Honey Bee Location- and Time-Linked Memory Use in Novel Foraging Situations: Floral Color Dependency
Abstract
:1. Introduction
2. Experimental Section

2.1. Blue and Yellow Flowers: Reward Odor Differing between Sites
| Exp. # | Flower Colors | Morning | Afternoon | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | |||||||||
| Treatment 1 | Treatment 2 | Treatment 3 | ||||||||
| Rewards | Odor | Flower Shape | Rewards | Odor | Flower Shape | Rewards | Odor | Flower Shape | ||
| 1 | Blue & Yellow | 1 M both colors | clove | flat | 1 M:2 M | clove | flat | 1 M both colors | mint | flat |
| 2 | Blue & Yellow | 1 M both colors | clove | flat | 1 M:2 M | clove | flat | 1 M both colors | mint | tube |
| 3 | Blue & White | 1 M both colors | clove | flat | 1 M:2 M | clove | flat | 1 M both colors | mint | flat |
| 4 | Blue & White | 1 M both colors | clove | flat | 1 M:2 M | clove | flat | 1 M both colors | mint | tube |
2.2. Blue and Yellow Flowers: Flower Shape and Reward Odor Differing between Sites
2.3. Blue and White Flowers: Reward Odor Differing between Sites
2.4. Blue and White Flowers: Flower Shape and Reward Odor Differing between Sites
2.5. Data Analysis
3. Results and Discussion
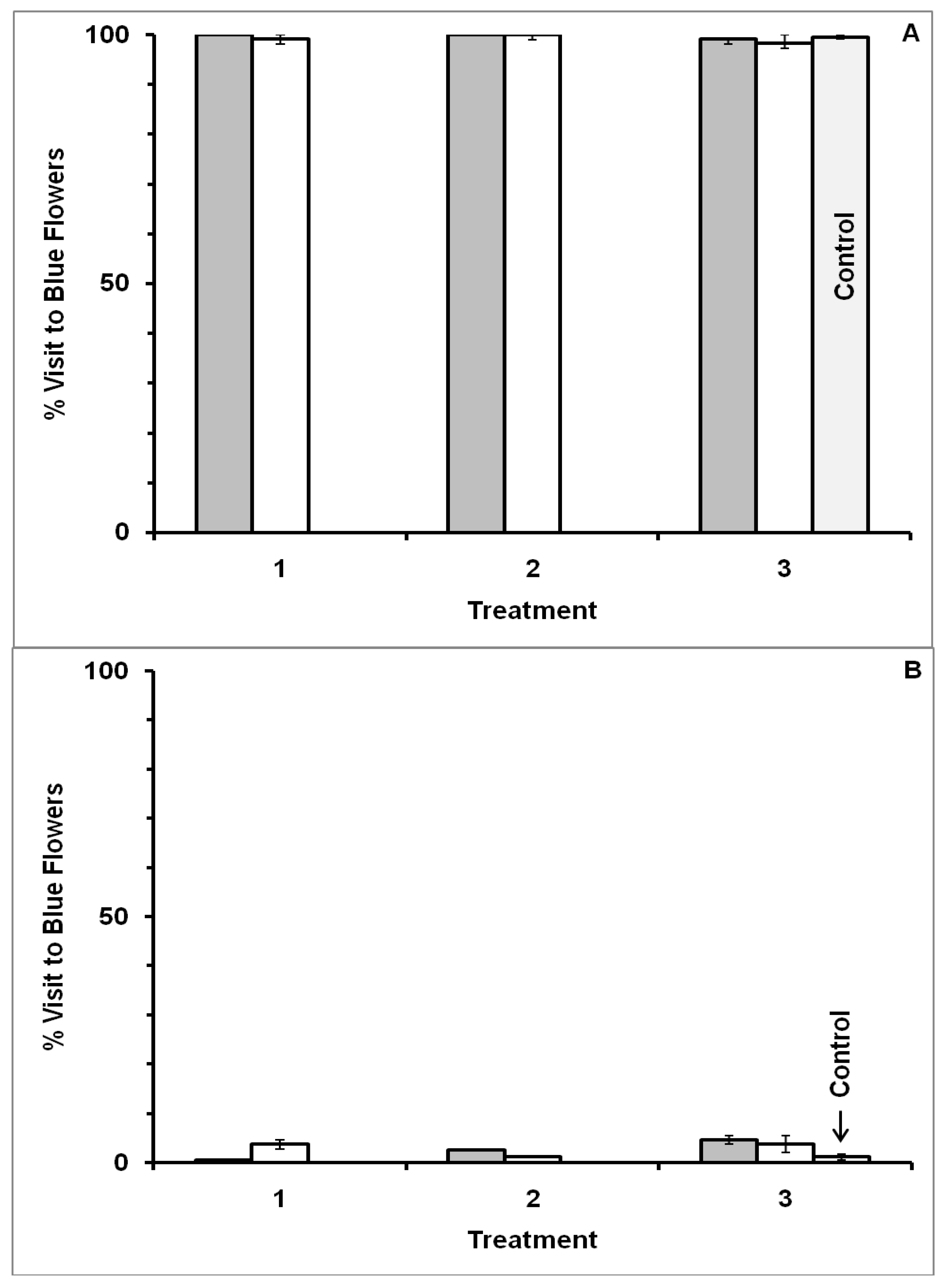
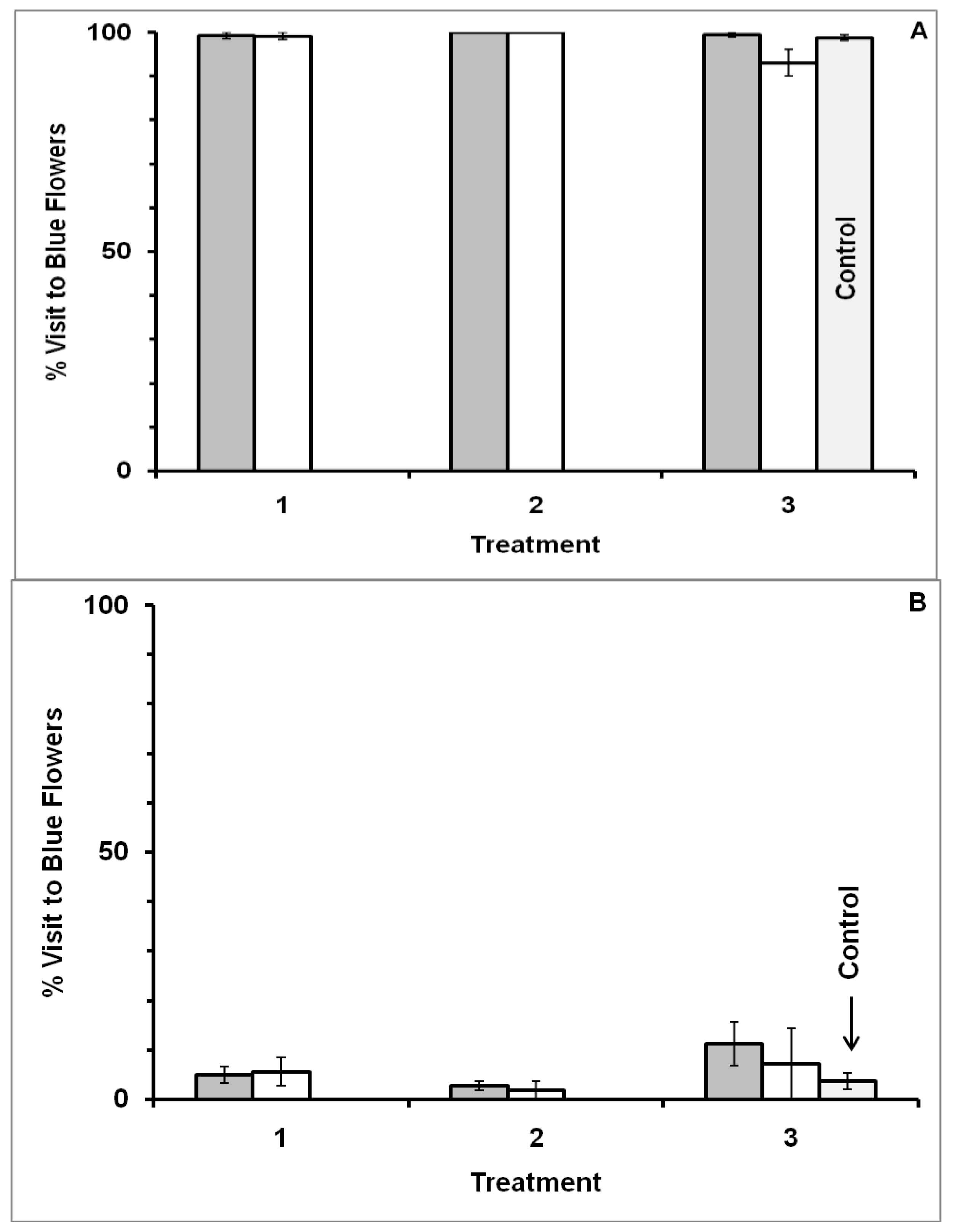
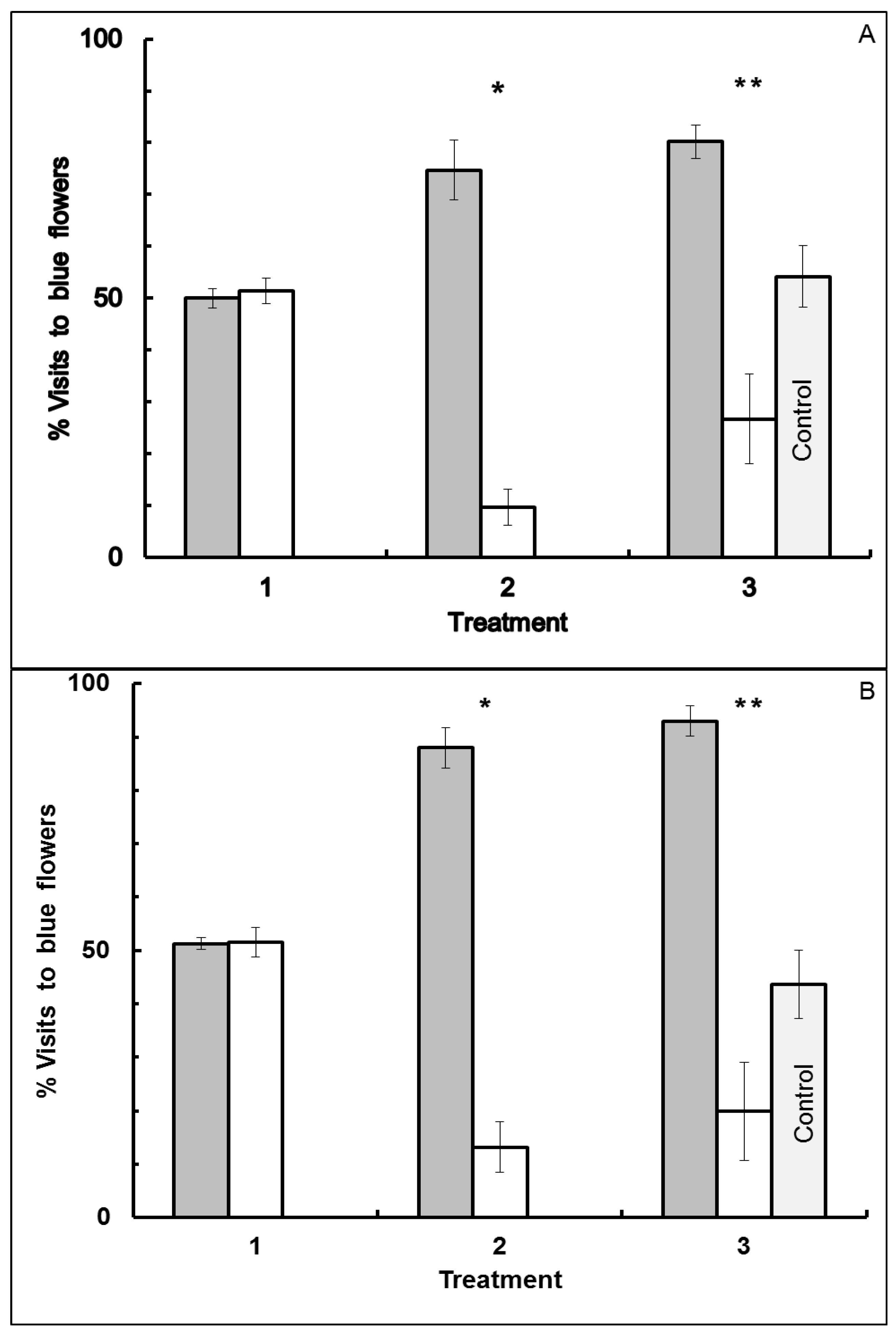
3.1. Blue-Yellow Dimorphic Flower Patches
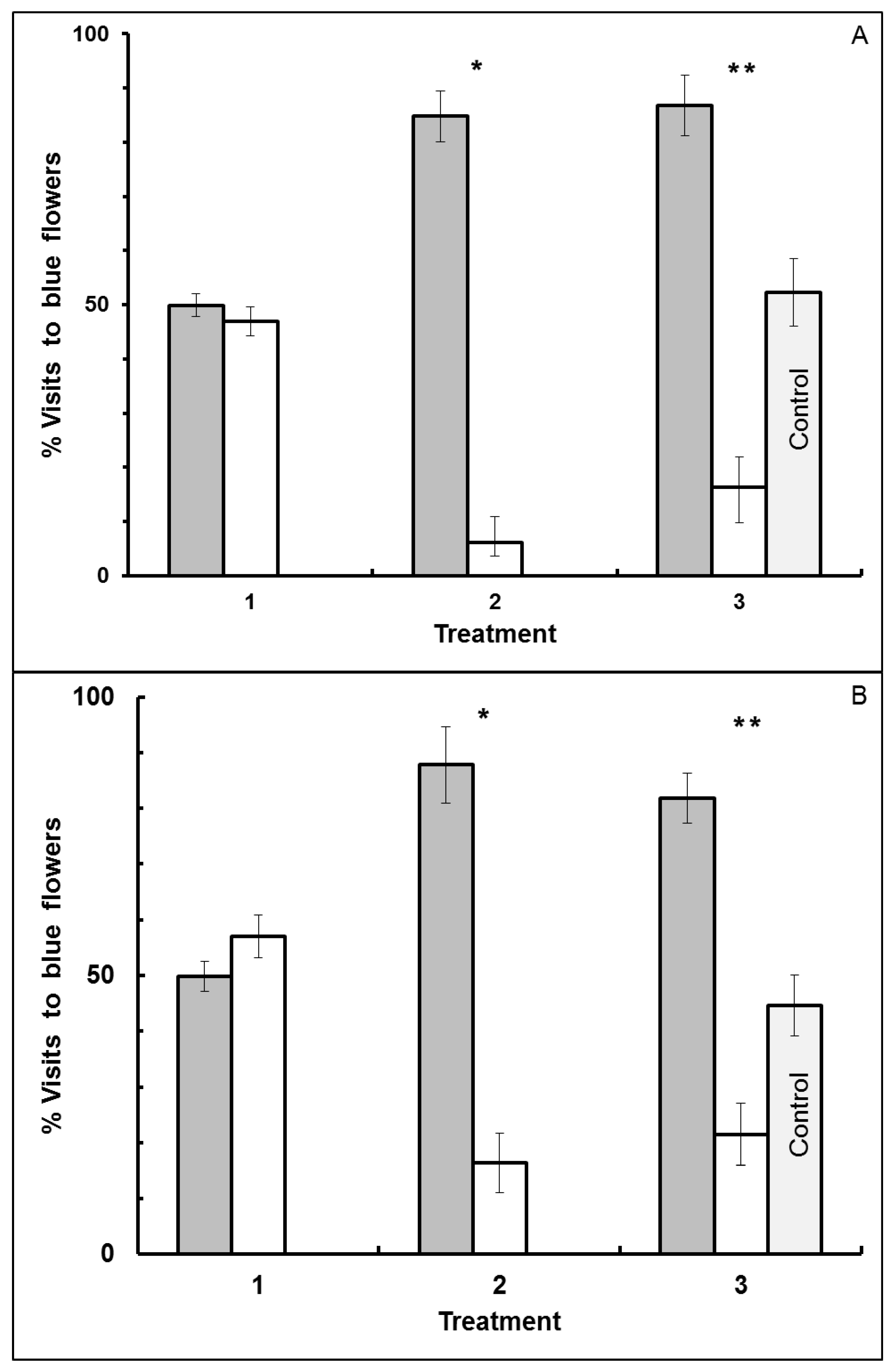
3.2. Blue-White Dimorphic Flower Patches
4. Conclusions
4.1. Blue-Yellow Dimorphic Flower Patches
4.2. Blue-White Dimorphic Flower Patches
4.3. Angiosperm Evolution
4.4. Agricultural Settings
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MA, USA, 2000; p. 992. [Google Scholar]
- Linnaeus, C. Calendarium Florae; Hojer: Uppsala, Sweden, 1756; p. 17. [Google Scholar]
- Percival, M. Floral Biology; Pergamon Press: Oxford, UK, 1965; p. 243. [Google Scholar]
- Endress, P.K. Diversity and Evolutionary Biology of Tropical Flowers; Cambridge University Press: Cambridge, MA, USA, 1994; p. 511. [Google Scholar]
- Heinrich, B. Bumblebee Economics; Harvard University Press: Cambridge, MA, USA, 1979; p. 245. [Google Scholar]
- Willmer, P.G.; Stone, G.N. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv. Study Behav. 2004, 34, 347–466. [Google Scholar] [CrossRef]
- Wcislo, W.T. Behavioral environments and evolutionary change. Annu. Rev. Ecol. Syst. 1989, 20, 137–169. [Google Scholar]
- Dukas, R. Evolutionary ecology of learning. In Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision Making; Dukas, R., Ed.; The University of Chicago Press: Chicago, IL, USA, 1998; pp. 129–174. [Google Scholar]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003; p. 794. [Google Scholar]
- Boisvert, M.J.; Sherry, D.F. Interval timing by an invertebrate, the bumble bee Bombus impatiens. Curr. Biol. 2006, 16, 1–5. [Google Scholar]
