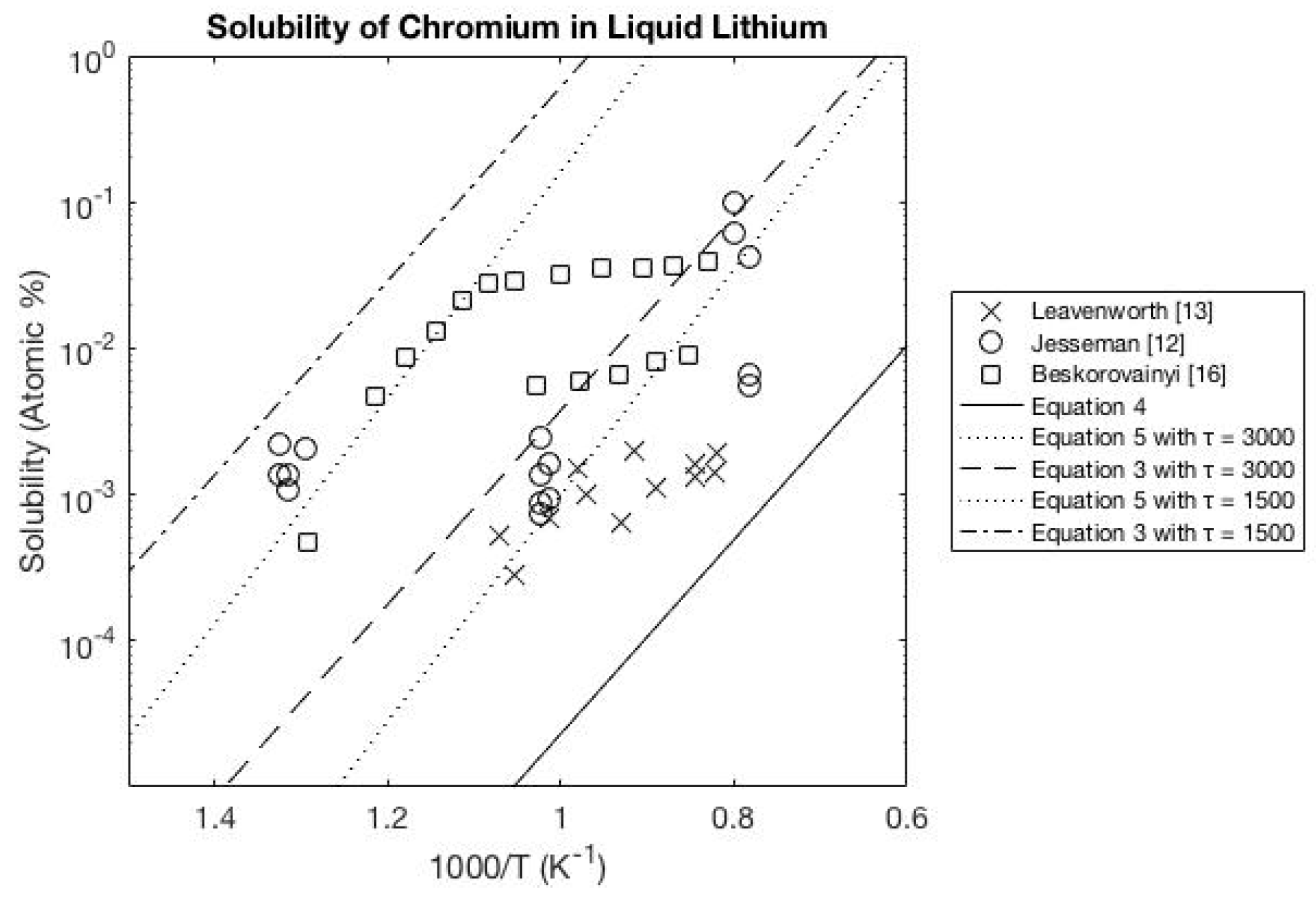
3.1. Lithium
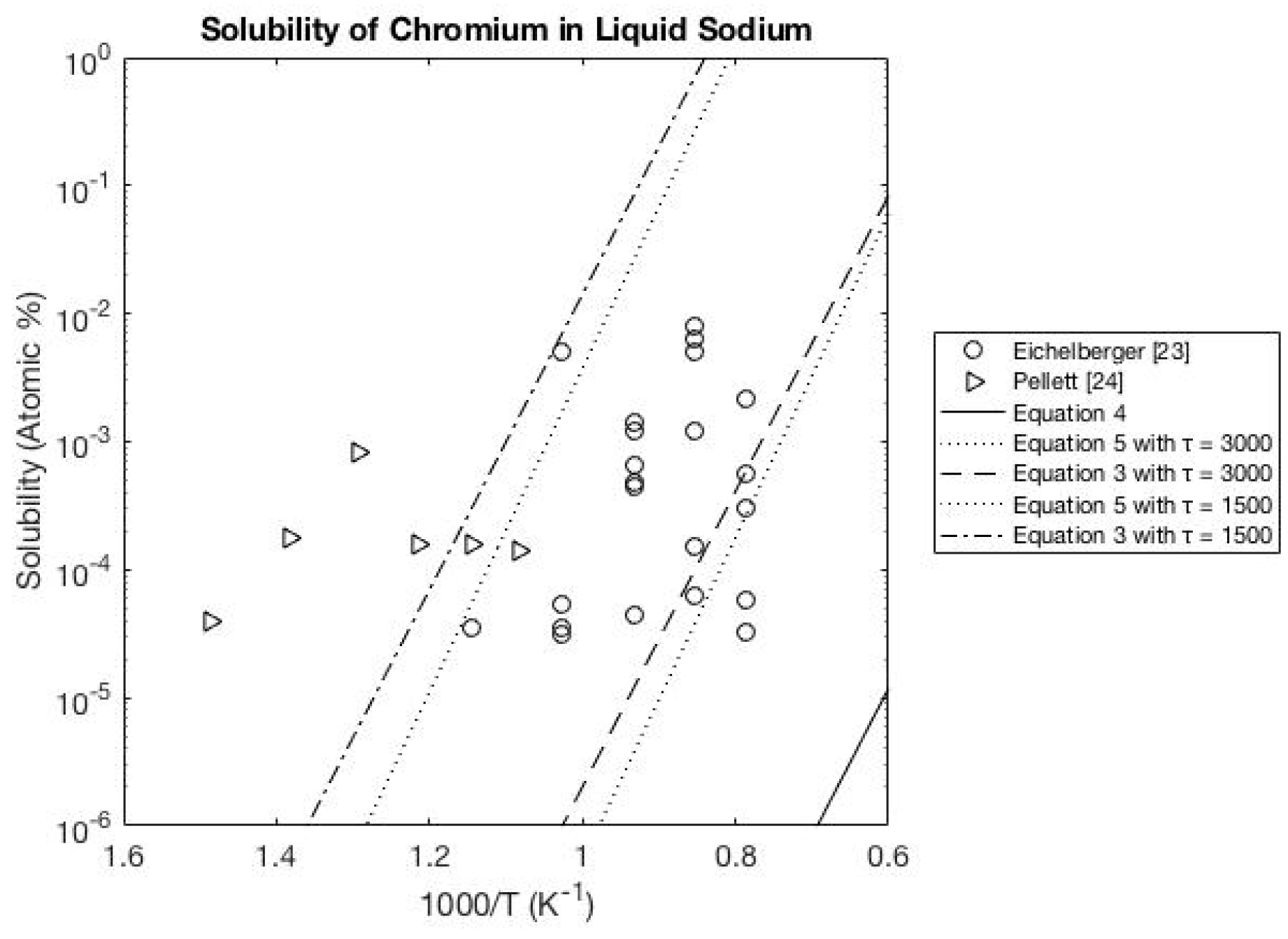
3.2. Sodium
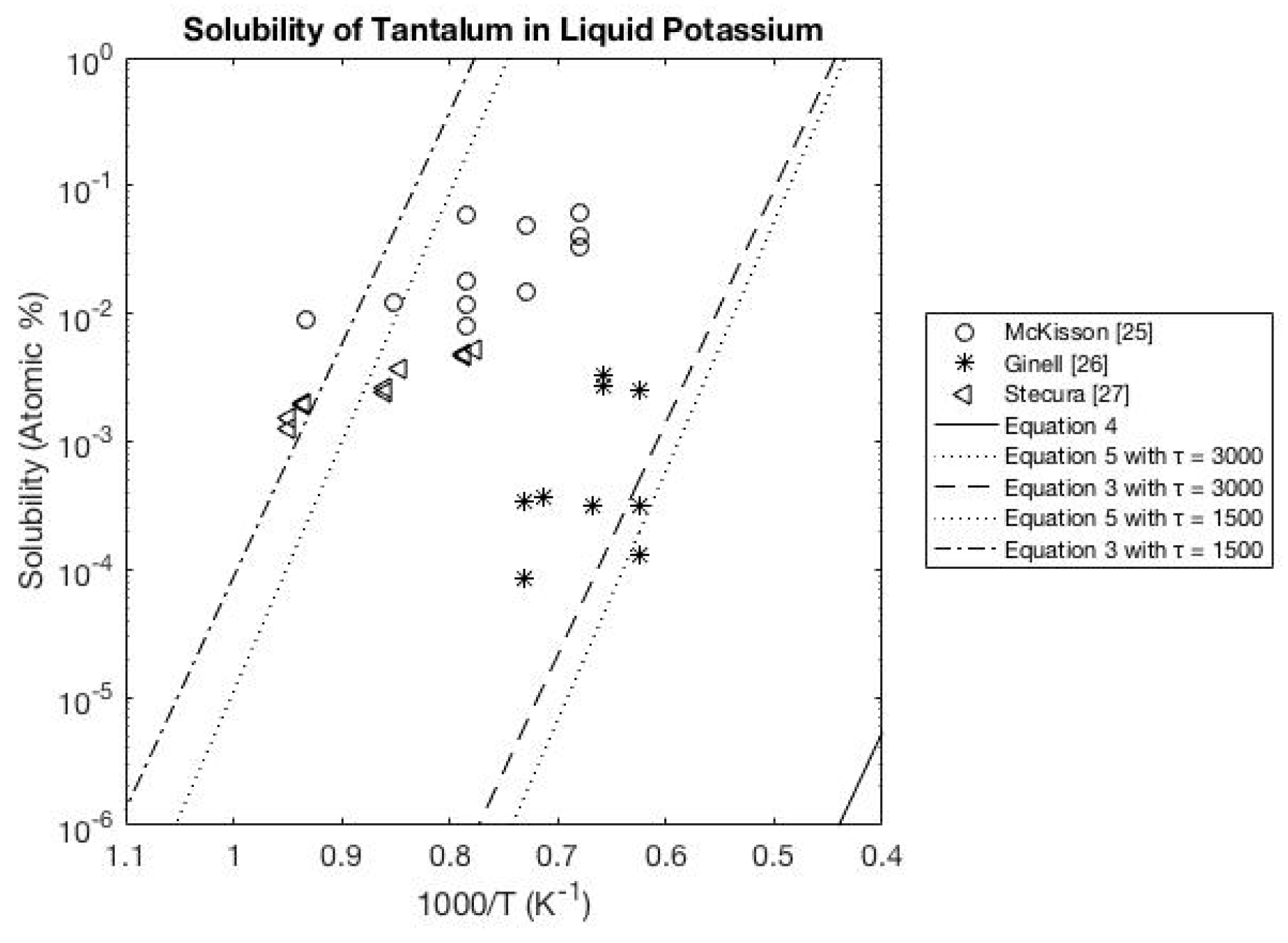
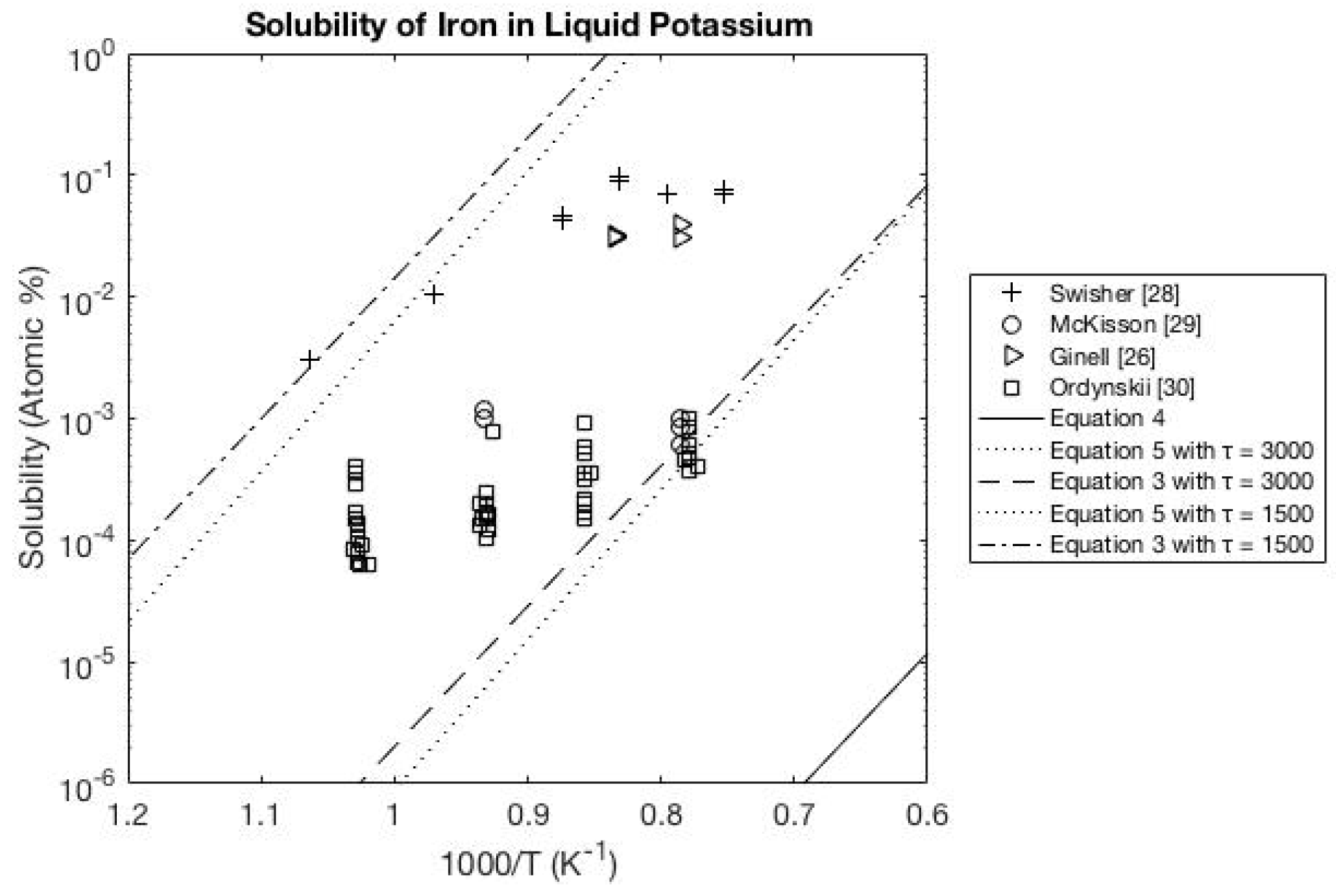
3.3. Potassium
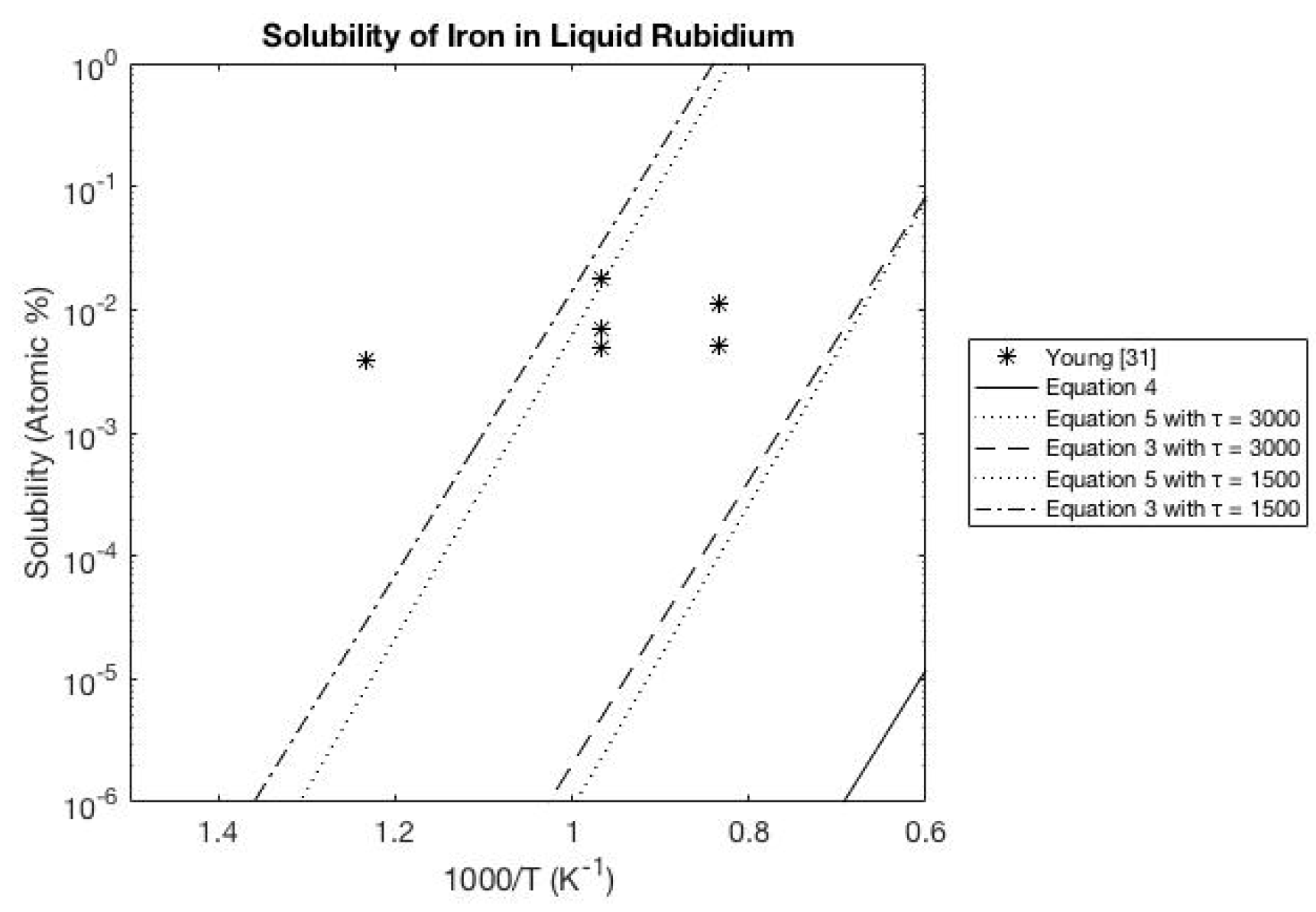
3.4. Rubidium
3.5. Cesium
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
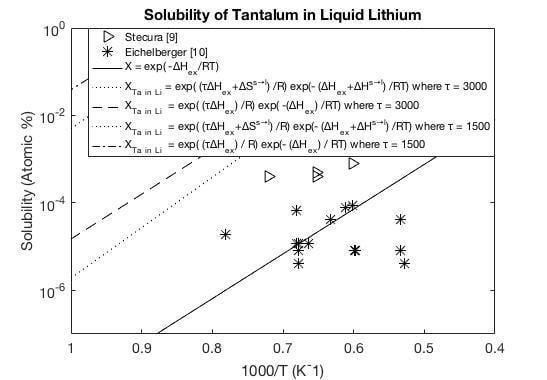
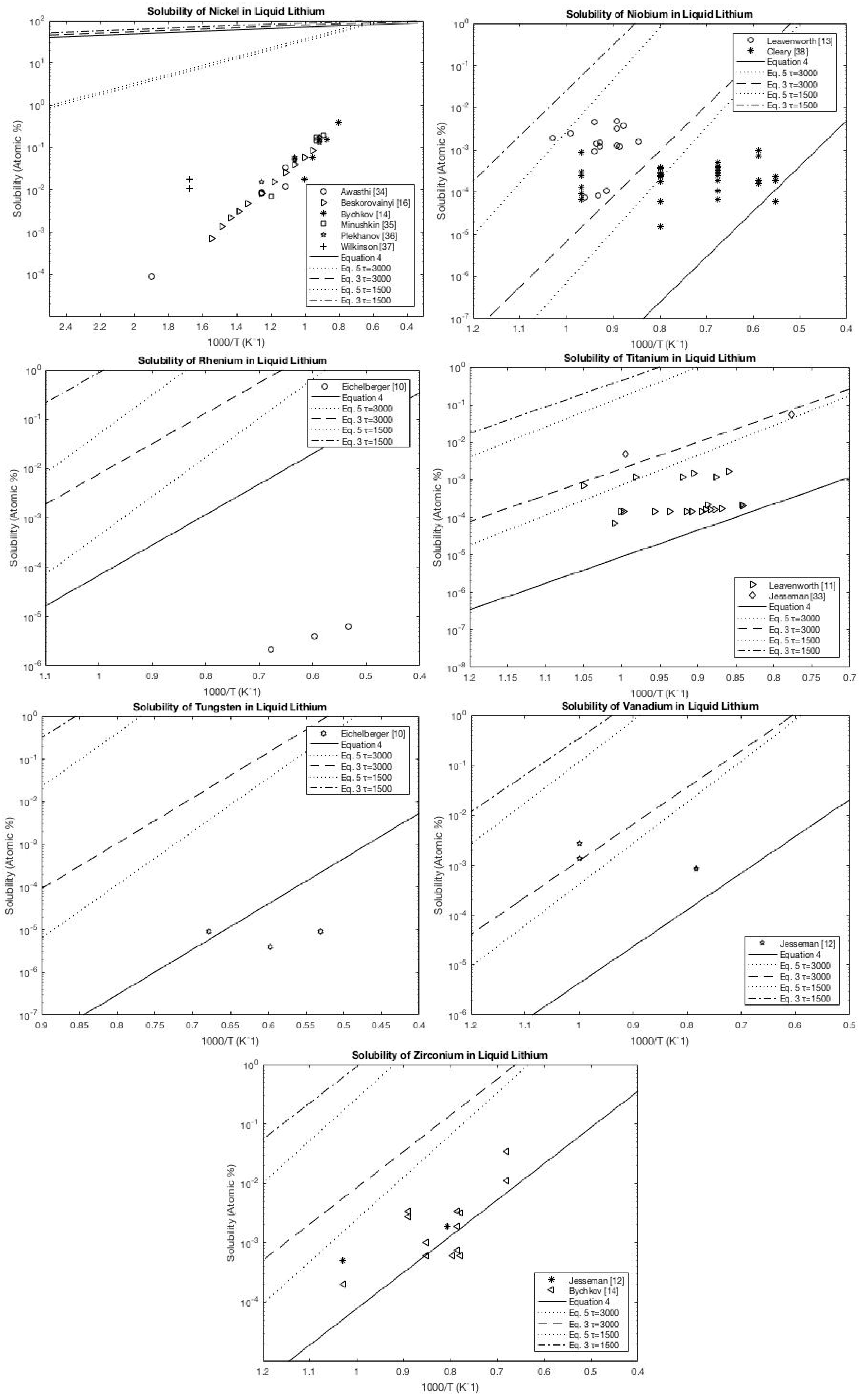
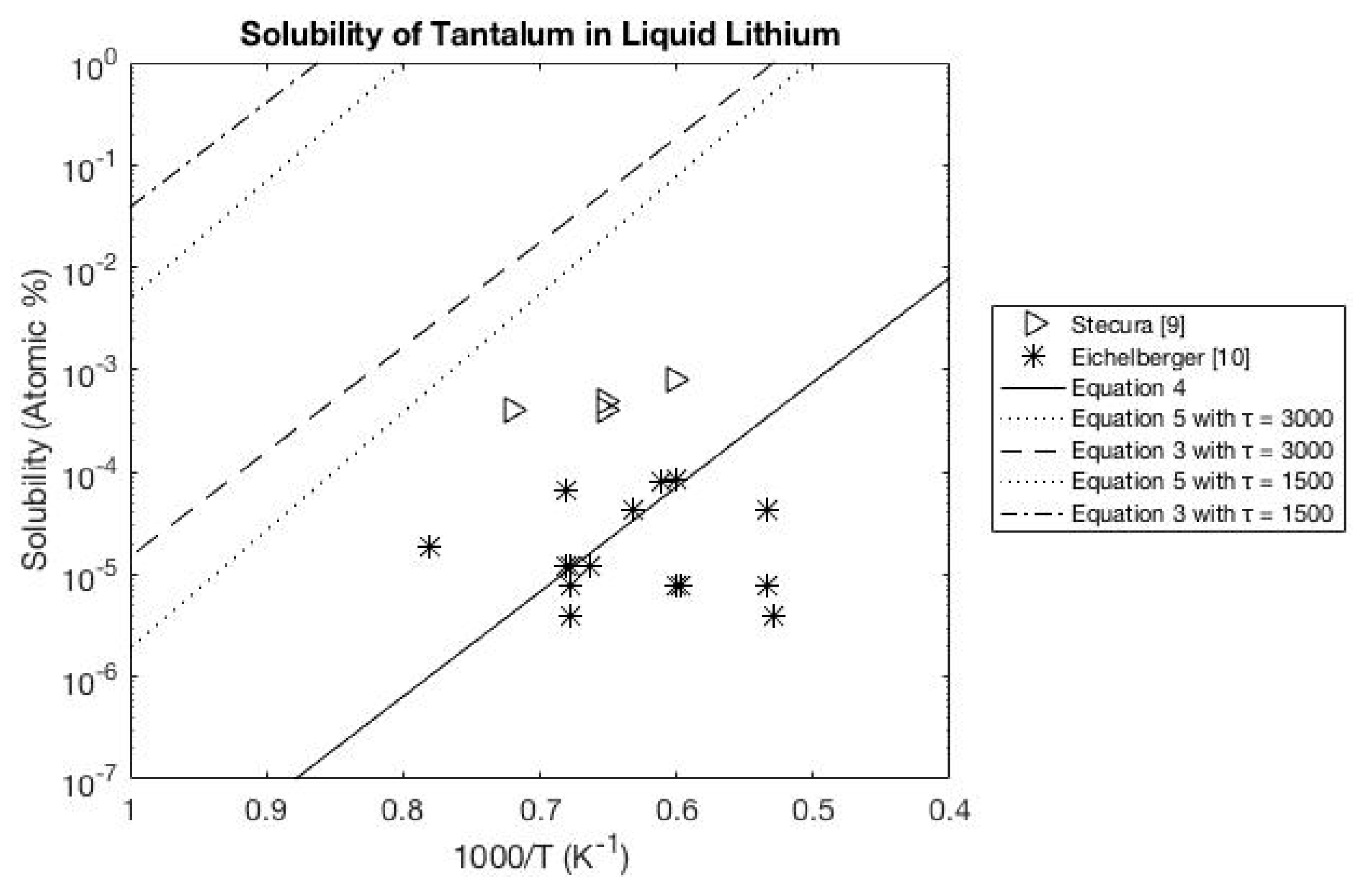
A.1. Lithium

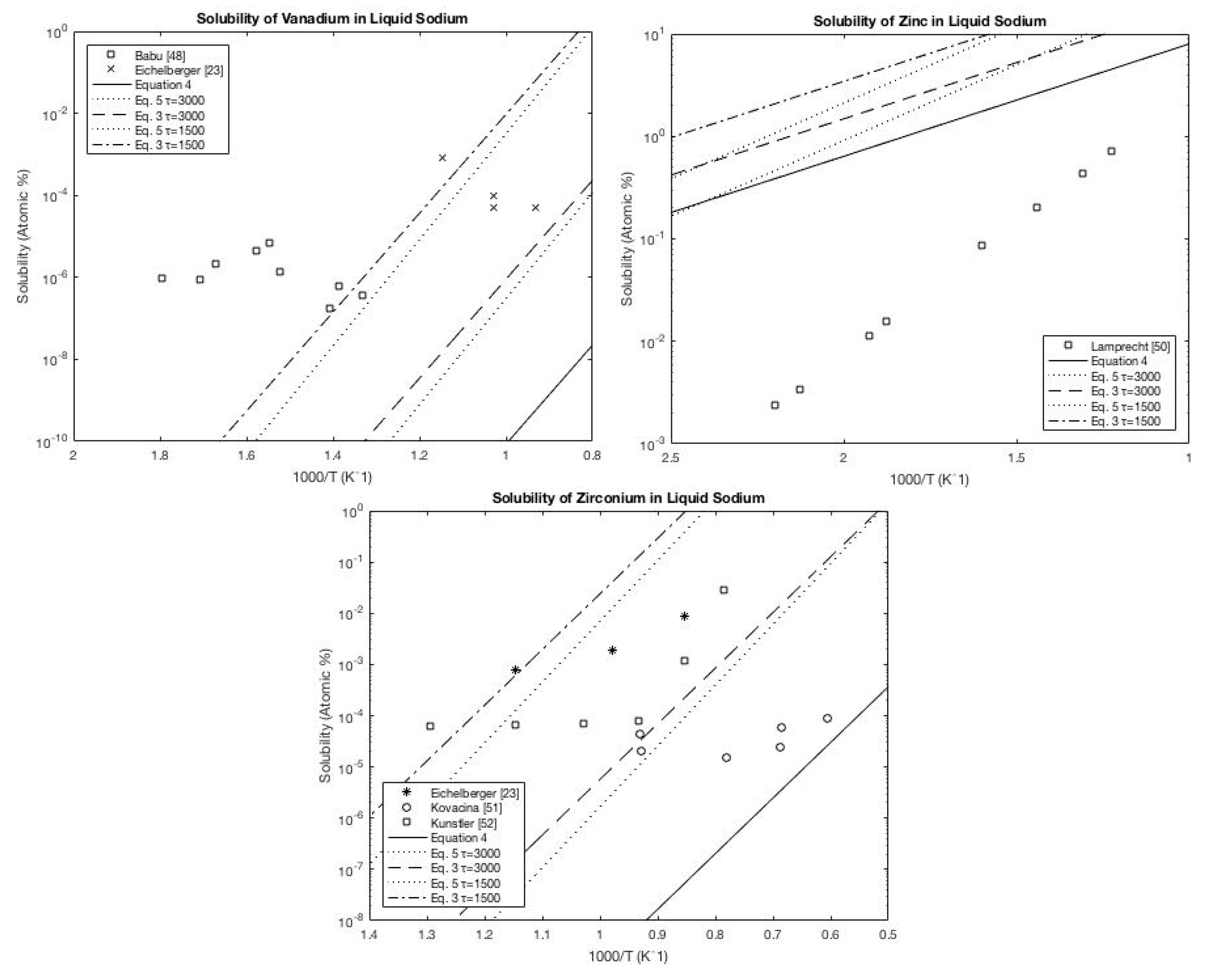
A.2. Sodium

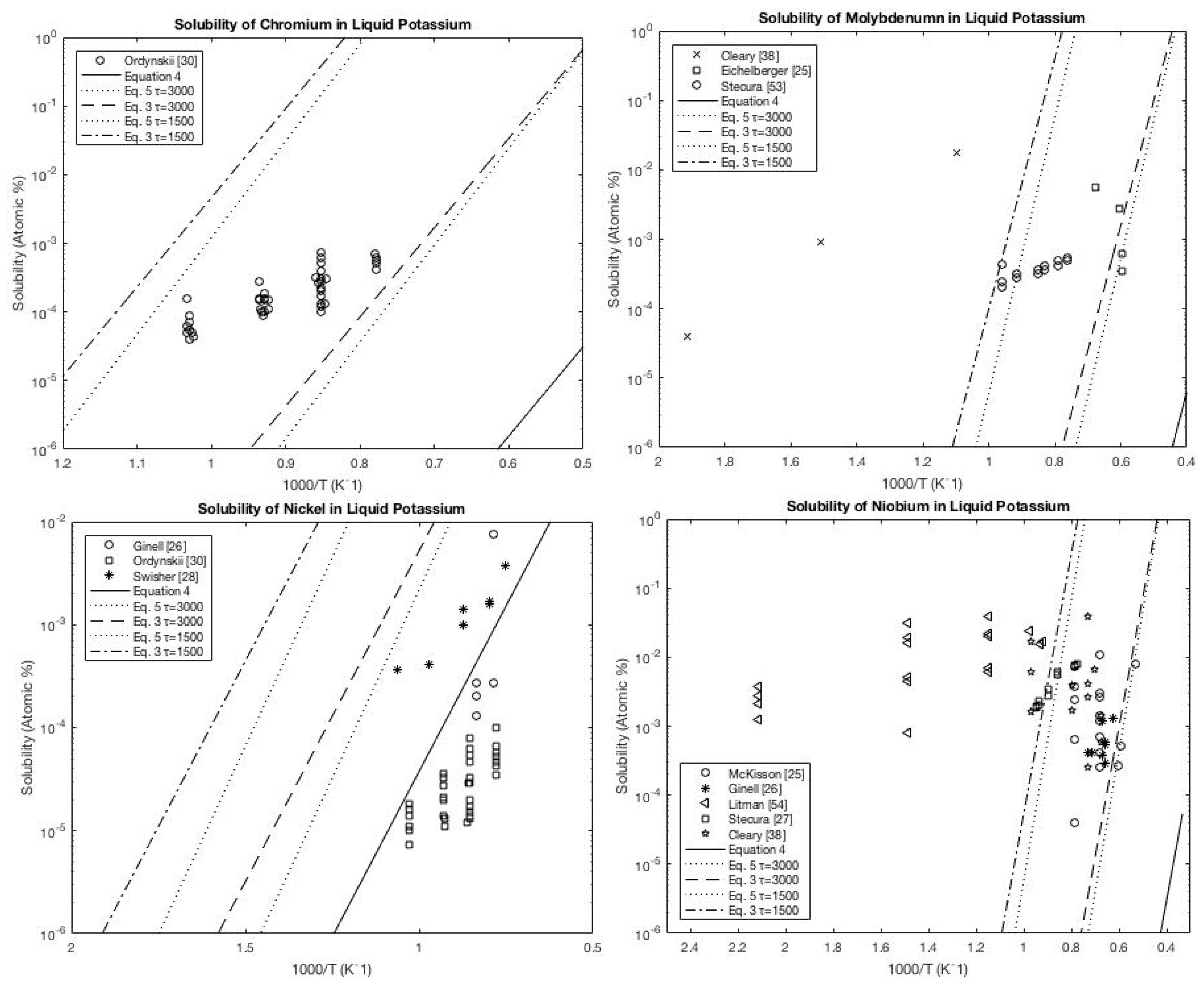
A.3. Potassium
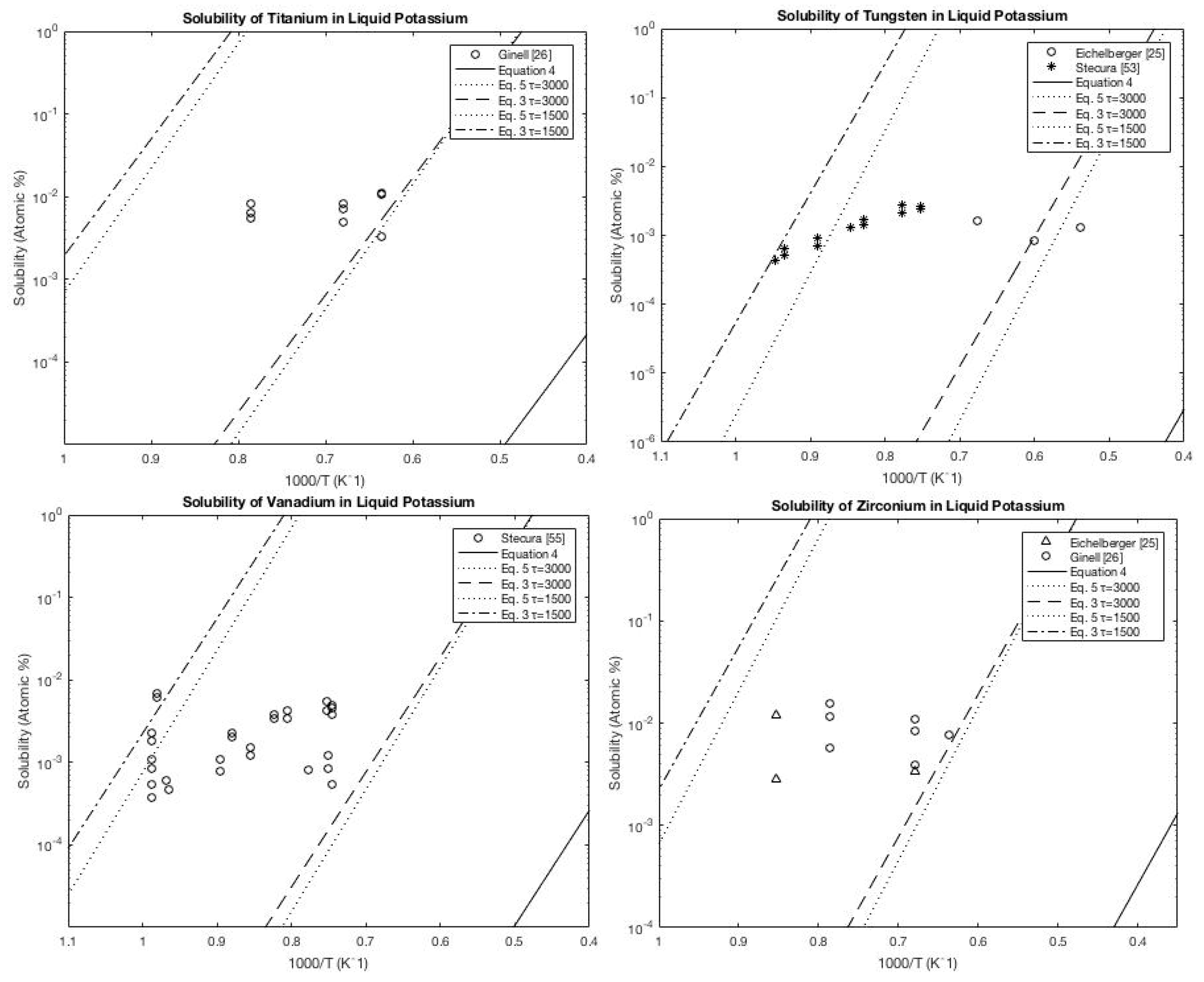
A.4. Rubidium
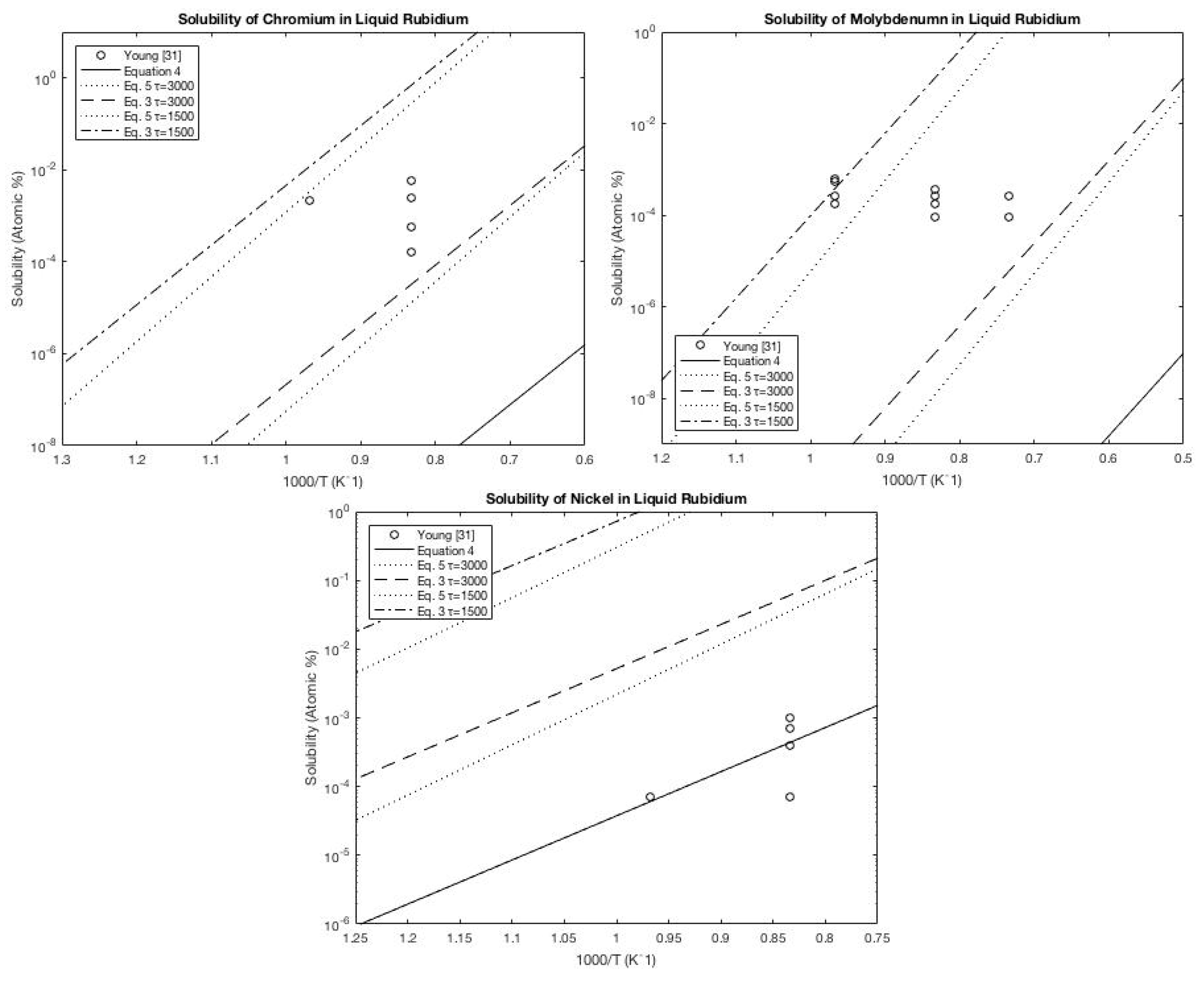
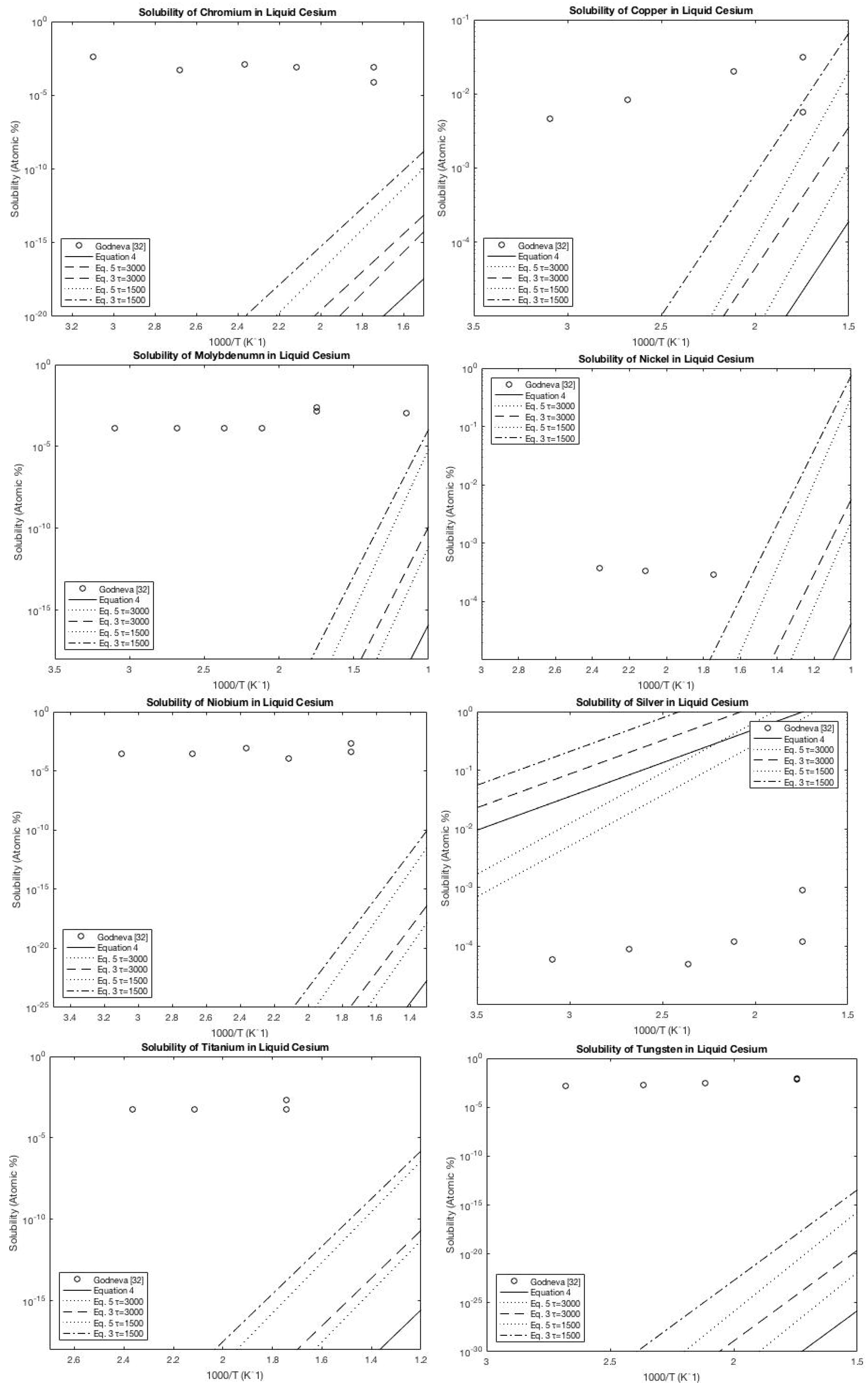
A.5. Cesium

References
- Borgstedt, H.U.; Guminski, C. Solubility Data Series: Metals in Liquid Alkali Metals Part 1: Be to Os; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- IAEA. Alkali Metal Coolants: Proceedings of the Symposium Held by the International Atomic Energy Agency in Vienna, 28 November–2 December 1966; International Atomic Energy Agency: Wien, Austria, 1967. [Google Scholar]
- Mariani, R.D.; Porter, D.L.; O’Holleran, T.P.; Hayes, S.L.; Kennedy, J.R. Lanthanides in metallic nuclear fuels: Their behavior and methods for their control. J. Nucl. Mater. 2011, 419, 263–271. [Google Scholar] [CrossRef]
- De Boer, F.R.; Boom, R.; Mattens, W.C.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals: Transition Metal Alloys; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Lyublinski, I.E.; Evtikhin, V.A.; Pankratov, V.Y.; Krasin, V.P. Numerical and experimental determination of metallic solubilities in liquid lithium, lithium-containing nonmetallic impurities, lead and lead-lithium eutectic. J. Nucl. Mater. 