Sperm Cells of a Primitive Strepsipteran
Abstract
:1. Introduction
2. Experimental Section
2.1. Collection of Insects
2.2. Preparation of Tissues for Light and Electron Microscopy
2.2.1. Preparation of Tissues for Sectioning
2.2.2. Preparation of Sperm Whole Mounts
3. Results and Discussion
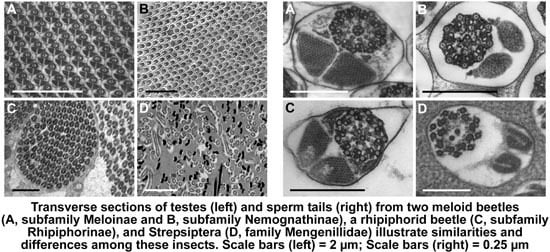
3.1. Testis Organization
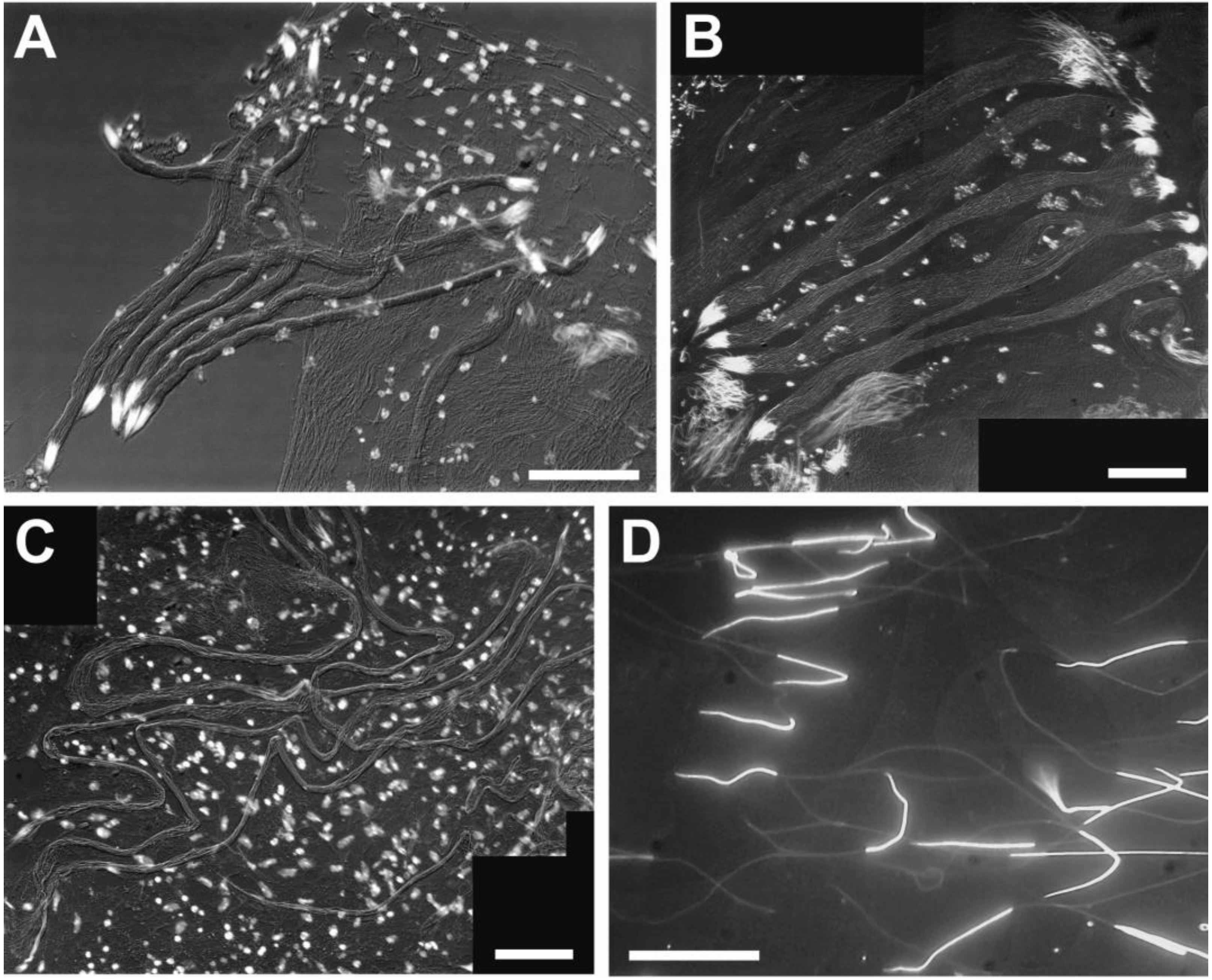
3.2. Sperm Architecture

| Order, Family | Species, Subfamily | Total Length ± s.d. (μm) | Head Length ± s.d. (μm) | ~Ratio of Tail Length:Head Length |
|---|---|---|---|---|
| Coleoptera, Meloidae | Zonitis flava Nemognathinae | 365.1 ± 22.3 (n = 4) | 13.3 ± 1.1 (n = 9) | ~27:1 |
| Coleoptera, Meloidae | Hycleus scutellatus Meloinae | 362.5 ± 13.4 (n = 8) | 18.1 ± 3.3 (n = 11) | ~19:1 |
| Coleoptera, Meloidae | Mylabris variabilis Meloinae | 603.3 ± 77.1 (n = 8) | 29.8 ± 4.4 (n = 32) | ~19:1 |
| Coleoptera, Rhipiphoridae | Macrosiagon tricuspidata Rhipiphorinae | 1,236.3 ± 38.1 (n = 4) | 32.8 ± 3.3 (n = 13) | ~37:1 |
| Strepsiptera, Mengenillidae | Eoxenos laboulbenei | 55.3 ± 7.8 (n = 6) | 17.6 ± 2.2 (n = 6) | ~2:1 |
3.3. Sperm Ultrastructure
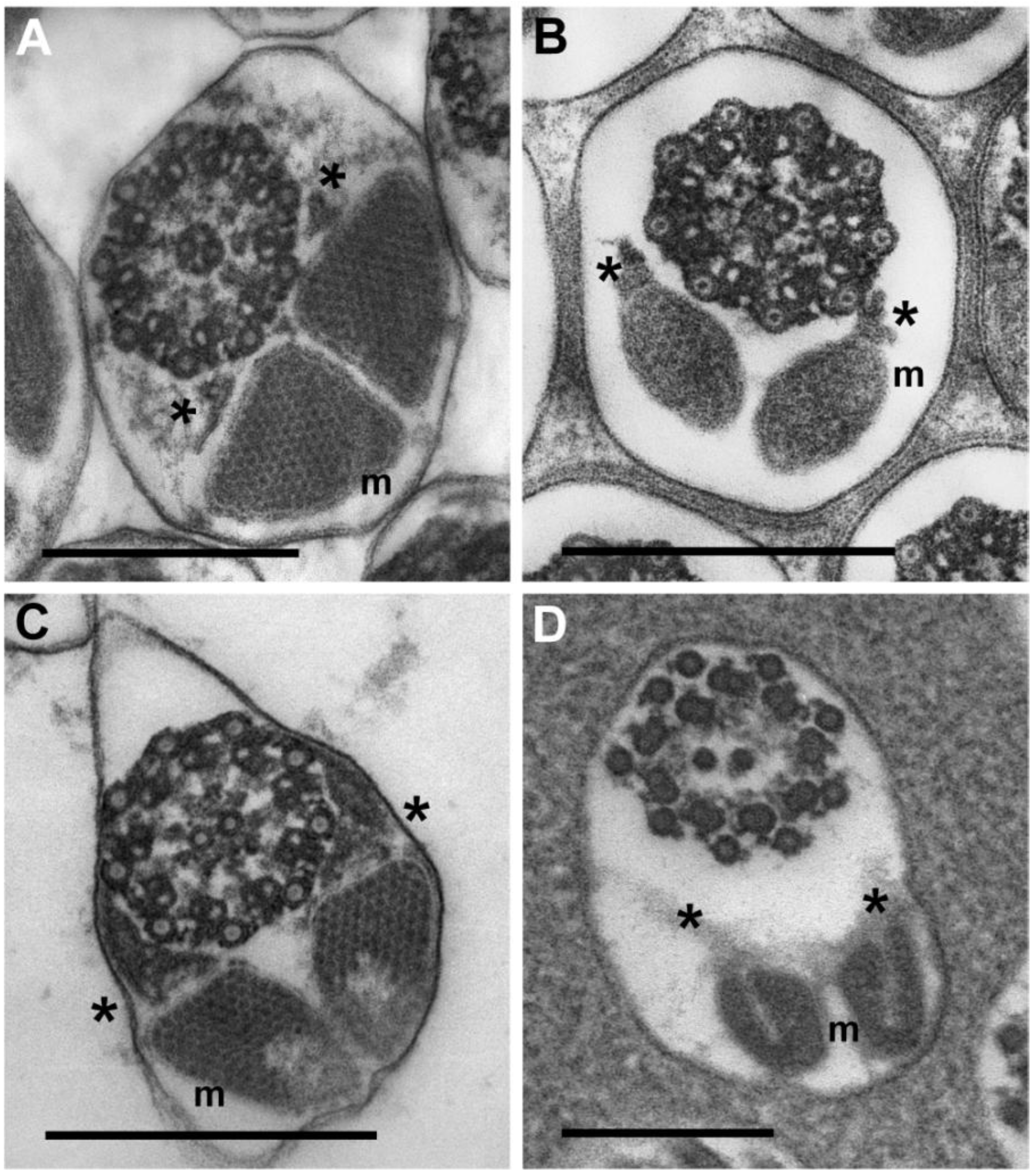
3.3.1. Sperm Heads: Nuclei and Acrosomes

3.3.2. Sperm Necks

3.3.3. Sperm Flagella: Axonemes, Mitochondrial Derivatives and Accessory Bodies
4. Conclusions
4.1. Distinctive Physiological Demands of Strepsipteran Sperm May Be Reflected in Sperm Structure
4.2. Sperm Structure and Phylogeny
Conflicts of Interest
References
- Dallai, R.; Beani, L.; Kathirithamby, J.; Lupetti, P.; Afzelius, B.A. New findings on sperm ultrastructure of Xenos vesparum (Rossi) (Strepsiptera, Insecta). Tissue Cell 2003, 35, 19–27. [Google Scholar] [CrossRef]
- Jamieson, B.G.M. The Ultrastructure and Phylogeny of Insect Spermatozoa; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Kinzelbach, R.K. Morphologische Befunde an Fächerflüglern und ihre phylogenetische Bedentung Insecta: Strepsiptera; Schwerzerbartsche: Stuttgart, Germany, 1971; p. 256. [Google Scholar]