- Boisvert, M.J.; Veal, A.J.; Sherry, D.F. Floral reward production is timed by an insect pollinator. Proc. R. Soc. Lond. B 2007, 274, 1831–1837. [Google Scholar] [CrossRef]
- Ohashi, K.; Leslie, A.; Thomson, J.D. Trapline foraging by bumble bees: V. Effects of experience and priority on competitive performance. Behav. Ecol. 2008, 19, 936–948. [Google Scholar] [CrossRef]
- Ohashi, K.; Leslie, A; Thompson, J.D. Trapline foraging by bumble bees: VII. Adjustments for foraging success following competitor removal. Behav. Ecol. 2012, 24, 768–778. [Google Scholar] [CrossRef] [Green Version]
- Lihoreau, M.; Chittka, L.; Raine, N.E. Travel optimization by foraging bumblebees through readjustments of traplines after discovery of new feeding locations. Am. Nat. 2010, 176, 744–757. [Google Scholar] [CrossRef]
- Lihoreau, M.; Raine, N.E.; Reynolds, A.M.; Stelzer, R.J.; Lim, K.S.; Smith, A.D.; Osborne, J.L.; Chittka, L. Radar tracking and motion-sensitive cameras on flowers reveal the development of pollinator multi-destination routes over large spatial scales. PLoS Biol. 2012, 10, e1001392. [Google Scholar] [CrossRef] [Green Version]
- Healy, S.D.; de Kort, S.R.; Clayton, N.S. The hippocampus, spatial memory and food hoarding: A puzzle revisited. Trends Ecol. Evol. 2005, 20, 17–22. [Google Scholar] [CrossRef]
- Raine, N.E.; Chittka, L. Pollen foraging: Learning a complex motor skill by bumblebees (Bombus terrestris). Naturwissenschaften 2007, 94, 459–464. [Google Scholar] [CrossRef]
- Raine, N.E.; Chittka, L. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. Lond. B 2008, 275, 803–808. [Google Scholar] [CrossRef]
- Mery, F.; Kawecki, T.J. An operating cost of learning in Drosophila melanogaster. Anim. Behav. 2004, 68, 589–598. [Google Scholar] [CrossRef]
- Dukas, R.; Bernays, E. Learning improves growth rate in grasshoppers. Proc. Natl. Acad. Sci. USA 2000, 97, 2637–2640. [Google Scholar] [CrossRef]
- Smith, C.C.; Reichman, O.J. The evolution of food caching by birds and mammals. Annu. Rev. Ecol. Syst. 1984, 15, 329–351. [Google Scholar]
- Rescorla, R.A. Behavioral studies of Pavlovian conditioning. Annu. Rev. Neurosci. 1988, 11, 329–352. [Google Scholar] [CrossRef]
- Stephens, D.W. Variance and the value of information. Am. Nat. 1989, 134, 128–140. [Google Scholar]
- Kerr, B.; Feldman, M.W. Carving the cognitive niche: Optimal learning strategies in homogeneous and heterogeneous environments. J. Theor. Biol. 2003, 220, 169–188. [Google Scholar] [CrossRef]
- Lewis, A.C. Memory constraints and flower choice in Pieris rapae. Science 1986, 232, 863–865. [Google Scholar]
- Dukas, R. Cost of memory: Ideas and predictions. J. Theor. Biol. 1999, 197, 41–51. [Google Scholar] [CrossRef]
- Heinrich, B. Bee flowers: A hypothesis on flower variety and blooming times. Evolution 1975, 29, 325–335. [Google Scholar] [CrossRef]
- Goulson, D. Are insects flower constant because they use search images to find flowers? Oikos 2000, 88, 547–552. [Google Scholar]