1995, 224, 288–292. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book (Eighth Edition); Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Bakker, H. Enthalpies in Alloys: Miedema’s Semi-Empirical Model; Enfield Publishing & Distribution Company: Enfield, NH, USA, 1998. [Google Scholar]
- Lupis, C.H.P. Chemical Thermodynamic of Materials; Elsevier Science Ltd.: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Stecura, S. Corrosion of oxygen-doped tantalum by lithium. Corros. Sci. 1976, 16, 233–241. [Google Scholar] [CrossRef]
- Eichelberger, R.L.; McKisson, R.L.; Johnson, B.G. Solubility of Refractory Metals and Alloys in Potassium and in Lithium; National Aeronautics and Space Administration: Washington, DC, USA, 1969. [Google Scholar]
- Leavenworth, H.W.; Cleary, R.E. The solubility of Ni, Cr, Fe, Ti and Mo in liquid lithium. Acta Metall. 1961, 9, 519–520. [Google Scholar] [CrossRef]
- Jesseman, D.S.; Roben, G.D.; Grunewald, A.L.; Fleshman, W.S.; Anderson, K.; Calkins, V.P. Preliminary Investigation of Metallic Elements in Molten Lithium; Technical Report NEPA-1465; Fairchild Engine and Airplane Corp.: Oak Ridge, TN, USA, 1950. [Google Scholar]
- Leavenworth, H.; Cleary, R.E.; Bratton, W.D. Solubility of Structural Metals in Lithium; Technical Report PWAC-356; Pratt and Whitney Aircraft: Middletown, CT, USA, 1961. [Google Scholar]
- Bychkov, Y.F.; Rozanov, A.N.; Yakovleva, V.B. The solubility of metals in liquid lithium. At. Energ. 1959, 7, 531–536. (In Russian) [Google Scholar]
