Observation of the Mating Behavior of Honey Bee (Apis mellifera L.) Queens Using Radio-Frequency Identification (RFID): Factors Influencing the Duration and Frequency of Nuptial Flights
Abstract
:1. Introduction
2. Experimental Section
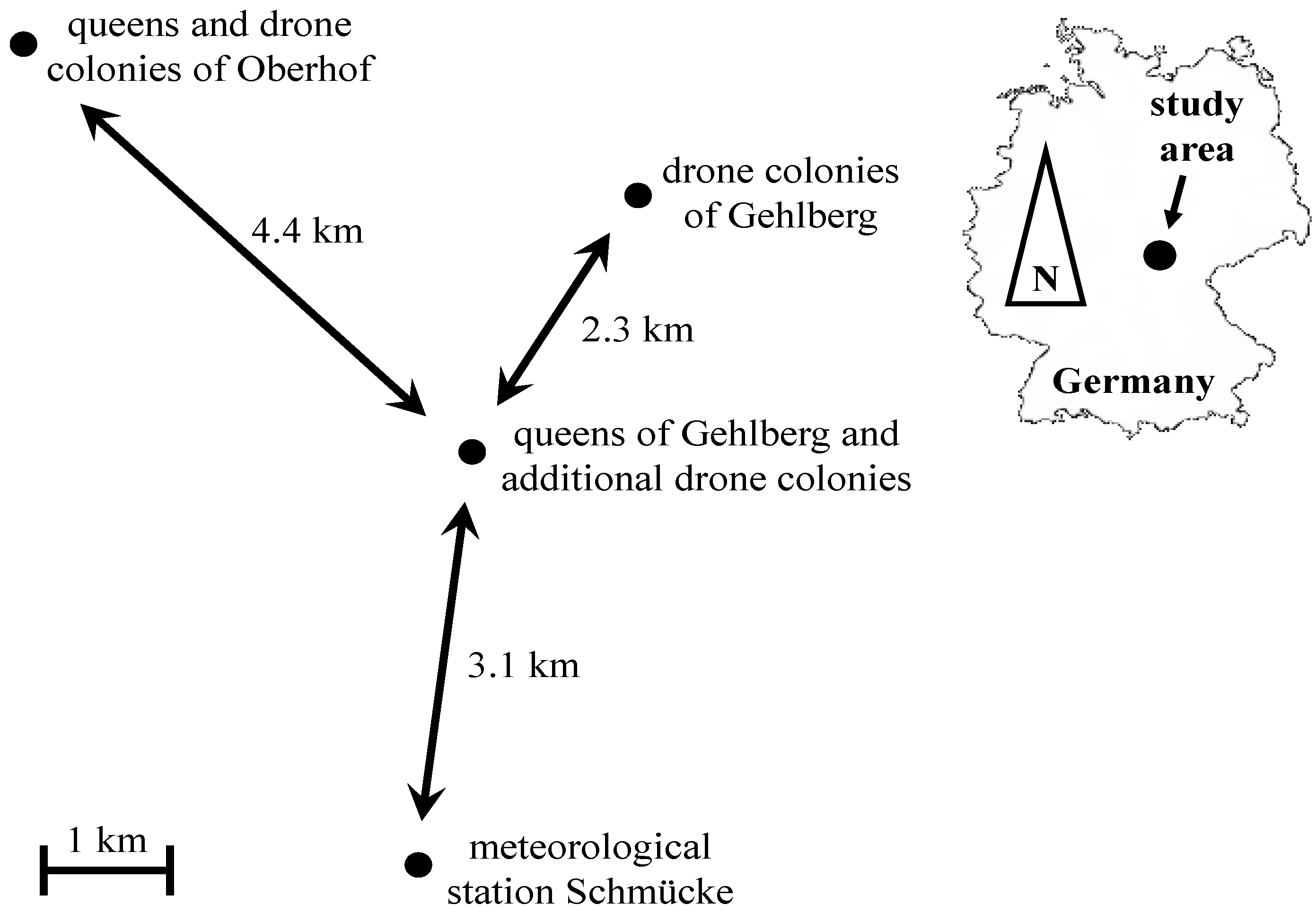
2.1. Field Work
| Calendar week | Gehlberg | Oberhof |
|---|---|---|
| 22 | 13 | 20 |
| 24 | 13 + 47 | 20 |
| 26 | 13 | 20 |
| 27 | 13 + 47 | 20 |
2.2. Data Analysis
| Factor | Category 1 | Category 2 | Category 3 | Category 4 |
|---|---|---|---|---|
| Age of the queens | 5 to 9 days | 10 to 13 days | 14 to 17 days | --- |
| Sequence of flights | 1st flights | 2nd flights | 3rd flights | 4th to 7th flights |
3. Results and Discussion
3.1. Survival Rates and General Flight Behavior of the Queens
| Calendar week | Total number of monitored queens | Queens with recorded flights | Mated queens | Lost queens |
|---|---|---|---|---|
| Gehlberg | ||||
| 22 | 8 | 7 | 7 | 1 |
| 24 | 8 | 7 | 7 | 1 |
| 26 | 8 | 5 | 5 | 3 |
| 27 | 8 | 3 | 3 | 5 |
| Oberhof | ||||
| 22 | 8 | 7 | 7 | 1 |
| 24 | 8 | 6 | 8 | 0 |
| 26 | 8 | 8 | 8 | 0 |
| 27 | 8 | 8 | 8 | 0 |

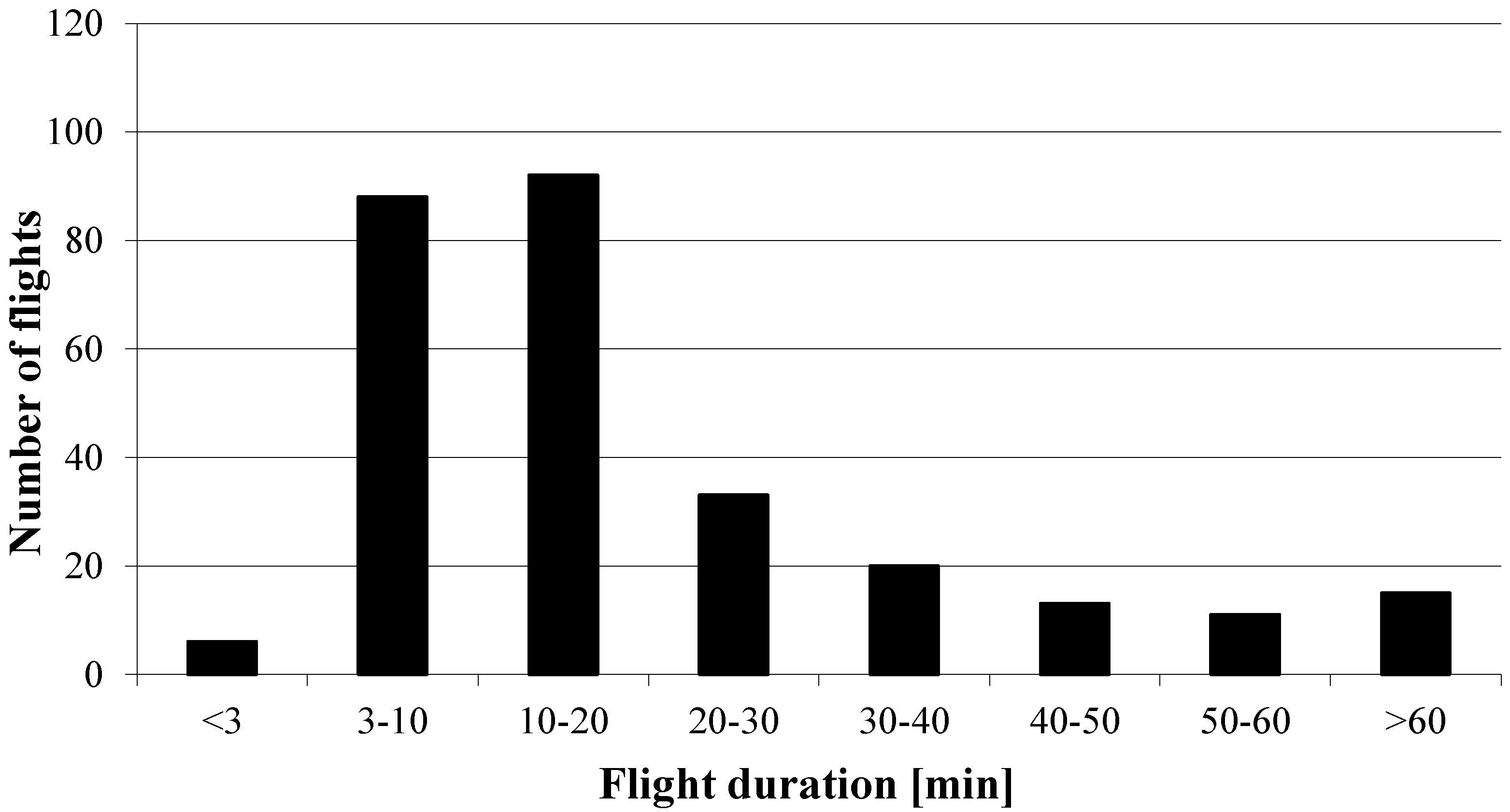
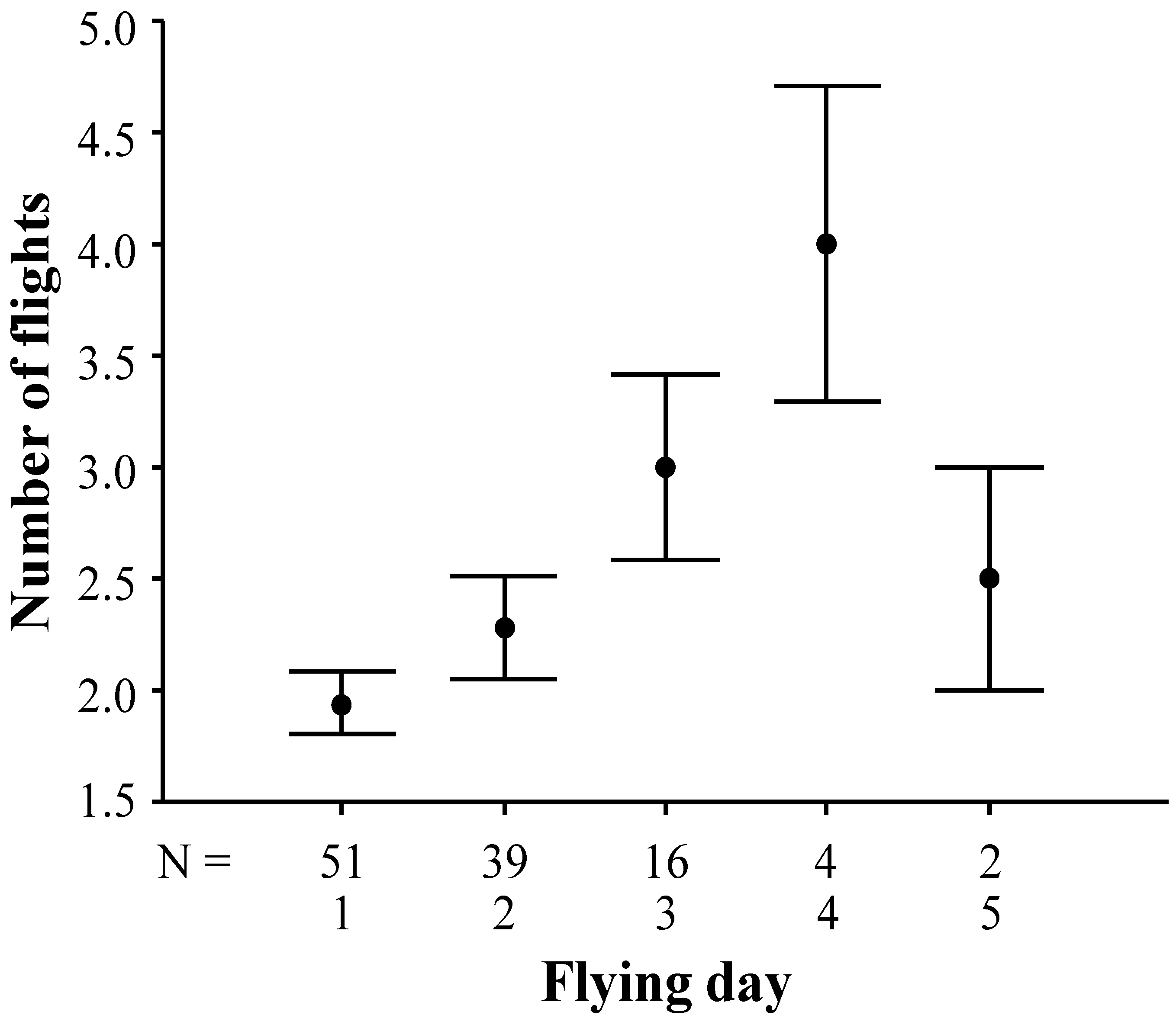
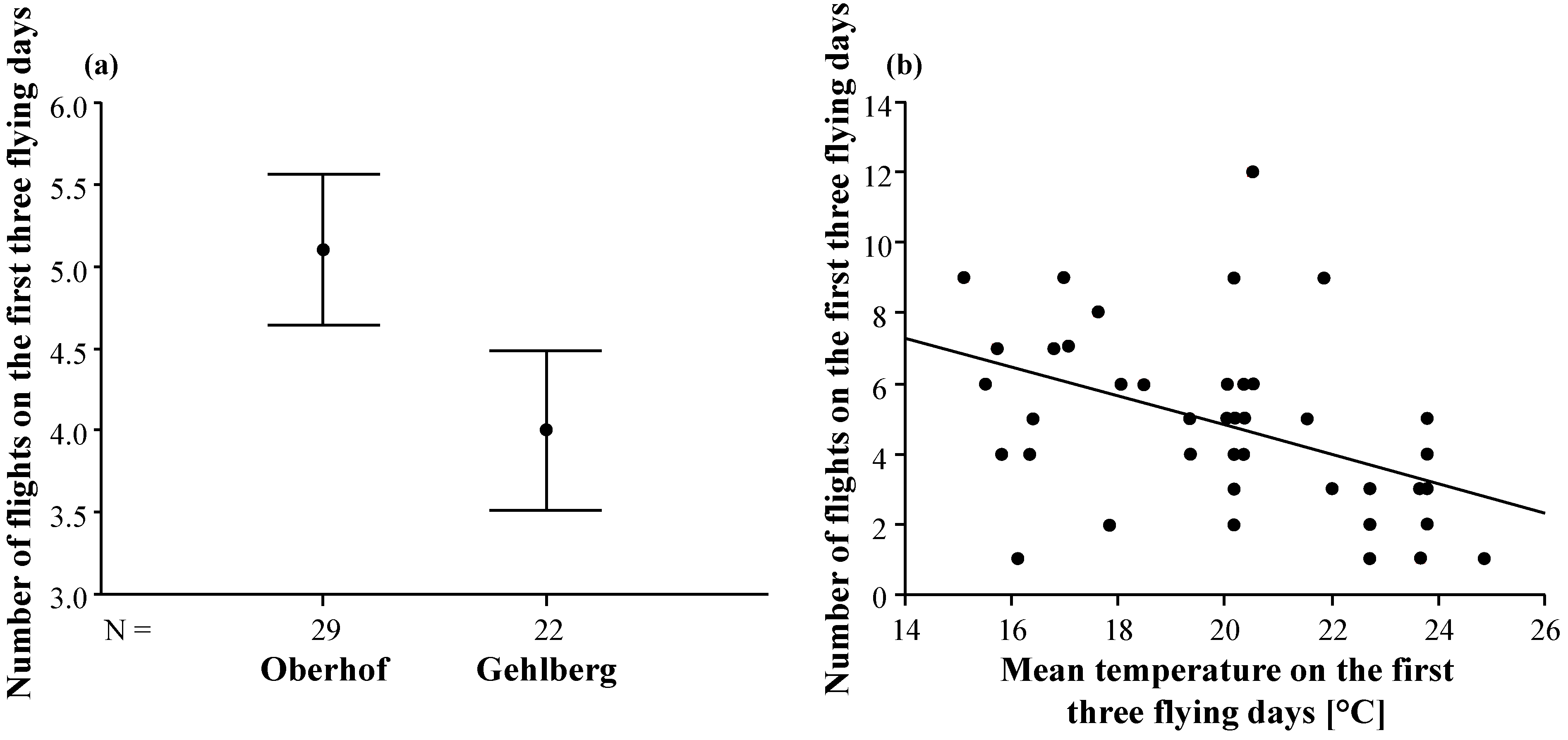
3.2. Duration and Frequency of Queen Nuptial Flights
| Source | Type III sum of squares | Den. d.f. | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 1197.26 | 5 | 239.45 | 57.75 | 0.000 |
| Mating apiary | 21.90 | 1 | 21.90 | 5.28 | 0.026 |
| Mean temperature | 19.89 | 1 | 19.89 | 4.80 | 0.034 |
| Number of drone colonies | 3.67 | 1 | 3.67 | 0.88 | 0.352 |
| Mating apiary x Number of drone colonies | 9.17 | 1 | 9.17 | 2.21 | 0.144 |
| Error | 190.74 | 46 | 4.15 | ||
| Total | 1388.00 | 51 | |||