- Chittka, L.; Gumbert, A.; Kunze, J. Foraging dynamics of bumble bees: Correlates of movements within and between plant species. Behav. Ecol. 1997, 8, 239–249. [Google Scholar] [CrossRef]
- Raine, N.E.; Chittka, L. Flower constancy and memory dynamics in bumblebees (Hymenoptera: Apidae: Bombus). Entomol. Gen. 2007, 29, 179–199. [Google Scholar] [CrossRef]
- Harley, C.B. Learning and the evolutionary stable strategy. J. Theor. Biol. 1981, 89, 611–633. [Google Scholar] [CrossRef]
- Dukas, R. Constraints on information processing and their effects on behavior. In Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision Making; Dukas, R., Ed.; The University of Chicago Press: Chicago, IL, USA, 1998; pp. 89–128. [Google Scholar]
- Mery, F.; Kawecki, T.J. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 2003, 270, 2465–2469. [Google Scholar] [CrossRef]
- Abramson, C.I.; Aquino, I.S. Behavioral studies of learning in the Africanized honey bee (Apis mellifera L.). Brain Behav. Evol. 2002, 59, 68–86. [Google Scholar]
- Abramson, C.I.; Aquino, I.S.; Silva, M.C.; Price, J.M. Learning in the Africanized honey bee: Apis mellifera L. Physiol. Behav. 1997, 62, 657–674. [Google Scholar] [CrossRef]
- Couvillon, M.J.; DeGrandi-Hoffman, G.; Gronenberg, W. Africanized honeybees are slower learners than their European counterparts. Naturwissenschaften 2010, 97, 153–160. [Google Scholar] [CrossRef]
- Waddington, K.D.; Holden, L.R. Optimal foraging: On flower selection by bees. Am. Nat. 1979, 114, 179–196. [Google Scholar]
- Wells, H.; Wells, P.H. Honey bee foraging ecology: Optimal diet, minimal uncertainty or individual constancy? J. Anim. Ecol. 1983, 52, 829–836. [Google Scholar] [CrossRef]
- Wells, H.; Wells, P.H. Optimal diet, minimal uncertainty and individual constancy in the foraging of honey bee Apis mellifera. J. Anim. Ecol. 1986, 55, 375–384. [Google Scholar]
- Wells, H.; Hill, P.S.; Wells, P.H. Nectarivore foraging ecology: Rewards differing in sugar types. Ecol. Entomol. 1992, 17, 280–288. [Google Scholar] [CrossRef]
- Greggers, U.; Menzel, R. Memory dynamics and foraging strategies of the honeybee. Behav. Ecol. Sociobiol. 1993, 32, 17–29. [Google Scholar]
- Greggers, U.; Mauelshagen, J. Matching behavior of honeybees in a multiple-choice situation: The differential effect of environmental stimuli on the choice process. Anim. Learn. Behav. 1997, 25, 458–472. [Google Scholar] [CrossRef]
- Cnaani, J.; Thomson, J.D.; Papaj, D.R. Flower choice and learning in foraging bumblebees: Effects of variation in nectar volume and concentration. Ethology 2006, 112, 278–285. [Google Scholar] [CrossRef]
- Marden, J.H.; Waddington, K.D. Floral choices by honey bees in relation to the relative distances to flowers. Physiol. Entomol. 1981, 6, 431–435. [Google Scholar] [CrossRef]