- Beskorovainyi, N.M.; Yakovlev, E.I. Metallurgy & Metallography of Pure Metals; Gordon & Breach: New York, NY, USA, 1962; pp. 189–206. [Google Scholar]
- Beskorovainyi, N.M.; Vasilev, V.K.; Lyublinskii, I.E. Determination of the solubility of Fe, Ni, and Cr in Li by absorption X-ray spectral analysis. Metall. Metalloved. Chist. Met. 1980, 14, 135–148. (In Russian) [Google Scholar]
- Bogard, A.D. The Solubility of Iron in Sodium Metal, Sodium-Sodium Oxide, and Sodium-Sodium Oxide-Sodium Hydroxide; Technical Report NRL-4131; Naval Research Lab.: Washington, DC, USA, 1953. [Google Scholar]
- Eichelberger, R.L.; McKisson, R.L. Studies of the Solubility of Iron in Sodium; Technical Report Al-AEC-12834; Atomics International: Canoga Park, CA, USA, 1969. [Google Scholar]
- Epstein, L.F. Studies on the solubility of iron in liquid sodium. Science 1950, 112, 426. [Google Scholar]
- Fleitman, A.H.; Isaacs, H.S. The solubility and corrosion of pure iron in liquid sodium containing dissolved oxygen. In Proceedings of the 1970 Metals Conference, Cleveland, OH, USA; 1970. [Google Scholar]
- Periaswami, G.; Ganesan, V.; Babu, S.R.; Mathews, C.K. Solubility of manganese and iron in sodium. In Material Behavior and Physical Chemistry in Liquid Metal Systems; Borgstedt, H.U., Ed.; Plenum Press: New York, NY, USA, 1982; pp. 411–420. [Google Scholar]
- Rodgers, S.J.; Mausteller, J.W.; Batutis, E.F. Iron and Nickel Concentrations in Sodium; Technical Report NP-5241; Mine Safety Appliances Co.: Cranberry Township, PA, USA, 1954. [Google Scholar]
