Brevibacillus laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species
Abstract
:1. Introduction
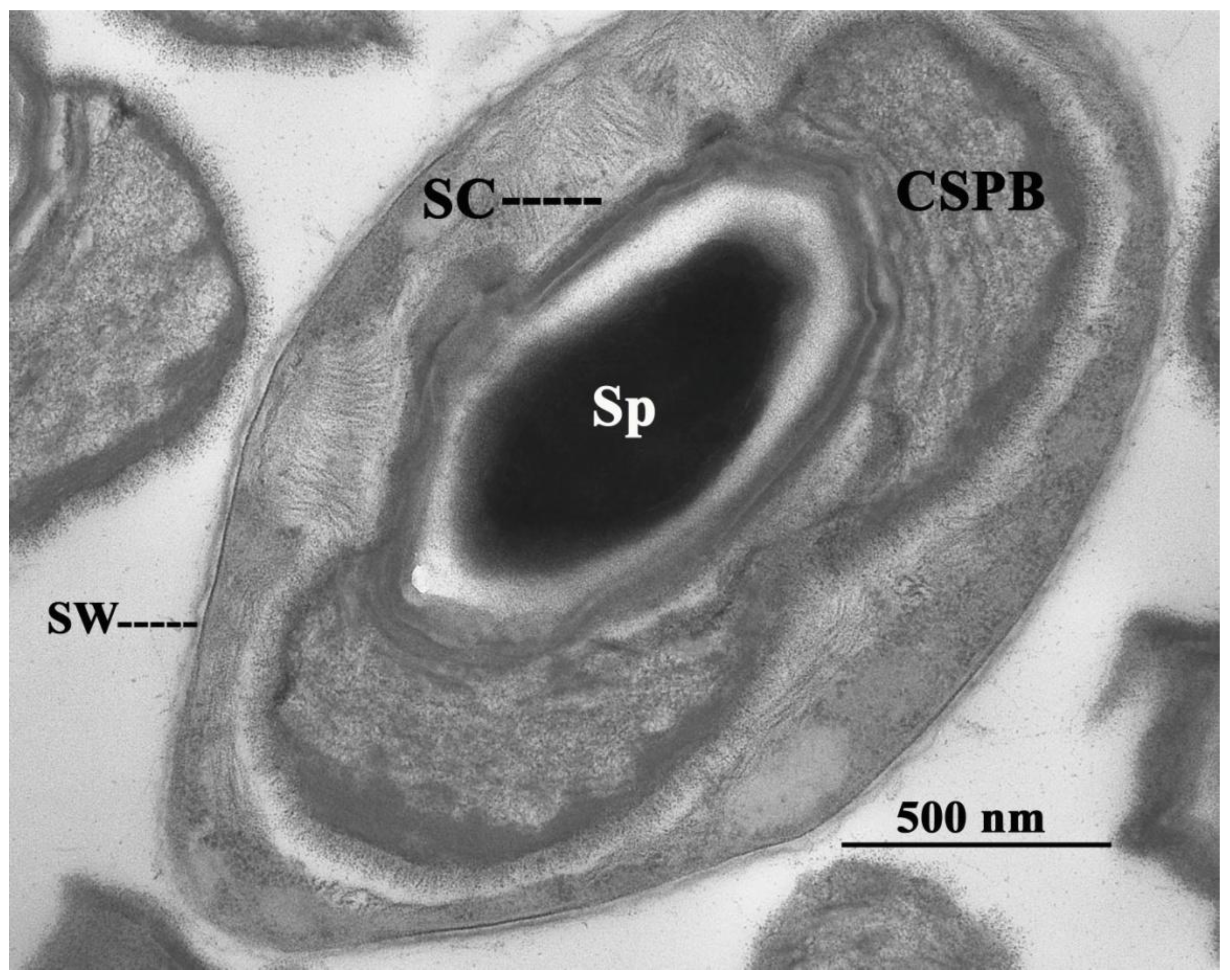
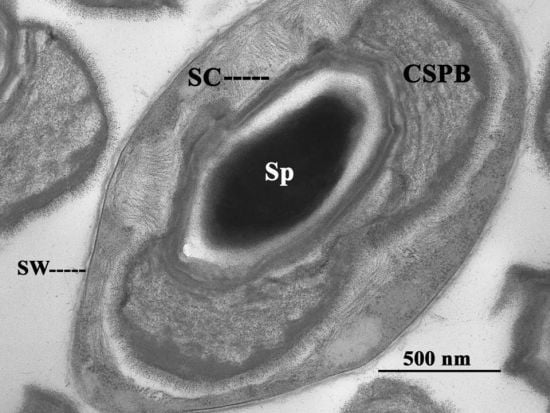
2. The Canoe-Shaped Parasporal Body (CSPB) and Other Parasporal Bodies (PBs)
3. Pathogenicity for Invertebrates
3.1. Insects
3.1.1. Coleoptera
3.1.2. Lepidoptera
3.1.3. Mosquitoes and Black Flies
3.1.4. House Fly
3.2. Nematodes
3.3. Mollusks
4. Antimicrobial Features
5. Other Properties and Uses
6. Conclusions
| Active compounds | Main activity | References |
|---|---|---|
| Complementary toxins ISP1 and ISP2 (=Vip1Da1; Vip2Ad1) | Insecticidal (Coleoptera) | [40,41] |
| Complementary toxins MIS and RAR (=Vip1Ba1 and Vip2Ba1) | Insecticidal (Coleoptera) | [42] |
| Insecticidal crystal proteins (PBs) | Mosquitocidal | [49] |
| Proteins from spores and CSPB | Insecticidal (mosquitoes, flies) | [34,43,45] |
| Low MW (2,900 Da) protein | Nematicidal | [59] |
| Alkaline protease BLG4 | Nematicidal | [60,61,62,63,64,65] |
| Chitinases (i.e., ChiA1, chitodextrinase) | Insecticidal, fungicidal | [79] |
| Gramicidin S and D | Molluscicidal | [69] |
| Diverse antimicrobial compounds | Antibacterial, antifungal | [71,72] |
| BL-A60 Antimicrobial peptide | Antibacterial, antifungal | [77] |
| Laterosporulin | Antibacterial | [80] |
| Antibiotics and drugs (i.e., laterosporamine, tupuseleiamides, basilikamides, loloatins, bogorols, taraumide, laterocidin, bacithrocins A-B-C, leuhistin, spergualin, cephalosporin acylase) | Antibacterial, antifungal, other therapeutic effects | [30,82,83,84,85,86,87] |
| Enzymes (i.e., lignin peroxidase, laccase, aminopyrine N-demethylase, NADH-DCIP reductase and malachite green reductase) | Decontamination, detoxification, bioremediation | [88,92,93,94,95,96,97,98] |
Acknowledgments
Conflicts of Interest
References
- Oliveira, E.J.; Rabinovitch, L.; Monnerat, R.G.; Passos, L.K.; Zahner, V. Molecular characterization of Brevibacillus laterosporus and its potential use in biological control. Appl. Environ. Microbiol. 2004, 70, 6657–6664. [Google Scholar] [CrossRef]
- Khan, M.R.; Saha, M.L.; Afroz, H. Microorganisms associated with gemstones. Bangladesh J. Bot. 2001, 30, 93–96. [Google Scholar]
- Raymundo, A.K.; Capistrano, B.G.; Aquino, A. Isolation, characterization and identification of bacteria from lahar. Philipp. Agric. Sci. 1997, 80, 57–64. [Google Scholar]