- Kathirithamby, J. Host-parasitoid associations in Strepsiptera. Annu. Rev. Ent. 2009, 54, 227–249. [Google Scholar] [CrossRef]
- McMahon, D.P.; Hayward, A.; Kathirithamby, J. The first molecular phylogeny of Strepsiptera (Insecta) reveals an early burst of molecular evolution correlated with the transition to endoparasitism. PLoS One 2011, 6, 1–7. [Google Scholar]
- McKenna, D.D.; Farrell, B.D. 9-genes reinforce the phylogeny of holometabola and yield alternative views on the phylogenetic placement of Strepsiptera. PLoS One 2010, 5, e11887. [Google Scholar] [CrossRef]
- Niehuls, O.; Hartig, G.; Grath, S.; Pohl, H.; Lehmann, J.; Tafer, H.; Donath, A.; Krauss, V.; Eisenhardt, C.; Hertel, J.; et al. Genomic and morphological evidence converge to resolve the enigma of Strepsiptera. Curr. Biol. 2012, 22, 1–5. [Google Scholar]
- Böving, A.G.; Craighead, F.C. An illustrated synopsis of the principal larval forms of the order Coleoptera. Entomol. Am. 1931, 11, 1–351. [Google Scholar]
- Kathirithamby, J. Review of the order Strepsiptera. Syst. Entomol. 1989, 14, 41–92. [Google Scholar] [CrossRef]
- Kinzelbach, R.K.; Pohl, H. Ordnung Strepsiptera, Fächerflüger. In Wirbellose Tiere. 5. Teil: Insecta; Dathe, H.H., Ed.; Spektrum Akademischer Verlag: Berlin, Heidelberg, Germany, 2003; pp. 526–539. [Google Scholar]
- Afzelius, B.A.; Dallai, R. Characteristics of the flagellar axoneme in Neuroptera, Coleoptera and Strepsiptera. J. Morph. 1994, 219, 15–20. [Google Scholar] [CrossRef]
- Heming, B.S. Insect Development and Evolution; Cornell University Press: Ithaca, NY, USA, 2003; pp. 6–28. [Google Scholar]
- Carcupino, M.; Mazzini, M.; Olmi, M.; Kathirithamby, J. The spermatozoon of Halictophagus chilensis Hofmann (Strepsiptera, Halictophagidae). Boll. Zool. 1993, 60, 361–365. [Google Scholar] [CrossRef]