- Eichelberger, R.L.; McKisson, R.L. Solubility Studies of Cr, Co, Mn, Mo, Ni, Nb, Ti, V, and Zr in Liquid Sodium; Technical Report AL-AEC-12955; Atomics International: Canoga Park, CA, USA, 1970. [Google Scholar]
- Pellett, C.R.; Thompson, R. Measurement of transition metal solubilities in liquid sodium; cobalt, nickel, and chromium. In Liquid Metal Engineering and Technology: Proceedings of the Third International Conference Held in Oxford on 9–13 April 1984; British Nuclear Energy Society: London, UK, 1984; Volume 3, pp. 43–48. [Google Scholar]
- McKisson, R.L.; Eichelberger, R.L.; Dahleen, R.C.; Scarborough, J.M.; Argue, G.R. Solubility Studies of Ultra Pure Alkali Metals; Technical Report NASA-CR-610; North American Aviation, Inc.: Canoga Park, CA, USA, 1966. [Google Scholar]
- Ginell, G.S.; Teitel, R.J. Determination of solubility of several transition metals in molten potassium. Trans. Am. Nucl. Soc. 1965, 8, 393–394. [Google Scholar]
- Stecura, S. Apparent Solubilities of Commercially Pure and Oxygen-Doped Tantalum and Niobium in Liquid Potassium; Technical Report NASA-TN-D-5875; National Aeronautics and Space Administration: Cleveland, OH, USA, 1970. [Google Scholar]
- Swisher, J.H. Solubility of Iron, Nickel, and Cobalt in Liquid Potassium and Effect of Oxygen Gettering Agents on Iron Solubility; Technical Report NASA-TN-D-2734; NASA Lewis Research Center: Cleveland, OH, USA, 1965. [Google Scholar]
- McKisson, R.L.; Eichelberger, R.L.; Dahleen, R.C.; Scarborough, J.M.; Argue, G.R. The Solubility of Cu, Mo, Nb, Fe, Ni, and Cr in High Purity Sodium; Technical Report CONF-650411; United States Atomic Energy Commission: Washington, DC, USA, 1965. [Google Scholar]