- Laubach, A.C. Studies on aerobic, sporebearing, non pathogenic bacteria. Spore bearing organism in water. J. Bacteriol. 1916, 1, 505–512. [Google Scholar]
- Suslova, M.Y.; Lipko, I.A.; Mamaeva, E.V.; Parfenova, V.V. Diversity of cultivable bacteria isolated from the water column and bottom sediments of the Kara Sea shelf. Mikrobiologiia (Russ. Federation) 2012, 81, 484–491. [Google Scholar]
- White, G.F. The cause of European foulbrood. US Dep. Agric. Bur. Entomol. 1912, 157, 1–15. [Google Scholar]
- Roy, D.K.; Singh, G.P.; Sahay, A.; Sahay, D.N.; Suryanarayana, N. Leaf surface microflora for tasar crop improvement. Indian Silk 2006, 45, 19–21. [Google Scholar]
- Sarkar, P.K.; Hasenack, B.; Nout, M.J.R. Diversity and functionality of Bacillus and related genera isolated from spontaneously fermented soybeans (Indian Kinema) and locust beans (African Soumbala). Int. J. Food Microbiol. 2002, 77, 175–186. [Google Scholar] [CrossRef]
- Adegunloye, D.V.; Adetuyi, F.C.; Akinyosoye, F.A.; Doyeni, M.O. Microbial analysis of compost using cowdung as booster. Pak. J. Nutr. 2007, 6, 506–510. [Google Scholar] [CrossRef]
- Varadaraj, M.C.; Devi, N.; Keshava, N.; Manjrekar, S.P. Antimicrobial activity of neutralized extracellular culture filtrates of lactic acid bacteria isolated from a cultured Indian milk product ('dahi'). Int. J. Food Microbiol. 1993, 20, 259–267. [Google Scholar] [CrossRef]
- Román-Blanco, C.; Sanz-Gómez, J.J.; López-Díaz, T.-M.; Otero, A.; García-López, M.-L. Numbers and species of Bacillus during the manufacture and ripening of Castellano cheese. Milchwissenschaft 1999, 54, 385–388. [Google Scholar]
- Iurlina, M.O.; Fritz, R. Characterization of microorganisms in Argentinean honeys from different sources. Int. J. Food Microbiol. 2005, 105, 297–304. [Google Scholar] [CrossRef]
- Fangio, M.F.; Roura, S.I.; Fritz, R. Isolation and identification of Bacillus spp. and related genera from different starchy foods. J. Food Sci. 2010, 75, M218–M221. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, C.L.S.N.; Ball, B.K.; Hasija, S.K. Characteristics of extracellular proteases produced by Bacillus laterosporus and Flavobacterium sp. isolated from gelatin-factory effluents. World J. Microbiol. Biotechnol. 1996, 12, 615–617. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, H.; Zhang, Y.; Deng, M.; Wu, Z.; Zhu, L.; Duan, Q.; Xu, B.; Liang, C.; Yue, Z.; Xiao, X. Analysis of the bacterial diversity existing on animal hide and wool: Development of a preliminary PCR-restriction fragment length polymorphism fingerprint database for identifying isolates. J. AOAC Int. 2012, 95, 1750–1754. [Google Scholar] [CrossRef]
