3.2. Cabbage Maggot Flight Dynamics
There was no literature to support that any other
Delia species is damaging to cabbage in the region of Ogulin and the flies on the YST were identified to genus level only. Workers from Croatia (1–6) always mentioned
Delia radicum as the main pest of cabbage and this species is always mentioned as the most serious pest of the brassica crops in Western Europe [
8,
19,
21]. Additionally, Sandrine
et al. 2006 [
23] indicated that the distribution of the most similar species,
Delia floralis is more restricted to Northern regions with lower temperatures and does not exist at latitudes below 50° N. Therefore, all
Delia flies on the traps were considered as
D. radicum.
Populations of cabbage maggot flies were much lower in 2007 than in the other three years. The average captures of the files per trap/day in 2007, 2008, 2009 and 2011 were 2.45, 10.64, 7.84 and 13.55, respectively. The differences were significant (LSD
p = 0.05 = 3.28). Despite the differences in average captures, populations could still be considered high in all years. Other authors reported maximal daily captures of between 1 and 14 flies [
12] and a maximal weekly capture of 8–15 flies per trap [
12]. However, they used trap size that was double as small.
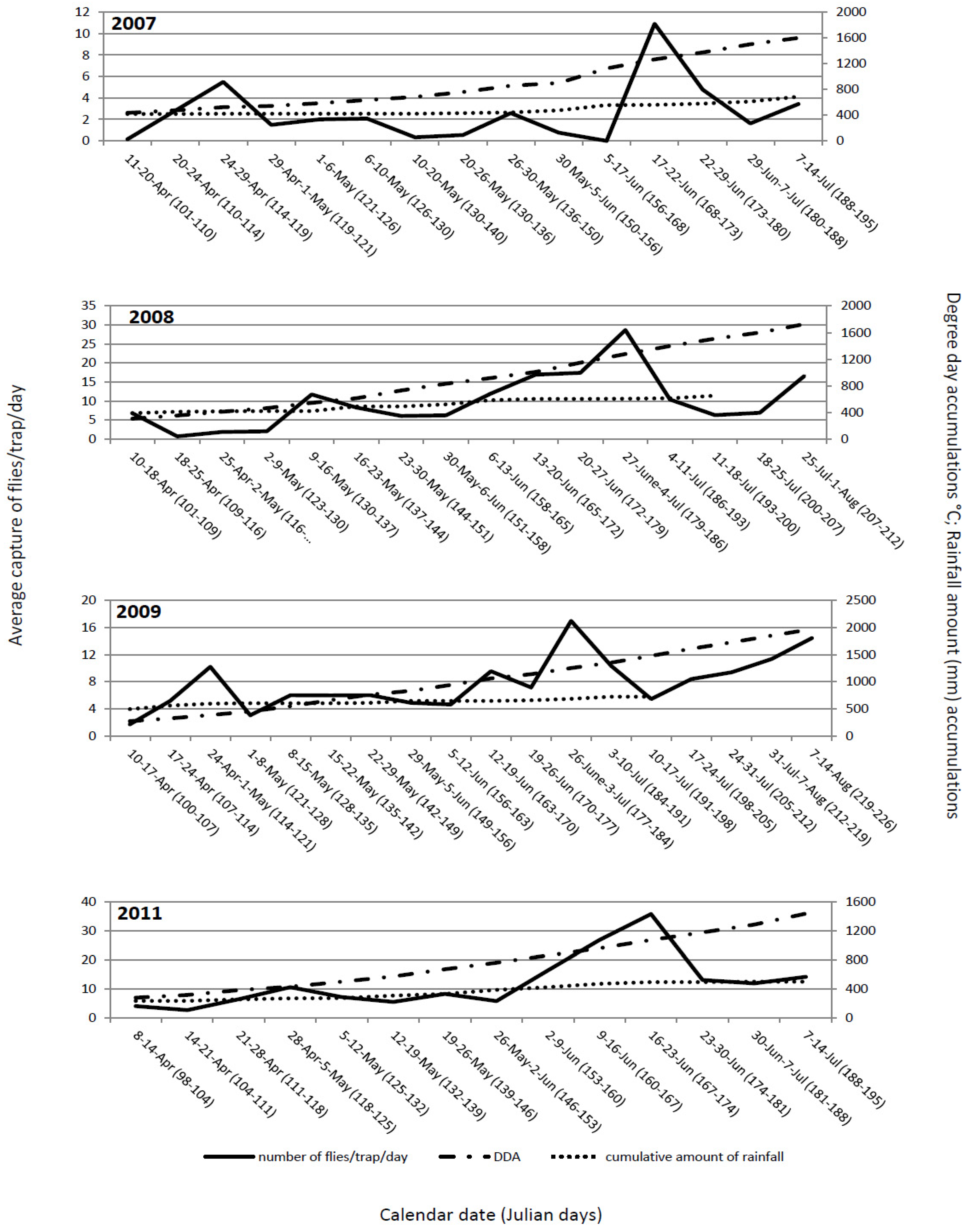
The flight dynamics in all four years mainly showed similar patterns. The first flies were observed immediately after placing out yellow sticky traps, suggesting that at the time of transplanting, the flies had already emerged from their overwintering sites and, immediately after cabbage was transplanted, migrated to the new fields. Generally, the flight dynamics had two peaks: one at the end of April and beginning of May and, the second between mid and the end of June (
Figure 1). These peaks probably correspond to two generations. The peak of the flight of overwintering cabbage maggot flies could be predicted with 15 to 16 days of delay or with ≈150 °C difference in DDA. It starts at 119 ± 7.5 Julian days and ends at 125.5 ± 8 Julian days. At that time, degree-day accumulation reaches 471.35 ± 74.97 °C. Some authors [
14,