- Ordynskii, A.M.; Popov, R.G.; Raikova, G.P.; Samsonov, N.V.; Tarbov, A.A. Solubility of components of stainless steel in liquid potassium. Teplofiz. Vys. Temp. 1981, 19, 1192–1197. (In Russian) [Google Scholar]
- Young, P.F.; Arabian, R.W. Determination of Temperature Coefficient of Solubility of Various Metals in Rubidium and the Corrosive Effects of Rubidium on Various Alloys at Temperatures from 1000 to 2000 °F; Technical Report AGN-8063; Aerojet-General Nucleonics: San Ramon, CA, USA, 1963. [Google Scholar]
- Godneva, M.M.; Sedelnikova, N.D.; Geizler, E.S. Solubilities and Change of Weight of Some Metals in Caesium. Zh. Prikl. Khim. 1974, 47, 2177–2180. (In Russian) [Google Scholar]
- Kirillov, V.B.; Krasin, V.P.; Lyublinskij, I.E.; Kuzin, A.N. Effect of nitrogen and oxygen impurities on dissolution and metal mass transfer in lithium and sodium melts. Zh. Fiz. Khim. 1988, 62, 3191–3195. [Google Scholar]
- Awasthi, S.P.; Borgstedt, H.U.; Frees, G. Solubility of metals in sodium and lithium. In Liquid Metal Engineering and Technology: Proceedings of the Third International Conference Held in Oxford on 9–13 April 1984; British Nuclear Energy Society: London, UK, 1984; Volume 1, pp. 265–269. [Google Scholar]
- Minushkin, B. Solution Rates and Equilibrium Solubility of Nickel and Iron in Liquid Lithium; Technical Report NDA-2141-1; United Nuclear Corp.: White Plains, NY, USA, 1961. [Google Scholar]