- Bagherzadeh Kasmani, F.; Karimi Torshizi, M.A.; Allameh, A.; Shariatmadari, F. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail. Poultry Sci. 2012, 91, 1846–1853. [Google Scholar] [CrossRef]
- McCray, A.H. Spore-forming bacteria of the apiary. J. Agric. Res. 1917, 8, 399–420. [Google Scholar]
- Smith, N.R.; Gordon, R.E.; Clark, F.E. Aerobic sporeforming bacteria. US Dep. Agric. Agric. Monograph. 1952, 16, 114–116. [Google Scholar]
- Steinhaus, E.A. An orientation with respect to members of the genus Bacillus pathogenic for insects. Bacteriol. Rev. 1946, 10, 51–61. [Google Scholar]
- Shida, O.; Takagi, H.; Kadowaki, K.; Komagata, K. Proposal for two new genera, Brevibacillus gen. nov. and Aneurinobacillus gen. nov. Int. J. Syst. Bacteriol. 1996, 46, 939–946. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Emerging entomopathogenic bacteria for insect pest management. Bull. Insectology 2013, 66, 181–186. [Google Scholar]
- Gilliam, M.; Valentine, D.K. Bacteria isolated from the intestinal contents of foraging worker honey bees, Apis mellifera: The Genus Bacillus. J. Invertebr. Pathol. 1976, 28, 275–276. [Google Scholar] [CrossRef]
- Bailey, L. The pathogenicity for honey-bee larvae of microorganisms associated with European foulbrood. J. Insect Pathol. 1963, 5, 198–205. [Google Scholar]
- Alippi, A.M. A comparison of laboratory techniques for the detection of significant bacteria of the honey bee, Apis mellifera, in Argentina. J. Apicult. Res. 1991, 30, 75–80. [Google Scholar]
- Shimanuki, H.; Knox, D.A. Diagnosis of honey bee diseases. US Dep. Agric. Agric. Handbook 1991, AH-690, 10–11. [Google Scholar]
- Djukic, M.; Poehlein, A.; Thürmer, A.; Daniel, R. Genome sequence of Brevibacillus laterosporus LMG 15441, a pathogen of invertebrates. J. Bacteriol. 2011, 193, 5535–5536. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, P.K.; Midha, S.; Ranjan, M.; Korpole, S.; Patil, P.B. Genome sequence of Brevibacillus laterosporus strain GI-9. J. Bacteriol. 2012, 194, 1279. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Porubcan, R.S. Administering Bacillus laterosporus to increase poultry feed conversion and weight gain. U.S. Patent 0,099,624, 29 May 2003. [Google Scholar]