- Hill, P.S.M.; Hollis, J.; Wells, H. Foraging decisions in nectarivores: Unexpected interactions between flower constancy and energetic rewards. Anim. Behav. 2001, 62, 729–737. [Google Scholar] [CrossRef]
- Heinrich, B. “Majoring” and “minoring” by foraging bumblebees, Bombus vagans: An experimental analysis. Ecology 1979, 60, 245–255. [Google Scholar] [CrossRef]
- Sanderson, C.E.; Orozco, B.S.; Hill, P.S.M.; Wells, H. Honeybee (Apis mellifera ligustica) response to differences in handling time, rewards, and flower colours. Ethology 2006, 112, 937–946. [Google Scholar] [CrossRef]
- Kacelnik, A.; Bateson, M. Risk-sensitivity: Crossroads for the theories of decision making. Trends Cogn. Sci. 1998, 1, 304–309. [Google Scholar] [CrossRef]
- Trimmer, P.C.; Houston, A.I.; Marshall, J.A.R.; Mendl, M.T.; Paul, E.S.; McNamara, J.M. Decision-making under uncertainty: Biases and Bayesians. Anim. Cogn. 2011, 14, 465–476. [Google Scholar] [CrossRef]
- Naug, D.; Arathi, H.S. Sampling and decision rules used by honey bees in a foraging arena. Anim. Cogn. 2007, 10, 117–124. [Google Scholar] [CrossRef]
- Sanderson, C.E.; Cook, P.; Hill, P.S.M.; Orozco, B.S.; Abramson, C.I.; Wells, H. Nectar quality perception by honey bees (Apis mellifera ligustica). J. Comp. Psychol. 2013, 127, 341–351. [Google Scholar] [CrossRef]
- Hill, P.S.M.; Wells, P.H.; Wells, H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 1997, 54, 615–627. [Google Scholar] [CrossRef]
- Wells, H.; Wells, P.H.; Contreras, D. Effect of flower-morph frequency and distribution on recruitment and behaviour of honeybees. J. Apic. Res. 1986, 25, 139–145. [Google Scholar]
- Wells, H.; Rathore, R.R.S. Foraging ecology of the Asian hive bee, Apis cerana indica, within artificial flower-patches. J. Apic. Res. 1994, 33, 219–230. [Google Scholar]
- Cakmak, I.; Wells, H. Honey bee forager individual constancy: Innate or learned? BeeScience 1995, 3, 165–173. [Google Scholar]
- Levin, D.A.; Anderson, W.W. Competition for pollinators between simultaneously flowering species. Am. Nat. 1970, 104, 455–467. [Google Scholar]
- Cakmak, I.; Song, D.S.; Mixson, T.A.; Serrano, E.; Clement, M.L.; Savitski, A.; Johnson, G.; Giray, T.; Abramson, C.I.; Barthell, J.F.; et al. Foraging responses of Turkish honey bee subspecies to flower choices and reward constancy. J. Insect Behav. 2010, 23, 100–116. [Google Scholar] [CrossRef]
- Lehrer, M.; Bischof, S. Detection of model flowers by honey bees: The role of chromatic and achromatic contrast. Naturwissenschaften 1995, 82, 145–147. [Google Scholar] [CrossRef]
- Hanson, C.H.; Graumann, H.O.; Elling, L.J.; Dudley, J.W.; Carnahan, H.L.; Kehr, W.R.; Davis, R.L.; Frosheiser, F.L.; Novin, A.W. Performance of Two-Clone Crosses in Alfalfa and an Unanticipated Self-Pollination Problem; U.S. Department of Agriculture ARS Technical Bulletin No. 1300: Washington, DC, USA, 1964; p. 46.
- Faulkner, G.J. The behavior of honeybees (Apis mellifera) on flowering Brussels sprout inbreds in production of F1 hybrid seed. Hortic. Res. 1971, 11, 60–62. [Google Scholar]