- Plekhanov, G.A.; Fedortsov-Lutikov, G.P.; Glushko, Y.V. The effect of non-metallic impurities on the solubility of steel components in liquid lithium. At. Energ. 1978, 45, 143–145. [Google Scholar] [CrossRef]
- Wilkinson, W.D.; Yaggee, F.L. Attack on Metals by Lithium; Technical Report ANL-4990; Argonne National Lab.: Lemont, IL, USA, 1950. [Google Scholar]
- Cleary, R.E.; Blecherman, S.S.; Corliss, J.E. Solubility of Refractory Metals in Lithium and Potassium; Technical Report TIM-850; Pratt and Whitney Aircraft: Middletown, CT, USA, 1965. [Google Scholar]
- Grand, J.A.; Baus, R.A.; Bogard, A.D.; Williams, D.D.; Lockhart, L.B.; Miller, R.R. The solubility of tantalum and cobalt in sodium by activation analysis. J. Phys. Chem. 1959, 63, 1192–1194. [Google Scholar]
- Sivasubramanian, K.; Mitragotri, D.S.; Bhat, N.P. Measurement of Solubility of Cobalt in Liquid Sodium; Bhabha Atomic Research Centre: Bombay, India, 1995; pp. 262–263. [Google Scholar]
- Eichelberger, R.L.; McKisson, R.L. Solubility of Copper in Sodium; Technical Report Al-AEC-12671; Atomics International: Canoga Park, CA, USA, 1968. [Google Scholar]
- Humphreys, J.R. Interdivision Document K-3-774; Los Alamos Scientific Laboratory: Los Alamos, NM, USA, 1958. [Google Scholar]
- Koenig, R.F. Corrosion of Zirconium and Its Alloys in Liquid Metals; Technical Report KAPL-982; Knolls Atomic Power Lab.: Niskayuna, NY, USA, 1953. [Google Scholar]
- Singer, R.M.; Weeks, J.R. On the Solubilities of Cu, Ni, and Fe in Liquid Sodium; Technical Report BNL-1307; Brookhaven National Lab.: Upton, NY, USA, 1969. [Google Scholar]