- Umezawa, K.; Takeuchi, T. Spergualin: A new antitumour antibiotic. Biomed. Pharmacother. 1987, 41, 227–232. [Google Scholar]
- Hannay, C.L. The parasporal body of Bacillus laterosporus Laubach. J. Biophys. Biochem. Cytol. 1957, 3, 1001–1010. [Google Scholar] [CrossRef]
- Fitz-James, P.C.; Young, I.E. Morphological and chemical studies of the spores and parasporal bodies of Bacillus laterosporus. J. Biophys. Biochem. Cytol. 1958, 4, 639–649. [Google Scholar] [CrossRef]
- Montaldi, F.A.; Roth, I.L. Parasporal bodies of Bacillus laterosporus sporangia. J. Bacteriol. 1990, 172, 2168–2171. [Google Scholar]
- Rivers, D.B.; Vann, C.N.; Zimmack, H.L.; Dean, D.H. Mosquitocidal activity of Bacillus laterosporus. J. Invertebr. Pathol. 1991, 58, 444–447. [Google Scholar] [CrossRef]
- Singer, S. The Utility of Morphological Group II Bacillus. Adv. Appl. Microbiol. 1996, 42, 219–261. [Google Scholar] [CrossRef]
- Salama, H.S.; Foda, M.S.; El-Bendary, M.A.; Abdel-Razek, A. Infection of red palm weevil, Rhynchophorus ferrugineus, by spore-forming bacilli indigenous to its natural habitat in Egypt. J. Pest Sci. 2004, 77, 27–32. [Google Scholar] [CrossRef]
- Echeverri-Molina, D.; Santolamazza-Carbone, S. Toxicity of synthetic and biological insecticides against adults of the Eucalyptus snout-beetle Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae). J. Pest Sci. 2010, 83, 297–305. [Google Scholar] [CrossRef]
- Du Rand, N.; Laing, M.D. Determination of insecticidal toxicity of three species of entomopathogenic spore-forming bacterial isolates against Tenebrio molitor L. (Coleoptera: Tenebrionidae). Afr. J. Microbiol. Res. 2011, 5, 2222–2228. [Google Scholar]
- Arnaut, G.; Boets, A.; Damme, N.; Van Rie, J. Toxins. U.S. Patent 7,919,609, 5 April 2011. [Google Scholar]
- Warren, G.W. Vegetative insecticidal proteins: Novel proteins for control of corn pests. In Advances in Insect Control: The Role of Transgenic Plants; Carozzi, N.B., Koziel, M.G., Eds.; Taylor & Francis: London, UK, 1997; pp. 109–121. [Google Scholar]
- Bacillus thuringiensis Toxin Nomenclature. Available online: http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/ (accessed on 17 July 2013).
