Molecular Identification of Diaspididae and Elucidation of Non-Native Species Using the Genes 28s and 16s
Abstract
:1. Introduction
2. Experimental Section
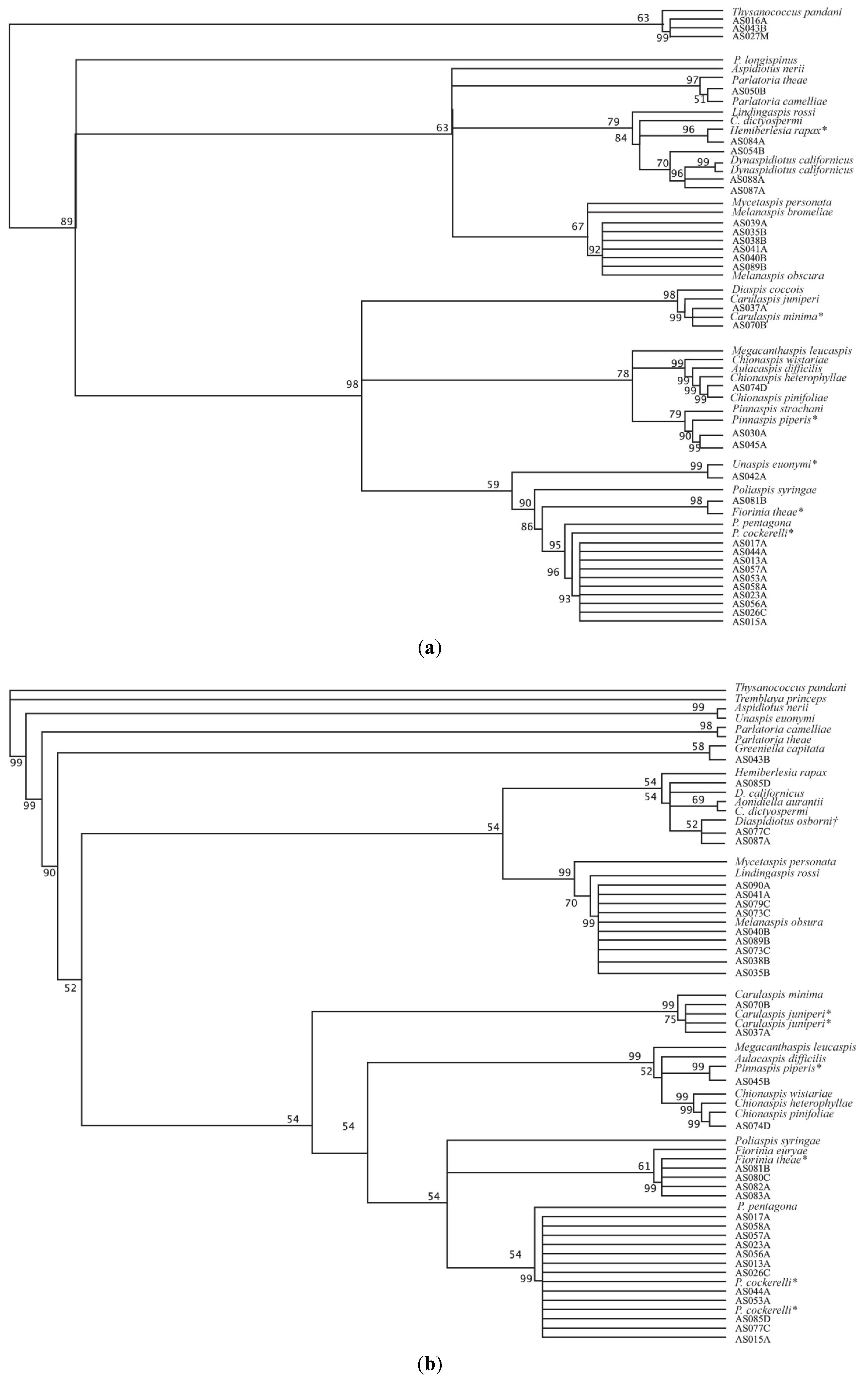
3. Results and Discussion
| Sample # | 28s | Accession Number | 16s | Accession Number |
|---|---|---|---|---|
| Phylogenetic ID | Phylogenetic ID | |||
| AS013 | Pseudalacaspis cockerelli* | KF887352 | Pseudalacaspis cockerelli* | KF887383 |
| AS015 | Pseudalacaspis cockerelli* | KF887353 | Pseudalacaspis cockerelli* | KF887384 |
| AS016 | undet Halimococcidae | KF887354 | --- | --- |
| AS017 | Pseudalacaspis cockerelli* | KF887355 | Pseudalacaspis cockerelli* | KF887385 |
| AS023 | Pseudalacaspis cockerelli* | KF887356 | Pseudalacaspis cockerelli* | KF887386 |
| AS026 | Pseudalacaspis cockerelli* | KF887357 | Pseudalacaspis cockerelli* | KF887387 |
| AS027 | undet. Halimococcidae | KF887358 | --- | --- |
| AS030 | Pinaspis piperis* | KF887359 | --- | --- |
| AS035 | Melanaspis obscura | KF887360 | Melanaspis obscura | KF887388 |
| AS037 | Carulaspis minima* | KF887361 | Carulaspis juniperi* | KF887389 |
| AS038 | Melanaspis obscura | KF887362 | Melanaspis obscura | KF887390 |
| AS039 | Melanaspis obscura | KF887363 | --- | --- |
| AS040 | Melanaspis obscura | KF887364 | Melanaspis obscura | KF887391 |
| AS041 | Melanaspis obscura | KF887365 | Melanaspis obscura | KF887392 |
| AS042 | Unaspis euonymi* | KF887366 | --- | --- |
| AS043 | undet Halimococcidae | KF887367 | Unknown Symbiotic bacteria | KF887393 |
| AS044 | Pseudalacaspis cockerelli* | KF887368 | Pseudalacaspis cockerelli* | KF887394 |
| AS045 | Pinaspis piperis* | KF887369 | Pinaspis piperis* | KF887395 |
| AS050 | Parlatoria sp. | KF887370 | --- | --- |
| AS053 | Pseudalacaspis cockerelli* | KF887371 | Pseudalacaspis cockerelli* | KF887396 |
| AS054 | † | KF887372 | --- | --- |
| AS056 | Pseudalacaspis cockerelli* | KF887373 | Pseudalacaspis cockerelli* | KF887397 |
| AS057 | Pseudalacaspis cockerelli* | KF887374 | Pseudalacaspis cockerelli* | KF887398 |
| AS058 | Pseudalacaspis cockerelli* | KF887375 | Pseudalacaspis cockerelli* | KF887399 |
| AS070 | Carulaspis minima* | KF887376 | Carulaspis juniperi* | KF887400 |