- Kathirithamby, J.; Carcupino, M.; Mazzini, M. Comparative spermatology of four species of Strepsiptera and comparison with a species of primitive Coleoptera (Rhipiphoridae). Int. J. Insect Morph. Embryol. 1993, 22, 459–470. [Google Scholar] [CrossRef]
- Jamieson, B.G.M.; Dallai, R.; Afzelius, B.A. Insects: Their Spermatozoa and Phylogeny; Science Publishers: Enfield, NH, USA, 1999. [Google Scholar]
- Mazzini, M.; Carcupino, M.; Kathirithamby, J. Fine structure of the spermatozoon of the Strepsipteran Xenos moutoni. Tissue Cell 1991, 23, 199–207. [Google Scholar] [CrossRef]
- Carcupino, M.; Profili, G.; Kathirithamby, J.; Mazzini, M. Sperm ultrastructure of Xenos vesparum (Rossi) and its significance in the taxonomy and phylogeny of Strepsiptera (Insecta). Advances in spermatozoal phylogeny and taxonomy. In Mémoires du Muséum National d’Histoire Naturelle; Jamieson, B.G.M., Ausio, J., Justine, J.-L., Eds.; G. Dufour: Paris, France, 1995; Volume 166, pp. 291–296. [Google Scholar]
- Kathirithamby, J.; Carcupino, M.; Mazzini, M. Ultrastructure of the spermatozoon of Elenchus japonicus and its bearing on the phylogeny of Strepsiptera. Tissue Cell 1992, 24, 437–442. [Google Scholar] [CrossRef]
- Beani, L.; Giusti, F.; Mercati, D.; Lupetti, P.; Paccagnini, E.; Turillazzi, S.; Dallai, R. Mating of Xenos vesparum (Rossi) (Strepsiptera, Insecta) revisited. J. Morph. 2005, 265, 291–303. [Google Scholar] [CrossRef]
- Silvestri, F. Studi sugli “Strepsiptera” (Insecta). III. Descrizione e biologia di 6 specie italiane di Mengenilla. Boll. Lab. Zool. Gen. Agric. Portici. 1943, 32, 197–282. [Google Scholar]
- Kathirithamby, J. Morphology of the female Myrmecolacidae (Strepsiptera) including the apron, and an associated structure analogous to the peritrophic matrix. Zool. J. Linn. Soc. 2000, 128, 269–287. [Google Scholar] [CrossRef]
- Whiting, M.F.; Carpenter, J.C.; Wheeler, Q.D.; Wheeler, W.C. The Strepsiptera problem: Phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst. Biol. 1997, 46, 1–68. [Google Scholar]