24] stated that even though DDA could predict fly emergence and activity with reasonable accuracy, the calendar date was comparable or better than DDA as a predictive tool. Our results do not support that the calendar date is better than DDA as a predictive tool. Transplanting early cabbage in the open fields in the region of Ogulin is regularly conducted between April 10 and 20. Obviously, the emergence of the flies occurs earlier and, at the time of transplanting, the flies are already emerged and ready to migrate to the newly planted cabbage fields. The importance of DDA is related to the conditions for fly emergence and the numbers of flies present at planting. The peak of the flight of the second generation could be predicted with a delay of 12 to 13 days or with ≈190 °C difference in DDA. It started between at 172.8 ± 6.1 Julian days and ended at 179.3 ± 6.7 Julian days. At that time, degree-day accumulations were 1,217.28 ± 96.12 °C.
In Oregon, which has the similar latitude as Ogulin but a lower elevation, cabbage maggot shows a bimodal spring emergence pattern. Bimodal spring emergence patterns may be driven by genetic variability,
i.e., population composition [
25]. Waagelbach
et al. [
26] showed that cabbage maggot populations consist of different ratios of early and later emerging individuals. If one biotype prevails, the population will not show a bimodal emergence pattern. For northern European populations, Finch and Collier [
27] found both biotypes, but large variation in the percentage of each biotype in local populations. We do not have data on the makeup of the fly population in the region of Ogulin, but if we compare our data on degree-day accumulations needed for the spring flight, we can conclude that in this region, one biotype prevails and it is most likely the early emerging biotype. There is evidence that in the region of Međimurje (northeast of Ogulin), the cabbage maggot adult shows different emergence and flight patterns [
6] compared to Ogulin. Therefore, if we wish to better understand emergence patterns in different regions of Croatia, additional research is needed, particularly to determine the developmental thresholds and to establish the percentage of early/late flies. As was suggested by Turnock and Boivin [
25], the results may indicate if the percentage early/late remains quite constant or changes over time in each particular region.