- Faulkner, G.J. Factors affecting field-scale production of seed of F1 hybrid Brussels sprout. Ann. Appl. Biol. 1974, 77, 181–190. [Google Scholar] [CrossRef]
- Free, J.B.; Williams, I.H. The pollination of hybrid kale (Brassica oleraceae L.). J. Agric. Sci. 1973, 81, 557–559. [Google Scholar]
- Free, J.B.; Williams, I.H. Foraging behavior of honeybees and bumblebees on Brussels sprout grown to produce hybrid seed. J. Agric. Res. 1983, 22, 94–97. [Google Scholar]
- Abramson, C.I.; Sanderson, C.; Painter, J.; Barnett, S.; Wells, H. Development of an ethanol model using social insects V: Honey bee foraging decisions under the influence of alcohol. Alcohol 2005, 36, 187–193. [Google Scholar] [CrossRef]
- Gori, D.F. Post-pollination phenomena and adaptive floral changes. In Handbook of ExperimentalPollination Biology, Scientific and Academic ed.; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1983; pp. 31–49. [Google Scholar]
- Abramson, C.I. Aversive conditioning in honey bees (Apis mellifera). J. Comp. Psychol. 1986, 100, 108–116. [Google Scholar] [CrossRef]
- Abramson, C.I. A Primer of Invertebrate Learning: The Behavioural Perspective; American Psychological Association: Washington, DC, USA, 1994; p. 273. [Google Scholar]
- Abramson, C.I.; Nolf, S.L.; Mixson, T.A.; Wells, H. Can honey bees learn the removal of a stimulus as a conditioning cue? Ethology 2010, 116, 843–854. [Google Scholar]
- Menzel, R.; Giurfa, M. Cognitive architecture of a mini-brain: The honeybee. Trends Cogn. Sci. 2001, 5, 62. [Google Scholar] [CrossRef]
- Gegear, R.J.; Laverty, T.M. Effect of a colour dimorphism on the flower constancy of honey bees and bumble bees. Can. J. Zool. 2004, 82, 587–593. [Google Scholar] [CrossRef]
- Chittka, L.; Wells, H. Color vision in bees: Mechanisms, ecology and evolution. In Complex Worlds from Simpler Nervous Systems; Prete, F., Ed.; MIT Press: Cambridge, MA, USA, 2004; pp. 165–191. [Google Scholar]
- Wells, P.H.; Wells, H. Ethological isolation of plants 2. Odour selection by honeybees. J. Apic. Res. 1986, 24, 86–92. [Google Scholar]
- Banschbach, V.S. Colour association influences honey bee choice between sucrose concentrations. J. Comp. Physiol. ANeuroethol. Sens. Neural Behav. Physiol. 1994, 175, 107–114. [Google Scholar]
- Darwin, C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom; Appleton: New York, NY, USA, 1985; p. 338. [Google Scholar]
- Chittka, L.; Shmida, A.; Troje, N.; Menzel, R. Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vis. Res. 1994, 34, 1489–1508. [Google Scholar] [CrossRef]
- Wagner, A.E.; van Nest, B.E.; Hobbs, C.N.; Moore1, D. Persistence, reticence and the management of multiple time memories by forager honey bees. J. Exp. Biol. 2013, 216, 1131–1141. [Google Scholar] [CrossRef]