- Schnepf, H.E.; Narva, K.E.; Stockhoff, B.A.; Lee, S.F.; Walz, M.; Sturgis, B. Pesticidal toxins and genes from Bacillus laterosporus strains. U.S. Patent 6,956,116, 18 October 2005. [Google Scholar]
- Favret, E.M.; Yousten, A.A. Insecticidal activity of Bacillus laterosporus. J. Invertebr. Pathol. 1985, 45, 195–203. [Google Scholar] [CrossRef]
- Erturk, O.; Demirbag, Z. Studies on bacterial flora and biological control agent of Cydia pomonella L. (Lepidoptera: Tortricidae). Afr. J. Biotechnol. 2006, 5, 2081–2085. [Google Scholar]
- Ruiu, L.; Floris, I.; Satta, A.; Ellar, D.J. Toxicity of a Brevibacillus laterosporus strain lacking parasporal crystals against Musca domestica and Aedes aegypti. Biol. Contr. 2007, 43, 136–143. [Google Scholar] [CrossRef]
- Smirnova, T.A.; Minenkova, I.B.; Orlova, M.V.; Lecadet, M.-M.; Azizbekyan, R.R. The crystal-forming strains of Bacillus laterosporus. Res. Microbiol. 1996, 147, 343–350. [Google Scholar] [CrossRef]
- Orlova, M.V.; Smirnova, T.A.; Ganushkina, L.A.; Yacubovich, V.Y.; Azizbekyan, R.R. Insecticidal activity of Bacillus laterosporus. Appl. Environ. Microbiol. 1998, 64, 2723–2725. [Google Scholar]
- Goldberg, L.J.; Margalit, J. A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univittatus, Aedes aegypti and Culex pipiens. Mosq. News 1977, 37, 355–358. [Google Scholar]
- Zubasheva, M.V.; Ganushkina, L.A.; Smirnova, T.A.; Azizbekyan, R.R. Larvicidal activity of crystal-forming strains of Brevibacillus laterosporus. Appl. Biochem. Microbiol. 2010, 46, 755–762. [Google Scholar] [CrossRef]
- Zubasheva, M.V.; Ganushkina, L.A.; Smirnova, T.A.; Azizbekyan, R.R. Enhancement of larvicidal activity of Brevibacillus laterosporus by bioincapsulation in Protozoa Tetrahymena pyriformis and Entamoeba moshkovskii. Appl. Biochem. Microbiol. 2011, 47, 762–766. [Google Scholar] [CrossRef]
- Ruiu, L.; Delrio, G.; Ellar, D.J.; Floris, I.; Paglietti, B.; Rubino, S.; Satta, A. Lethal and sub-lethal effects of Brevibacillus laterosporus on the housefly (Musca domestica). Entomol. Exp. Appl. 2006, 118, 137–144. [Google Scholar] [CrossRef]
- Bravo, A.; Gill, S.S.; Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Observations on house fly larvae midgut ultrastructure after Brevibacillus laterosporus ingestion. J. Invertebr. Pathol. 2012, 111, 211–216. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Ultrastructural changes in the gut of adult flies after Brevibacillus laterosporus ingestion. In Proceedings of the 43rd Annual Meeting of the Society for Invertebrate Pathology, Trabzon, Turkey, 11–15 July 2010; Contributed paper 149. p. 97.