| AS073 | --- | --- | Melanaspis obscura | KF887401 |
| AS074 | Chionaspis pinifoliae | KF887377 | Chionaspis pinifoliae | KF887402 |
| AS077 | --- | --- | Diaspidiotus osborni† | KF887403 |
| AS079 | --- | --- | Melanaspis obscura | KF887404 |
| AS080 | --- | --- | Fiorinia theae* | KF887405 |
| AS081 | Fiorinia theae* | KF887378 | Fiorinia theae* | KF887406 |
| AS082 | --- | --- | Fiorinia theae* | KF887407 |
| AS083 | --- | --- | Fiorinia theae* | KF887408 |
| AS084 | Hemiberlesia rapax* | KF887379 | --- | --- |
| AS085 | --- | --- | † | KF887409 |
| AS087 | Dynaspidiotus californicus | KF887380 | Diaspidiotus osborni† | KF887410 |
| AS088 | Dynaspidiotus californicus | KF887381 | --- | --- |
| AS089 | Melanaspis obscura | KF887382 | Melanaspis obscura | KF887411 |
| AS090 | --- | --- | Melanaspis obscura | KF887412 |
- (1)
- Obtain DNA sequence of 28S and 16S from either in a lab utilizing the methods outlined above or by sending it to a contract lab.
- (2)
- When accurate DNA sequences for one or both of the loci outlined above have been obtained, sequences should be imported into an already prepared, unaligned data file (FASTA file) in the form of a text document. The file is available for download through corresponding author’s faculty webpage and the unknown diaspidid sequences in question can be added after the end of the last sequence listed in the document. The sequence should be entered in this format: <28Suknown1, i.e., left arrow, followed by the sequence name without spaces followed by a single return and the DNA sequence in all lowercase letters. Data files for both 16S and 28S genes can be copied from the following site, [20], under the publications tab, directly below citation for this publication.
- (3)
- The entire data file should then be pasted in the data window on the online sequence alignment server, MAFFT [21]. In the segments below the window, the following settings should be selected. (a) UPPERCASE/lowercase—Amino Acid….. (b) Direction of nucleotide sequences—Adjust direction according to the first sequence (accurate for most cases) (c) Output order—aligned. An email address should be provided to MAAFT as well.