| R2 = 0.863; R2 corrected = 0.848 | |||||
| Source | Type III sum of squares | Den. d.f. | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 83,767.52 | 10 | 8376.75 | 50.24 | 0.000 |
| Age of the queens | 1198.90 | 2 | 599.45 | 3.60 | 0.029 |
| Mating apiary | 34.24 | 1 | 34.24 | 0.21 | 0.651 |
| Number of drone colonies | 116.40 | 1 | 116.40 | 0.70 | 0.404 |
| Rank of flight | 1359.03 | 3 | 453.01 | 2.72 | 0.045 |
| Temperature | 650.91 | 1 | 650.91 | 3.90 | 0.049 |
| Mating apiary x Number of drone colonies | 47.45 | 1 | 47.45 | 0.29 | 0.594 |
| Error | 41,187.13 | 247 | 166.75 | ||
| Total | 124,954.65 | 257 | |||
| R2 = 0.670; R2 corrected = 0.657 | |||||

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taber, S. The frequency of multiple mating of queen honey bees. J. Econ. Entomol. 1954, 47, 995–998. [Google Scholar]
- Taber, S. Concerning the number of times queen bees mate. J. Econ. Entomol. 1958, 51, 786–789. [Google Scholar]
- Tryasko, V.V. Repeated and multiple mating of queens. Pchelovodstvo 1956, 33, 43–50. [Google Scholar]
- Woyke, J. Multiple mating of the honeybee queen (apis mellifica l.) in one nuptial flight. Bull. L'Academie Pol. Des. Sci. II 1955, 3, 175–180. [Google Scholar]
- Laidlaw, H.H., Jr.; Page, R.E., Jr. Polyandry in honey bees (apis mellifera l.): Sperm utilization and intracolony genetic relationships. Genetics 1984, 108, 985–997. [Google Scholar]
- Zmarlicki, C.; Morse, R.A. Drone congregation areas. J. Apic. Res. 1963, 2, 64–66. [Google Scholar]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer-Verlag: Berlin, Germany, 1988; p. 284. [Google Scholar]
- Ruttner, F. Drohnen-sammelplätze. Bienenvater 1962, 83, 1–2. [Google Scholar]
- Jean-Prost, P. Observations sur le vol nuptial des reines d’abeilles. Comptes Rendus l'Académie Sci. 1957, 245, 2107–2110. [Google Scholar]