- Ruiu, L.; Satta, A.; Floris, I. Immature house fly (Musca domestica) control in breeding sites with a new Brevibacillus laterosporus formulation. Environ. Entomol. 2008, 37, 505–509. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Comparative applications of azadirachtin and Brevibacillus laterosporus based formulations for house fly management experiments in dairy farms. J. Med. Entomol. 2011, 48, 345–350. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Susceptibility of the house fly pupal parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae) to the entomopathogenic bacteria Bacillus thuringiensis and Brevibacillus laterosporus. Biol. Contr. 2007, 43, 188–194. [Google Scholar] [CrossRef]
- Ruiu, L.; Floris, I.; Satta, A. Susceptibility of the honeybee (Apis mellifera L.) to entomopathogenic bacterial toxins used for the biological control. Redia 2007, 90, 87–90. [Google Scholar]
- Bone, L.W.; Singer, S. Control of parasitic nematode ova/larvae with a Bacillus laterosporus. U.S. Patent 5,045,314, 3 September 1991. [Google Scholar]
- Huang, X.; Tian, B.; Niu, Q.; Yang, J.; Zhang, L.; Zhang, K. An extracellular protease from Brevibacillus laterosporus G4 without parasporal crystals can serve as a pathogenic factor in infection of nematodes. Res. Microbiol. 2005, 156, 719–727. [Google Scholar] [CrossRef]
- Maizels, R.M.; Blaxter, M.L.; Selkir, M.E. Forms and functions of nematode surfaces. Exp. Parasitol. 1993, 77, 380–384. [Google Scholar] [CrossRef]
- Lian, L.H.; Tian, B.Y.; Xiong, R.; Zhu, M.Z.; Xu, J.; Zhang, K.Q. Proteases from Bacillus: A new insight into the mechanism of action for rhizobacterial suppression of nematode populations. Lett. Appl. Microbiol. 2007, 45, 262–269. [Google Scholar] [CrossRef]
- Tian, B.; Li, N.; Lian, L.; Liu, J.; Yang, J.; Zhang, K.Q. Cloning, expression and deletion of the cuticle-degrading protease BLG4 from nematophagous bacterium Brevibacillus laterosporus G4. Arch. Microbiol. 2006, 186, 297–305. [Google Scholar] [CrossRef]
- Tian, B.; Ke, C.R.; Huang, W.; Zhang, K.-Q.; Huang, J.-Z. Direct visualization of bacterial infection process in nematode hosts by an improved immunocytochemical method. World J. Microbiol. Biotechnol. 2009, 25, 909–912. [Google Scholar] [CrossRef]
- Tian, B.; Huang, W.; Huang, J.; Jiang, X.; Qin, L. Investigation of protease-mediated cuticle-degradation of nematodes by using an improved immunofluorescence-localization method. J. Invertebr. Pathol. 2009, 101, 143–146. [Google Scholar] [CrossRef]
- Tian, B.; Yang, J.; Lian, L.; Wang, C.; Li, N.; Zhang, K.Q. Role of an extracellular neutral protease in infection against nematodes by Brevibacillus laterosporus strain G4. Appl. Microbiol. Biotechnol. 2007, 74, 372–380. [Google Scholar] [CrossRef]
- Singer, S.; Bair, T.B.; Hammill, T.B.; Berte, A.M.; Correa-Ochoa, M.M.; Stambaugh, A.D. Fermentation and toxin studies of the molluscicidal strains of Bacillus brevis. J. Ind. Microbiol. 1994, 13, 112–119. [Google Scholar] [CrossRef]
- Strayer, D.L.; Hattala, K.A.; Kahnle, A.W. Effects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Can. J. Fish. Aquat. Sci. 2004, 61, 924–941. [Google Scholar] [CrossRef]
- Singer, S.; Van Fleet, A.L.; Viel, J.J.; Genevese, E.E. Biological control of the zebra mussel Dreissena polymorpha and the snail Biomphalaria glabrata, using gramicidin S and D and molluscicidal strains of Bacillus. J. Ind. Microbiol. Biotechnol. 1997, 18, 226–231. [Google Scholar] [CrossRef]
- Chandel, S.; Allan, E.J.; Woodward, S. Biological control of Fusarium oxysporum f.sp. lycopersici on tomato by Brevibacillus brevis. J. Phytopathol. 2010, 158, 470–478. [Google Scholar]
- Saikia, R.; Gogoi, D.K.; Mazumder, S.; Yadav, A.; Sarma, R.K.; Bora, T.C.; Gogoi, B.K. Brevibacillus laterosporus strain BPM3, a potential biocontrol agent isolated from a natural hot water spring of Assam, India. Microbiol. Res. 2011, 166, 216–225. [Google Scholar] [CrossRef]
- Song, Z.; Liu, K.; Lu, C.; Yu, J.; Ju, R.; Liu, X. Isolation and characterization of a potential biocontrol Brevibacillus laterosporus. Afr. J. Microbiol. Res. 2011, 5, 2675–2681. [Google Scholar]
- Idris, H.A.; Labuschagne, N.; Korsten, L. Suppression of Pythium ultimum root rot of sorghum by rhizobacterial isolates from Ethiopia and South Africa. Biol. Contr. 2008, 45, 72–84. [Google Scholar]
- Zhang, S.; Reddy, M.S.; Kokalis-Burelle, N.; Wells, L.W.; Nightengale, S.P.; Kloepper, J.W. Lack of induced systemic resistance in peanut to late leaf spot disease by plant growth-promoting rhizobacteria and chemical elicitors. Plant Dis. 2001, 85, 879–884. [Google Scholar]
- Yobo, K.S.; Laing, M.D.; Hunter, C.H. Effect of commercially available rhizobacteria strains on growth and production of lettuce, tomato and pepper. S. Afr. J. Plant Soil 2004, 21, 230–235. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Dong, X.; Wang, G.; Jia, Y. Bioactivity quantification of a novel antimicrobial peptide by agar diffusion bioassay. In Proceedings of the 2011 International Conference on New Technology of Agricultural Engineering, Zibo, China, 27 May 2011; Article number 5943979. pp. 1096–1099.