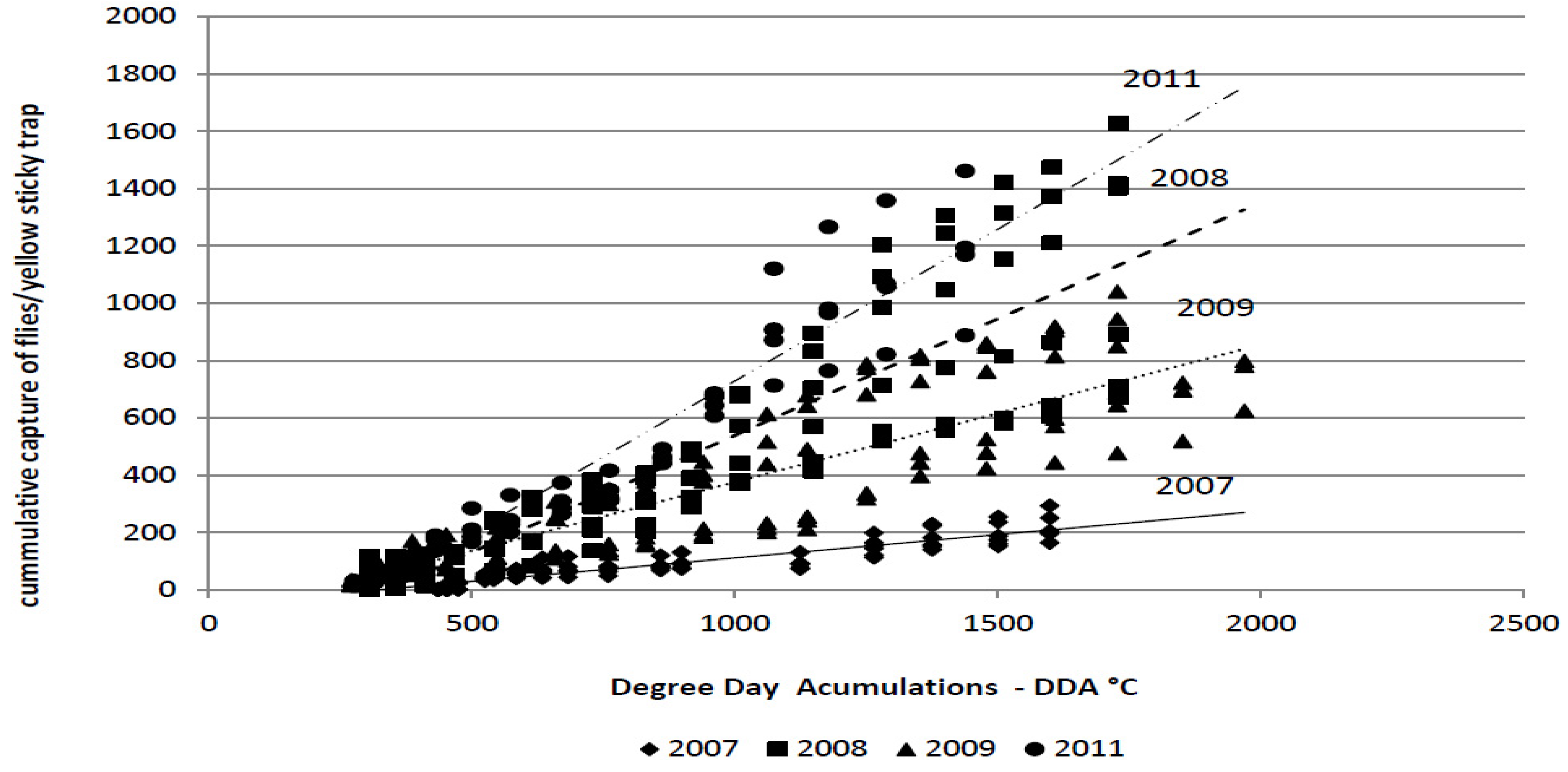
The average cumulative captures of the flies during the monitoring period in 2007, 2008, 2009 and 2011 were as follows: 220.32; 1,121; 840.33; and, 1,178.5 flies/trap, respectively. The cumulative captures depend on degree-day accumulations,
i.e., temperature (
Figure 2). The spring flight of the flies in cabbage fields was observed soon after transplanting in all years. At that time, degree-day accumulation had reached 270–435 °C.
Figure 1.