- Ruttner, H.; Ruttner, F. Investigations on the flight activity and the mating behaviour of drones—V. Drone congregation areas and mating distance. Apidologie 1972, 3, 203–232. [Google Scholar] [CrossRef]
- Böttcher, F.K. Contributions to the knowledge of the mating flicht of the noenybee (apis mellifica mellifica l.). Apidologie 1975, 6, 233–281. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G. Reproductive isolation among species of the genus apis. Apidologie 2000, 31, 313–339. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Gries, M.; Tingek, S. Drone competition at drone congregation areas in four apis species. Apidologie 2005, 36, 211–221. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G. Mating flight duration of apis mellifera queens: As short as possible, as long as necessary. Apidologie 2007, 38, 606–611. [Google Scholar] [CrossRef]
- Fletscher, D.J.C.; Tribe, G.D. Natural emergency queen rearing by apis mellifera adansonii ii. In African Bees: Taxonomy, Biology and Economic Use; Fletscher, D.J.C., Ed.; Apimondia: Pretoria, South Africa, 1977; pp. 132–140. [Google Scholar]
- Koeniger, G. Reproduction and mating behaviour. In Bee Genetics and Breeding; Rinderer, T.E., Ed.; Academic Press Inc.: London, UK, 1986; pp. 255–280. [Google Scholar]
- Schlüns, H.; Moritz, R.F.A.; Neumann, P.; Kryger, P.; Koeniger, G. Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 2005, 70, 125–131. [Google Scholar] [CrossRef]
- Woyke, J. Natural and artificial insemination of queen honeybees. Pszczel. Zesz. Nauk. 1960, 4, 183–275. [Google Scholar]
- Franck, P.; Solignac, M.; Vautrin, D.; Cornuet, J.M.; Koeniger, G.; Koeniger, N. Sperm competition and last-male precedence in the honeybee. Anim. Behav. 2002, 64, 503–509. [Google Scholar] [CrossRef]