- Ishiwata, K.; Sasaki, G.; Ogawa, J.; Miyata, T.; Su, Z.-H. Phylogenetic relationships among insect orders on three nuclear-coding gene sequences. Mol. Phylogenet. Evol. 2011, 58, 169–180. [Google Scholar] [CrossRef]
- Friedrich, F.; Beutel, R.G. Goodbye Halteria? The thoracic morphology of Endopterygota (Insecta) and its phylogenetic implications. Cladistics 2010, 26, 1–34. [Google Scholar] [CrossRef]
- Longhorn, S.J.; Pohl, H.; Vogler, A.P. Ribosomal protein genes of holometabolan insects reject the Halteria, instead revealing a close affinity of Strepsiptera with Coleoptera. Mol. Phylogenet. Evol. 2010, 55, 846–850. [Google Scholar] [CrossRef]
- Wiegmann, B.M.; Trautwein, M.D.; Kim, J.-W.; Cassel, B.K.; Bertone, M.A.; Winterton, S.L.; Yeates, D.K. Single-copy nuclear genes resolve the phylogeny of the holometabolous insects. BMC Biol. 2009, 7, 34. [Google Scholar] [CrossRef]
- Hayward, D.C.; Truman, J.W.H.; Bastiani, M.J.; Ball, E.E. The structure of the USP/PXR of Xenos pecki indicates that Strepsiptera are not closely related to Diptera. Dev. Genes Evol. 2005, 215, 213–219. [Google Scholar] [CrossRef]
- Rokas, A.; Kathirithamby, J.; Holland, P.W.H. Intron insertion as a phylogenetic character: The engrailed homeobox of Strepsiptera does not indicate affinity with Diptera. Insect Mol. Biol. 1999, 8, 527–530. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nardi, J.B.; Delgado, J.A.; Collantes, F.; Miller, L.A.; Bee, C.M.; Kathirithamby, J. Sperm Cells of a Primitive Strepsipteran. Insects 2013, 4, 463-475. https://doi.org/10.3390/insects4030463
Nardi JB, Delgado JA, Collantes F, Miller LA, Bee CM, Kathirithamby J. Sperm Cells of a Primitive Strepsipteran. Insects. 2013; 4(3):463-475. https://doi.org/10.3390/insects4030463
Chicago/Turabian StyleNardi, James B., Juan A. Delgado, Francisco Collantes, Lou Ann Miller, Charles M. Bee, and Jeyaraney Kathirithamby. 2013. "Sperm Cells of a Primitive Strepsipteran" Insects 4, no. 3: 463-475. https://doi.org/10.3390/insects4030463