- (4)
- Species identification can now by estimated three ways. (a) The output in MAFFT has two files to view. One is the aligned data set, which will have the most similar sequences next to each other. Viewing and comparing original data to provided data in the aligned data set can elucidate similarities between sequences. A high enough similarity infers a potential species match. (b) MAFFT also allows for viewing a rough phylogenetic tree. This tree depicts the sequences most closely related to the original data, and therefore provides an estimate of species identity. (c) The aligned data set can be exported from MAFFT and subjected to a rigorous phylogenetic analysis by uploading the data set into an online phylogenetics utility site such as CypresPortal and obtain a tree with higher specificity.
| Sequence Name | Accession # | 16s |
|---|---|---|
| 28s | ||
| Aonidiella aurantii | --- | GQ424836.1 |
| Aspidiotus nerii | DQ145297.2 | AY279402.1 |
| Aulacaspis difficilis | DQ145298.2 | DQ868801.1 |
| Carulaspis juniperi | DQ145303.2 | DQ868804.1 |
| Carulaspis minima | DQ145302.2 | GQ424837.1 |
| Chionaspis heterophylae | GU349426.1 | GQ424897.1 |
| Chionaspis pinifoliae | GQ325459.1 | DQ868807.1 |
| Chionaspis wistariae | DQ145308.2 | DQ868808.1 |
| Chrysomphalus dictyospermi | DQ145310.2 | GQ424913.1 |
| Diaspidiotus osborni | --- | GQ424913.1 |
| Diaspis coccois | DQ145317.2 | --- |
| Dynaspidiotus californicus | DQ145322.2 | --- |
| Fiorinia euryae | --- | DQ868822.1 |
| Fiorinia theae | DQ145332.2 | DQ868859.1 |
| Greeniella capitata | --- | GQ424922.1 |
| Hemiberlesia rapax | DQ145344.2 | GQ4249859.1 |
| Lindingaspis rossi | GQ325507.1 | GQ424933.1 |
| Megacanthaspis leucaspis | DQ145359.2 | GQ424869.1 |
| Melanaspis bromiliae | DQ145360.2 | DQ868835.1 |
| Melanaspis obscura | GQ325511.1 | GQ424850.1 |
| Mycetaspis personata | DQ145366.2 | DQ868838.1 |
| Nuculaspis californica | GQ325515.1 | GQ424839.1 |
| Parlatoria camelliae | DQ145371.2 | DQ868843.1 |
| Parlatoria theae | DQ145373.2 | DQ868841.1 |
| Pinnaspis piperis | DQ145377.2 | DQ868849.1 |
| Pinaspis strachani | DQ145378.2 | --- |
| Poliaspis syringae | GQ325539.1 | GQ424948.1 |
| Pseudalacaspis cockerelli | DQ145384.2 | DQ868851.1 |
| Pseudalacaspis pentagona | DQ145385.2 | DQ868852.1 |
| Pseudococcus longispinus* | AY427400.1 | JF714175.1 |
| Thysancoccus pandani | DQ145391.2 | GQ424871.1 |
| Unaspis euonymi | DQ14593.1 | DQ868855.1 |
4. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kondo, T.; Gullan, P.J.; Williams, D.J.; El, C. Coccidology. The study of scale insects (Hemiptera: Sternorrhyncha: Coccoidea). Cienc. y Tecnol. Agropecu. 2008, 9, 55–61. [Google Scholar]
- Miller, D. Selected scale insects groups (Hemiptera: Coccoidae) in the southern region of the United States. Florida Entomol. 2005, 88, 482–501. [Google Scholar] [CrossRef]
- Beardsley, J.W.; Gonzalez, R.H. The biology and ecology of armored scales. Annu. Rev. Entomol. 1975, 20, 47–73. [Google Scholar] [CrossRef]
- Takagi, S. The adult female. In Armored Scale Insects: Their Biology, Natural Enemies, and Control; Rosen, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 5–20. [Google Scholar]
- Brown, S.W.; Chandra, H.S. Chromosome imprinting and the differential regulation of homologous chromosomes [Insects]. Cell Biol. 1977, 1, 109–189. [Google Scholar]
- Normark, B.B. The evolution of alternative genetic systems in insects. Annu. Rev. Entomol. 2003, 48, 397–423. [Google Scholar] [CrossRef]
- Foldi, I. The scale cover. In Armored Scale Insects: Their Biology, Natural Enemies, and Control; Rosen, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 43–54. [Google Scholar]
- Foldi, I. Internal anatomy. In Armored Scale Insects: Their Biology, Natural Enemies, and Control; Rosen, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 65–80. [Google Scholar]
- Veilleux, K.; Miller, D.R.; Ben-Dov, Y. Scale Net. Available online: http://www.sel.barc.usda.gov/scalecgi/chklist.exe?Family=Diaspididae&genus= (accessed on 13 June 2013).