- Woyke, J. Causes of repeated mating flights by queen honeybees. J. Apic. Res. 1964, 3, 17–23. [Google Scholar]
- Roberts, W.C. Multiple mating of queen bees proved by progeny and flight tests. Glean. Bee Cult. 1944, 72, 281–283. [Google Scholar]
- Ruttner, F. Mehrfache begattung der bienenkönigin. Zool. Anz. 1954, 153, 99–105. [Google Scholar]
- Alber, W.; Jordan, R.; Ruttner, F.; Ruttner, H. Von der paarung der honigbiene. Z. Bienenforsch. 1955, 3, 1–28. [Google Scholar]
- Ruttner, F. The mating of the honeybee. Bee World 1956, 37, 3–15. [Google Scholar]
- Ruttner, F. Die sexualfunktionen der honigbienen im dienste ihrer sozialen gemeinschaft. Z. Vgl. Physiol. 1957, 39, 577–600. [Google Scholar] [CrossRef]
- Estoup, A.; Solignac, M.; Cornuet, J.-M. Precise assessment of the number of patrilines and of genetic relatedness in honeybee colonies. Proc. R. Soc. Lond. B 1994, 258, 1–7. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Nielsen, R.; Nielsen, D.I. A scientific note on the revised estimates of effective paternity frequency. Insectes Sociaux 2004, 51, 203–204. [Google Scholar] [CrossRef]
- Neumann, P.; Moritz, R.F.A.; van Praagh, J.P. Queen mating frequency in different types of honey bee mating apiaries. J. Apic. Res. 1999, 38, 11–18. [Google Scholar]
- Woyke, J. Wovon hängt die zahl der spermien in der samenblase der auf natürlichem wege begattetten königinnen ab? Z. Bienenforsch. 1966, 8, 236–248. [Google Scholar]
- Tarpy, D.R.; Page, R.E., Jr. No behavioral control over mating frequency in queen honey hees (apis mellifera l.): Implications for the evolution of extreme polyandry. Am. Nat. 2000, 55, 820–827. [Google Scholar] [CrossRef]