- Sall, F.; Lehman, A. JMP IN; SAS Institute, Inc., Ducksberry Press: Belmont, CA, USA, 1996; p. 1. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; W.H. Freeman and Company: New York, NY, USA, 1995; p. 850. [Google Scholar]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, MA, USA, 1986; p. 247. [Google Scholar]
- Parker, G.A.; Maynard Smith, J. Optimality theory in evolutionary biology. Nature 1990, 348, 27–33. [Google Scholar] [CrossRef]
- McNamara, J.M.; Houston, A.I. The application of statistical decision theory to animal behaviour. J. Theor. Biol. 1980, 85, 673–690. [Google Scholar] [CrossRef]
- Gigerenzer, G.; Hoffrage, U. How to improve Bayesian reasoning without instruction: Frequency formats. Psychol. Rev. 1995, 102, 684–704. [Google Scholar] [CrossRef]
- Zhu, L.; Gigerenzer, G. Children can solve Bayesian problems: The role of representation in mental computation. Cognition 2006, 98, 287–308. [Google Scholar] [CrossRef]
- Kahneman, D.; Tversky, A. Subjective probability: A judgment of representativeness. Cogn. Psychol. 1972, 3, 430–454. [Google Scholar] [CrossRef]
- Waite, T.A. Preference for oddity: Uniqueness heuristic or hierarchical choice process? Anim. Cogn. 2008, 11, 707–713. [Google Scholar] [CrossRef]
- Dyer, A.G.; Dorin, A.; Reinhardt, V.; Rosa, M.G.P. Colour reverse learning and animal personalities: The advantage of behavioural diversity assessed with agent-based simulations. Available online: http://hdl.handle.net/10101/npre.2012.7037.1/ (accessed on 10 December 2013).
- Zhang, S.; Bock, F.; Si, A.; Tautz, J.; Srinivasan, M.V. Visual working memory in decision making by honey bees. Proc. Natl. Acad. Sci. USA 2005, 102, 5250–5255. [Google Scholar] [CrossRef]
- Butler, C.G. The influence of various physical and biological factors of the environment on honeybee activity. An examination of the relationship between activity and nectar concentration and abundance. J. Exp. Biol. 1945, 21, 5–12. [Google Scholar]
- Visscher, P.K.; Seeley, T.D. Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 1982, 63, 1790–1801. [Google Scholar] [CrossRef]
- Bogdany, F.J. Linking of learning signals in honeybee orientation. Behav. Ecol. Sociobiol. 1978, 3, 323–336. [Google Scholar] [CrossRef]
- Wenner, A.M.; Wells, P.H.; Johnson, D.L. Honeybee recruitment to food sources: Olfaction or language? Science 1969, 164, 84–86. [Google Scholar]
- Farina, W.M.; Gruter, C.; Diaz, P.C. Social learning of floral odours inside the honeybee hive. Proc. R. Soc. Lond. B 2005, 272, 1923–1928. [Google Scholar] [CrossRef]
- Johnson, D.L.; Wenner, A.M. A relationship between conditioning and communication in honey bees. Anim. Behav. 1966, 14, 261–265. [Google Scholar] [CrossRef]
- Reinhard, J.; Srinivasan, M.V.; Zhang, S. Scent-triggered navigation in honeybees. Nature 2004, 427, 411. [Google Scholar] [CrossRef]
- Prete, F.R.; Wells, H.; Wells, P.H.; Hurd, L.E. The Praying Mantids: Research Perspectives; Johns Hopkins University Press: Baltimore, MD, USA, 1999; p. 362. [Google Scholar]