- Walker, R.A.; Pratt, J.N. The solubility of copper in liquid sodium. J. Nucl. Mater. 1969, 32, 340–345. [Google Scholar] [CrossRef]
- Stanaway, W.P.; Thompson, R. Solubility of Metals, Iron and Manganese in Sodium. In Proceedings of the Second International Conference on Liquid Metal Technology in Energy Production, Richland, WA, USA, 20–24 April 1980; Dahlke, J.M., Ed.; American Nuclear Society: Washington, DC, USA, 1980. [Google Scholar]
- Stanaway, W.P.; Thompson, R. The solubility of transition metals, Mn and Co in liquid sodium. In Material Behavior and Physical Chemistry in Liquid Metal Systems; Borgstedt, H.U., Ed.; Plenum Press: New York, NY, USA, 1982; pp. 421–427. [Google Scholar]
- Babu, S.R.; Periaswami, C.; Geetha, R.; Mahalingam, T.R. Solubility of molybdenum and vanadium in liquid sodium. In Liquid Metal Engineering and Technology: Proceedings of the Third International Conference Held in Oxford on 9–13 April 1984; British Nuclear Energy Society: London, UK, 1984; Volume 1, pp. 271–275. [Google Scholar]
- Kunstler, K.; Baum, H. Deposition of Niobium in the System Sodium-Steel; Technical Report ZFK-340; German Democratic Republic: Dresden, Germany, 1977; pp. 47–48. (In German) [Google Scholar]
- Lamprecht, G.J.; Crowther, P. Solubility of metals in liquid sodium: The systems sodium-silver, sodium-zinc, and sodium-cerium. Trans. AIME 1968, 242, 2169–2171. [Google Scholar]
- Kovacina, T.A.; Miller, R.R. The Solubility of Columbian-1% Zirconium in Sodium by Activation Analysis; Technical Report NRL-6051; Naval Research Lab.: Washington, DC, USA, 1964. [Google Scholar]