- Lensky, Y.; Demter, M. Mating flights of the queen honeybee (apis mellifera) in a subtropical climate. Comp. Biochem. Physiol. 1985, 81A, 229–241. [Google Scholar] [CrossRef]
- Oertel, E. Observations on the flight of drone honey bees. Ann. Entomol. Soc. Am. 1956, 49, 497–500. [Google Scholar]
- Verbeek, B. Investigation of the flight activity of young honeybee queens under continental and insular conditions by means of photoelectronic control. Apidologie 1976, 7, 151–168. [Google Scholar]
- Koeniger, G. The role of the mating sign in honey bees, apis mellifera l.: Does it hinder or promote multiple mating? Anim. Behav. 1990, 39, 444–449. [Google Scholar] [CrossRef]
- Woyke, J. The process of mating in the honeybee. Pszczel. Zesz. Nauk. 1958, 2, 1–42. [Google Scholar]
- Neumann, P.; van Praagh, J.P.; Moritz, R.F.A.; Dustmann, J.H. Testing reliability of a potential island mating apiary using DNA microsatellite. Apidologie 1999, 30, 251–276. [Google Scholar]
- Shuck, S.A. Mating of a queen bee. Am. Bee J. 1882, 18, 789. [Google Scholar]
- Langstroth, L.L. Copulation of the queen bee. Am. Bee J. 1861, 1, 65–66. [Google Scholar]
- Demaree, G.W. Fertilization in confinement. Am. Bee J. 1881, 17, 1. [Google Scholar]
- Janscha, A. Vollständige Lehre von der Bienenzucht; G. Münzberg: Wien, Austria, 1775; p. 236. [Google Scholar]
- Gary, N.E.; Marston, J. Mating behaviour of drone honey bees with queen models (apis mellifera l.). Anim. Behav. 1971, 19, 299–304. [Google Scholar] [CrossRef]
- Williams, J.L. Wind-directed pheromone trap for drone honey bees (hymenoptera: Apidae). J. Econ. Entomol. 1987, 80, 532–536. [Google Scholar]