- Wells, P.H.; Wells, H. Can honey bees change foraging patterns? Ecol. Entomol. 1984, 9, 407–473. [Google Scholar]
- Brown, M.F.; McKeon, D.; Curley, T.; Weston, B.; Lambert, C.; Lebowitz, B. Working memory for color in honeybees. Anim. Learn. Behav. 1998, 26, 264–271. [Google Scholar] [CrossRef]
- Amaya-Márquez, M.; Wells, H. Social complexity and learning foraging tasks in bees. Caldasia 2008, 30, 469–477. [Google Scholar]
- Raine, N.E.; Chittka, L. No trade-off between learning speed and associative flexibility in bumblebees: A reversal learning test with multiple colonies. PLoS One 2012, 7, e45096. [Google Scholar] [CrossRef] [Green Version]
- Menzel, R.; Erber, J. Learning and memory in bees. Sci. Am. 1978, 239, 102–110. [Google Scholar] [CrossRef]
- Abramson, C.I. Aversive conditioning in honey bees (Apis mellifera). J. Comp. Psychol. 1986, 100, 108–116. [Google Scholar] [CrossRef]
- Pahl, M.; Zhu, H.; Pix, W.; Tautz, J.; Zhang, S. Circadian timed episodic-like memory—A bee knows what to do when, and also where. J. Exp. Biol. 2007, 210, 3559–3567. [Google Scholar] [CrossRef]
- Zhang, S.W.; Lehrer, M.; Srinivasan, M.V. Honeybee memory: Navigation by associative grouping and recall of visual stimuli. Neurobiol. Learn. Mem. 1999, 72, 180–201. [Google Scholar] [CrossRef]
- Zhang, S.; Schwarz, S.; Pahl, M.; Zhu, H.; Tautz, J. Honeybee memory: A honeybee knows what to do and when. J. Exp. Biol. 2006, 209, 4420–4428. [Google Scholar] [CrossRef]
- Motro, U.; Shmida, A. Near-far search—An evolutionarily stable foraging strategy. J. Theor. Biol. 1995, 73, 15–22. [Google Scholar] [CrossRef]
- Burns, J.G.; Thomson, J.D. A test of spatial memory and movement patterns of bumblebees at multiple spatial and temporal scales. Behav. Ecol. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- Collett, T.S.; Fauria, K.; Dale, K.; Baron, J. Places and patterns—A study of context learning in honeybees. J. Comp. Physiol. A 1997, 181, 343–353. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987; p. 281. [Google Scholar]
- Horridge, G.A.; Zhang, S.W. Pattern vision in honeybees (Apis mellifera): Flower-like patterns with no predominant orientation. J. Insect Physiol. 1995, 41, 681–688. [Google Scholar] [CrossRef]
- Waser, N.M. Flower constancy: Definition, cause, and measurement. Am. Nat. 1986, 127, 593–603. [Google Scholar]
- Kunze, J.; Gumbert, A. The combined effect of color and odor on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 2001, 12, 447–456. [Google Scholar] [CrossRef]
- Gumbert, A.; Kunze, J. Colour similarity to rewarding model plants affects pollination in a food deceptive orchid, Orchis boryi. Biol. J. Linn. Soc. 2001, 72, 419–433. [Google Scholar] [CrossRef]