- Kunstler, K.; Heyne, H. Solubility of zirconium in liquid sodium. In Material Behavior and Physical Chemistry in Liquid Metal Systems; Borgstedt, H.U., Ed.; Plenum Press: New York, NY, USA, 1995; pp. 311–319. [Google Scholar]
- Stecura, S. Solubilities of Molybdenum and Tungsten in Liquid Potassium; Technical Report NASA-TN-D-5504; NASA Lewis Research Center: Cleveland, OH, USA, 1969. [Google Scholar]
- Litman, A.P. The Effect of Oxygen on the Corrosion of Niobium by Liquid Potassium; Technical Report ORNL-3751; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1965. [Google Scholar]
- Stecura, S. Solubilities of molybdenum, tungsten, vanadium, titanium, and zirconium in liquid potassium. In Corrosion by Liquid Metals; Draley, J.E., Ed.; Springer: New York, NY, USA, 1970; pp. 601–611. [Google Scholar]
- Awasthi, S.P.; Borgstedt, H.U. An Assessment of solubility of some transition metals (Fe, Ni, Mn, and Cr) in liquid sodium. J. Nucl. Mater. 1983, 116, 103–111. [Google Scholar] [CrossRef]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isler, J.; Zhang, J. Examination of Solubility Models for the Determination of Transition Metals within Liquid Alkali Metals. Metals 2016, 6, 144. https://doi.org/10.3390/met6070144
Isler J, Zhang J. Examination of Solubility Models for the Determination of Transition Metals within Liquid Alkali Metals. Metals. 2016; 6(7):144. https://doi.org/10.3390/met6070144
Chicago/Turabian StyleIsler, Jeremy, and Jinsuo Zhang. 2016. "Examination of Solubility Models for the Determination of Transition Metals within Liquid Alkali Metals" Metals 6, no. 7: 144. https://doi.org/10.3390/met6070144