- Ruttner, H. Investigations on the flight avtivity and the mating nehaviour of the drones—vi. Flight on and over mountain ridges. Apidologie 1976, 7, 331–341. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Pechhacker, H. The nearer the better? Drones (apis mellifera) prefer nearer drone congregation areas. Insectes Sociaux 2005, 52, 31–35. [Google Scholar] [CrossRef]
- Loper, G.M.; Wolf, W.W.; Taylor, O.R., Jr. Radar detection of drones responding to honeybee queen pheromone. J. Chem. Ecol. 1993, 19, 1929–1939. [Google Scholar] [CrossRef]
- Loper, G.M.; Wolf, W.W.; Taylor, O.R., Jr. Honey bee drone flyways and congregation areas—Radar observations. J. Kans. Entomol. Soc. 1992, 65, 223–230. [Google Scholar]
- Loper, G.M.; Wolf, W.W.; Taylor, O.R., Jr. Detection and monitoring of honeybee drone congregation areas by radar. Apidologie 1987, 18, 163–172. [Google Scholar] [CrossRef]
- Witherell, P.C. Flight activity and natural mortality of normal and mutant drone honeybees. J. Apic. Res. 1972, 11, 65–75. [Google Scholar]
- Verbeek, B.; Verbeek, R. A method for recording the arrival and departure flights of individual bees. Apidologie 1974, 5, 289–293. [Google Scholar] [CrossRef]
- Moreau, M.; Arrufat, P.; Latil, G.; Jeanson, R. Use of radio-tagging to map spatial organization and social interactions in insects. J. Exp. Biol. 2011, 214, 17–21. [Google Scholar] [CrossRef]
- Sumner, S.; Lucas, E.; Barker, J.; Isaac, N. Radio-tagging technology reveals extreme nest-drifting behavior in a eusocial insect. Curr. Biol. 2007, 17, 140–145. [Google Scholar] [CrossRef]
- Molet, M.; Chittka, L.; Stelzer, R.J.; Streit, S.; Raine, N.E. Colony nutritional status modulates worker responses to foraging recruitment pheromone in the bumblebee bombus terrestris. Behav. Ecol. Sociobiol. 2008, 62, 1919–1926. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. Rfid tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of apis mellifera. PLoS One 2012, 7, 1–9. [Google Scholar]