- Renner, S.S. Rewardless flowers in the angiosperms and the role of insect cognition in their evolution. In Plant-Pollinator Interactions: From Specialization to Generalization; Waser, N.M., Ollerton, J., Eds.; The University of Chicago Press: Chicago, IL, USA, 2006; pp. 123–144. [Google Scholar]
- Chittka, L.; Menzel, R. The evolutionary adaptation of flower colours and the insect pollinator’s colour vision. J. Comp. Physiol. A 1992, 171, 171–181. [Google Scholar]
- Bernays, E.A.; Wcislo, W.T. Sensory capabilities, information processing, and resource specialization. Q. Rev. Biol. 1994, 69, 187–204. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Their Behavior and Ecology; Oxford University Press: New York, NY, USA, 2003; p. 236. [Google Scholar]
- Menzel, R. Behavioral and neural mechanisms of learning and memory as determinants of flower constancy. In Cognitive Ecology of Pollination: Animal Behaviour and Floral Evolution; Chittka, L., Thomson, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 21–40. [Google Scholar]
- Menzel, R. Learning in honey bees in an ecological and behavioral context. In Experimental Behavioral Ecology and Sociobiology:in Memoriam Karl von Frisch 1886–1982; Hölldobler, B., Lindauer, M., Eds.; Sinauer Associates: Sunderland, MA, USA, 1985; pp. 55–74. [Google Scholar]
- Free, J.B. The foraging behaviour of bees and its effects on the isolation and speciation of plants. In Reproductive Biology and Taxonomy of Vascular Plants; BSBI Conference Report No. 9; Hawkes, J.G., Ed.; Pergamon Press: New York, NY, USA, 1966; pp. 76–92. [Google Scholar]
- Free, J.B. Insect Pollination of Crops; Academic Press: New York, NY, USA, 1970; p. 544. [Google Scholar]
- Grant, V. Pollination systems as isolating mechanisms in angiosperms. Evolution 1949, 3, 82–97. [Google Scholar] [CrossRef]
- Grant, V. The flower constancy of bees. Bot. Rev. 1950, 16, 379–398. [Google Scholar] [CrossRef]
- Bateman, A.J. The taxonomic discrimination of bees. Heredity 1951, 5, 271–278. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Amaya-Márquez, M.; Hill, P.S.M.; Abramson, C.I.; Wells, H. Honey Bee Location- and Time-Linked Memory Use in Novel Foraging Situations: Floral Color Dependency. Insects 2014, 5, 243-269. https://doi.org/10.3390/insects5010243
Amaya-Márquez M, Hill PSM, Abramson CI, Wells H. Honey Bee Location- and Time-Linked Memory Use in Novel Foraging Situations: Floral Color Dependency. Insects. 2014; 5(1):243-269. https://doi.org/10.3390/insects5010243
Chicago/Turabian StyleAmaya-Márquez, Marisol, Peggy S. M. Hill, Charles I. Abramson, and Harrington Wells. 2014. "Honey Bee Location- and Time-Linked Memory Use in Novel Foraging Situations: Floral Color Dependency" Insects 5, no. 1: 243-269. https://doi.org/10.3390/insects5010243
APA StyleAmaya-Márquez, M., Hill, P. S. M., Abramson, C. I., & Wells, H. (2014). Honey Bee Location- and Time-Linked Memory Use in Novel Foraging Situations: Floral Color Dependency. Insects, 5(1), 243-269. https://doi.org/10.3390/insects5010243