- Zhao, J.; Guo, L.; Zeng, H.; Yang, X.; Yuan, J.; Shi, H.; Xiong, Y.; Chen, M.; Han, L.; Qiu, D. Purification and characterization of a novel antimicrobial peptide from Brevibacillus laterosporus strain A60. Peptides 2012, 33, 206–211. [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar]
- Prasanna, L.; Eijsink, V.G.H.; Meadow, R.; Gaseidnes, S. A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl. Microbiol. Biotechnol. 2013, 97, 1601–1611. [Google Scholar]
- Singh, P.K.; Chittpurna; Ashish; Sharma, V.; Patil, P.B.; Korpole, S. Identification, purification and characterization of laterosporulin, a novel bacteriocin produced by Brevibacillus sp. strain GI-9. PLoS One 2012, 7, e31498. [Google Scholar]
- Alippi, A.M.; Reynaldi, F.J. Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources. J. Invertebr. Pathol. 2006, 91, 141–146. [Google Scholar]
- Shoji, J.; Sakazaki, R.; Wakisaka, Y.; Koizumi, K.; Mayama, M. Isolation of a new antibiotic, laterosporamine. (Studies on antibiotics from the genus Bacillus. XIII). J. Antibiot. 1976, 29, 390–393. [Google Scholar]
- Barsby, T.; Kelly, M.T.; Andersen, R.J. Tupuseleiamides and basiliskamides, new acyldipeptides and antifungal polyketides produced in culture by a Bacillus laterosporus isolate obtained from a tropical marine habitat. J. Nat. Prod. 2002, 65, 1447–1451. [Google Scholar]
- Desjardine, K.; Pereira, A.; Wright, H.; Matainaho, T.; Kelly, M.; Andersen, R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: Structure elucidation and synthesis. J. Nat. Prod. 2007, 70, 1850–1853. [Google Scholar]
- Qin, C.; Xu, C.; Zhang, R.; Niu, W.; Shang, X. On-resin cyclization and antimicrobial activity of Laterocidin and its analogues. Tetrahedron Lett. 2010, 51, 1257–1261. [Google Scholar] [CrossRef]
- Kamiyama, T.; Umino, T.; Nakamura, Y.; Itezono, Y.; Sawairi, S.; Satoh, T.; Yokose, K. Bacithrocins A, B and C, novel thrombin inhibitors. J. Antibiot. 1994, 47, 959–968. [Google Scholar] [CrossRef]
- Aoyagi, T.; Yoshida, S.; Matsuda, N.; Ikeda, T.; Hamada, M.; Takeuchi, T. Leuhistin, a new inhibitor of aminopeptidase M, produced by Bacillus laterosporus BMI156–14F1. I. Taxonomy, production, isolation, physico-chemical properties and biological activities. J. Antibiot. 1991, 44, 573–578. [Google Scholar] [CrossRef]
- Aramori, I.; Fukagawa, M.; Tsumura, M.; Iwami, M.; Yokota, Y.; Kojo, H.; Kohsaka, M.; Ueda, Y.; Imanaka, H. Isolation of soil strains producing new cephalosporin acylases. J. Ferment. Bioeng. 1991, 72, 227–231. [Google Scholar] [CrossRef]
- Wolfenden, R.E.; Pumford, N.R.; Morgan, M.J.; Shivaramaiah, S.; Wolfenden, A.D.; Pixley, C.M.; Green, J.; Tellez, G.; Hargis, B.M. Evaluation of selected direct-fed microbial candidates on live performance and Salmonella reduction in commercial turkey brooding houses. Poultry Sci. 2011, 90, 2627–2631. [Google Scholar]