- Decourtye, A.; Devillers, J.; Aupinel, P.; Brun, F.; Bagnis, C.; Julie, F.; Gauthier, M. Honeybee tracking with microchips: A new methodology to measure the effects of pesticides. Ecotoxicology 2011, 20, 429–437. [Google Scholar] [CrossRef]
- Rice, W. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Ruttner, F. Reproductive behaviour in honeybees. In Fortschritte der Zoologie Band 31: Experimental Behavioural Ecology; Hölldobler, B., Lindauer, M., Eds.; Gustav Fischer Verlag Stuttgart: New York, NY, USA, 1985; pp. 225–236. [Google Scholar]
- Koeniger, G.; Koeniger, N.; Pechhacker, H.; Ruttner, F.; Berg, S. Assortative mating in a mixed population of European honeybees, apis mellifera ligustica and apis mellifera carnica. Insectes Sociaux 1989, 36, 129–138. [Google Scholar] [CrossRef]
- Taber, S. Factors influencing the circadian flight rhytm of drone honeybees. Ann. Entomol. Soc. Am. 1964, 57, 769–775. [Google Scholar]
- Drescher, W. Die flugaktivität von drohnen der rasse apis mellifica carnica l. Und a. Mell. Ligustica l. In abhängigkeit von lebensalter und witterung. Z. Bienenforsch 1969, 9, 390–409. [Google Scholar]
- Bol’Shakova, M.D. The flight of honey bee drones, apis mellifera l. (hymenoptera, apidae) to the queen in reletion to various ecological factors. Entomol. Rev. 1978, 56, 53–56. [Google Scholar]
- Ruttner, F. Drohnensammelplätze: Ein beispiel von paarungsverhalten bei insekten. Anz. Schädlingskde Pflanzenschutz Umweltschutz 1974, 47, 40–42. [Google Scholar]
- Hayworth, M.K.; Johnson, N.G.; Wilhelm, M.E.; Gove, R.P.; Metheny, J.D.; Rueppel, O. Added weights lead to reduced flight behaviour and mating sucess in polyandrous honey bee queens (apis mellifera). Ethology 2009, 115, 698–706. [Google Scholar] [CrossRef]