- Miller, D.; Miller, G.; Hodges, G.; Davidson, J. Introduced scale insects (Hemiptera: Coccoidae) of the United States and their impact on U.S. agriculture. Proc. Entomol. Soc. Wash. 2005, 107, 123–158. [Google Scholar]
- Burger, H.C.; Ulenberg, S.A. Quarantine problems and procedures. In Armored Scale Insects: Their Biology, Natural Enemies, and Control; Rosen, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 313–327. [Google Scholar]
- McClure, M. Patterns of host specificity. In Armored Scale Insects: Their Biology, Natural Enemies, and Control; Rosen, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 301–303. [Google Scholar]
- Andersen, J.C.; Wu, J.; Gruwell, M.E.; Gwiazdowski, R.; Santana, S.E.; Feliciano, N.M.; Morse, G.E.; Normark, B.B. A phylogenetic analysis of armored scale insects (Hemiptera: Diaspididae), based upon nuclear, mitochondrial, and endosymbiont gene sequences. Mol. Phylogenet. Evol. 2010, 57, 992–1003. [Google Scholar] [CrossRef]
- Gwiazdowski, R.; Vea, I.; Andersen, J.C.; Normark, B.B. Discovery of cryptic species among North American pine-feeding Chionaspis scale insects (Hemiptera: Diaspididae). Biol. J. Linn. Soc. 2011, 104, 47–62. [Google Scholar] [CrossRef]
- Morse, G.E.; Normark, B.B. A molecular phylogenetic study of armoured scale insects (Hemiptera: Diaspididae). Syst. Entomol. 2006, 31, 338–349. [Google Scholar] [CrossRef]
- Provencher, L.M.; Morse, G.E.; Weeks, A.R.; Normark, B.B. Parthenogenesis in the Aspidiotus nerii complex (Hemiptera: Diaspididae): A single origin of a worldwide, polyphagous lineage associated with Cardinium bacteria. Annu. Entomol. Soc. Am. 2005, 98, 629–635. [Google Scholar] [CrossRef]
- Gruwell, M.; Morse, G.; Normark, B. Phylogenetic congruence of armored scale insects (Hemiptera: Diaspididae) and their primary endosymbionts from the phylum Bacteroidetes. Mol. Phylogenet. Evol. 2007, 44, 267–280. [Google Scholar] [CrossRef]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Wheeler, D.L. GenBank: Update. Nucleic Acids Res. 2004, 32, D23. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Gruwell. Penn State Behrend Faculty Resource Page. Available online: http://psbehrend.psu.edu/school-of-science/faculty-staff-directory/biology/matthew-gruwell-ph-d/ (accessed on 25 June 2014).
- Katoh Standley 2013. MAFFT Version 7 multiple alignment program for amino acid or nucleotide sequences, online version. Available online: http://mafft.cbrc.jp/alignment/server/ (accessed on 15 June 2014).
- Van Lenterew, J.C.; DeBach, P. Host discrimination in three ectoparasites (Aphytis coheni, A. lingnanensis and A. melinus) of the oleander scale (Aspidiotus nerii). Neth. J. Zool. 1980, 31, 504–532. [Google Scholar] [CrossRef]
- Einhorn, J.; Guerrero, A.; Ducrot, P.H.; Boyer, F.D.; Gieselmann, M.; Roelofs, W. Sex pheromone of the oleander scale, Aspidiotus nerii: Structural characterization and absolute configuration of an unusual functionalized cyclobutane. Proc. Natl. Acad. Sci. USA 1998, 95, 9867–9872. [Google Scholar] [CrossRef]
- Ross, L.; Hardy, N.B.; Okusu, A.; Normark, B.B. Large population size predicts the distribution of asexuality in scale insects. Evolution 2013, 67, 196–206. [Google Scholar]
- BOLD Barcode of Life Database. http://www.boldsystems.org/index.php/Public_SearchTerms?query=Diaspididae[tax]/ (accessed on 12 June 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Campbell, A.M.; Lawrence, A.J.; Hudspath, C.B.; Gruwell, M.E. Molecular Identification of Diaspididae and Elucidation of Non-Native Species Using the Genes 28s and 16s. Insects 2014, 5, 528-538. https://doi.org/10.3390/insects5030528
Campbell AM, Lawrence AJ, Hudspath CB, Gruwell ME. Molecular Identification of Diaspididae and Elucidation of Non-Native Species Using the Genes 28s and 16s. Insects. 2014; 5(3):528-538. https://doi.org/10.3390/insects5030528
Chicago/Turabian StyleCampbell, Alexander M., Andrew J. Lawrence, Caleb B. Hudspath, and Matthew E. Gruwell. 2014. "Molecular Identification of Diaspididae and Elucidation of Non-Native Species Using the Genes 28s and 16s" Insects 5, no. 3: 528-538. https://doi.org/10.3390/insects5030528