- Kuznetsova, N.I.; Azizbekyan, R.R.; Konyukhov, I.V.; Pogosyan, S.I.; Rubin, A.B. Inhibition of photosynthesis in cyanobacteria and plankton algae by the bacterium Brevibacillus laterosporus metabolites. Dokl. Biochem. Biophys. 2008, 421, 181–184. [Google Scholar]
- Wen, J.; XiangHu, H.; ChangLing, L.; JiaHui, Z. Research on algicidal effect of bioactive metabolites of Brevibacillus laterosporus on Oscillattoria sp. in shrimp pond. J. Fish. Chin. 2013, 37, 465–472. [Google Scholar]
- Lim, J.G.; Park, D.H. Degradation of polyvinyl alcohol by Brevibacillus laterosporus: Metabolic pathway of polyvinyl alcohol to acetate. J. Microbiol. Biotechnol. 2001, 11, 928–933. [Google Scholar]
- Gomare, S.S.; Govindwar, S.P. Brevibacillus laterosporus MTCC 2298: A potential azo dye degrader. J. Appl. Microbiol. 2009, 106, 993–1004. [Google Scholar]
- Jeyaseelan, A.; Sivashanmugam, K.; Jayaraman, K. Comparative applications of bioreactor and shake flask system for the biodegradation of tannin and biotreatment of composite tannery effluents. Pollut. Res. 2008, 27, 371–375. [Google Scholar]
- Reda, A.B.; Ashraf, T.A.-H. Optimization of bacterial biodegradation of toluene and phenol under different nutritional and environmental conditions. J. Appl. Sci. Res. 2010, 6, 1086–1095. [Google Scholar]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Holail, H.; Al-Bahadly, A.; Olama, Z. Detoxification of hexavalent chromium Cr(VI) by Bacillus laterosporus and its application in Lebanese waste water. WIT Trans. Ecol. Environ. 2011, 153, 233–242. [Google Scholar] [CrossRef]
- Kamika, I.; Momba, M.N.B. Comparing the tolerance limits of selected bacterial and protozoan species to nickel in wastewater systems. Sci. Total Environ. 2011, 410–411, 172–181. [Google Scholar] [CrossRef]
- Zahner, V.; Rabinovitch, L.; Suffys, P.; Momen, H. Genotypic diversity among Brevibacillus laterosporus strains. Appl. Environ. Microbiol. 1999, 65, 5182–5185. [Google Scholar]
- Arulmani, M.; Aparanjini, K.; Vasanthi, K.; Arumugam, P.; Arivuchelvi, M.; Kalaichelvan, P.T. Purification and partial characterization of serine protease from thermostable alkalophilic Bacillus laterosporus-AK1. World J. Microbiol. Biotechnol. 2007, 23, 475–481. [Google Scholar]
- Usharani, B.; Muthuraj, M. Production and characterization of protease enzyme from Bacillus laterosporus. Afr. J. Microbiol. Res. 2010, 4, 1057–1063. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruiu, L. Brevibacillus laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species. Insects 2013, 4, 476-492. https://doi.org/10.3390/insects4030476
Ruiu L. Brevibacillus laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species. Insects. 2013; 4(3):476-492. https://doi.org/10.3390/insects4030476
Chicago/Turabian StyleRuiu, Luca. 2013. "Brevibacillus laterosporus, a Pathogen of Invertebrates and a Broad-Spectrum Antimicrobial Species" Insects 4, no. 3: 476-492. https://doi.org/10.3390/insects4030476