- Ruttner, F.; Ruttner, H. Untersuchungen über die flugaktivität und das paarungsverhalten der drohnen. 3. Flugweite und flugrichtung der drohnen. Z. Bienenforsch. 1966, 8, 332–354. [Google Scholar]
- Drescher, W. Untersuchungen zur zuflugsicherheit der inselbelegstelle mellum. Z. Bienenforsch. 1965, 8, 49–54. [Google Scholar]
- Woyke, J. Anatomo-physiological changes in queen-bees returning from mating flights, and the process of multiple mating. Bull. L'Academie Pol. Des. Sci. II 1956, 4, 81–87. [Google Scholar]
- Streit, S.; Bock, F.; Pirk, C.W.W.; Tautz, J. Automatic life-long monitoring of individual insect behaviour now possible. Zoology 2003, 106, 169–171. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Heidinger, I.M.M.; Meixner, M.D.; Berg, S.; Büchler, R. Observation of the Mating Behavior of Honey Bee (Apis mellifera L.) Queens Using Radio-Frequency Identification (RFID): Factors Influencing the Duration and Frequency of Nuptial Flights. Insects 2014, 5, 513-527. https://doi.org/10.3390/insects5030513
Heidinger IMM, Meixner MD, Berg S, Büchler R. Observation of the Mating Behavior of Honey Bee (Apis mellifera L.) Queens Using Radio-Frequency Identification (RFID): Factors Influencing the Duration and Frequency of Nuptial Flights. Insects. 2014; 5(3):513-527. https://doi.org/10.3390/insects5030513
Chicago/Turabian StyleHeidinger, Ina Monika Margret, Marina Doris Meixner, Stefan Berg, and Ralph Büchler. 2014. "Observation of the Mating Behavior of Honey Bee (Apis mellifera L.) Queens Using Radio-Frequency Identification (RFID): Factors Influencing the Duration and Frequency of Nuptial Flights" Insects 5, no. 3: 513-527. https://doi.org/10.3390/insects5030513