Delia radicum (L.) adult captures per trap per day on yellow sticky traps in early cabbage fields in Ogulin, Croatia compared with DDA (base 4.3 °C base threshold), and the cumulative amount of precipitation over four field seasons (2007 to 2009, 2011).
Figure 1.
Delia radicum (L.) adult captures per trap per day on yellow sticky traps in early cabbage fields in Ogulin, Croatia compared with DDA (base 4.3 °C base threshold), and the cumulative amount of precipitation over four field seasons (2007 to 2009, 2011).
Figure 2.
Linear regression analysis of the degree-day accumulations (base threshold 4.3 °C) vs. the cumulative capture of the cabbage maggot adults (Delia radicum (L.))on yellow sticky traps over four field seasons (2007 to 2009, 2011).
Figure 2.
Linear regression analysis of the degree-day accumulations (base threshold 4.3 °C) vs. the cumulative capture of the cabbage maggot adults (Delia radicum (L.))on yellow sticky traps over four field seasons (2007 to 2009, 2011).
The amount of variability was measured using the coefficient of determination (R
2) (
Table 4). The coefficient of determination indicates that the cumulative capture of flies on yellow sticky traps could be accurately predicted by the DDA in all years of the study (R
2 = 0.933–0.977). The significant influence of the cycle,
i.e., weather conditions, on the regression line is clear. The regression coefficient and the coefficient of determination were significantly lower in 2007 compared with all other years.
Table 4.
Data obtained in the linear regression analysis (coefficients of determination (±SE), probability, n, and regression coefficients (±SE) of the degree-day accumulations (above 4.3 °C and below 30 °C) (x) versus the cumulative number of Delia radicum (L.) flies /yellow sticky trap (y) over four field seasons (2007 to 2009, 2011).
Table 4.
Data obtained in the linear regression analysis (coefficients of determination (±SE), probability, n, and regression coefficients (±SE) of the degree-day accumulations (above 4.3 °C and below 30 °C) (x) versus the cumulative number of Delia radicum (L.) flies /yellow sticky trap (y) over four field seasons (2007 to 2009, 2011).
| Year | Coefficient of determination R2 (±SEM) | n ** | p | Regression coefficient b (±SEM) | Average daily capture of flies per trap/day |
|---|
| 2007 | 0.933 ± 0.017 b * | 96 | 0.0001 | 0.163 ± 0.036 c | 2.45 c |
| 2008 | 0.977 ± 0.0035 a | 96 | 0.0001 | 0.814 ± 0.326 ab | 10.64 ab |
| 2009 | 0.971 ± 0.182 a | 96 | 0.0001 | 0.541 ± 0.134 b | 7.84 b |
| 2011 | 0.965 ± 0.024 a | 56 | 0.0001 | 1.059 ± 0.240 a | 13.55 a |
| LSDp = 0.05 | 0.019 | | | 0.275 | 3.28 |
The linear regression analysis showed that there is significant impact of the year of the study, on the slope of the regression line,
i.e., on the regression coefficient (b). It was the lowest in 2007. April of 2007 was extremely dry and very warm. As the mean monthly temperatures in January, February and March in 2007 also were above average, it is possible that the flies emerged very early. It is known that cabbage maggot development may be stimulated by rainfall [
28]. After emergence, flies feed on pollen and nectar produced by various plants [
9], and start to mate and lay eggs approximately one week after emergence. Cabbage maggot adults deprived of host plants are less selective in choosing oviposition sites compared to females able to continuously lay eggs on host plants [
29]. In 2007, cabbage was transplanted as usual in April. It is possible that the flies had emerged earlier than the transplanting date in 2007, were deprived of host plants and therefore were less selective in choosing oviposition sites than in typical years.
Regression coefficients were higher in the years when the total numbers of adults captured on yellow sticky traps were higher. It is expected because the higher number of adult means larger sample what result in highest accuracy and lower amount of variability.
3.3. Cabbage Maggot Infestation
The first egg laying was observed two to three weeks after transplanting, with the first eggs found on May 2, May 1 and April 21 in 2008, 2009 and 2011, respectively. Although an average of up to four eggs were observed on individual plants, because some plants had no eggs, the average number of eggs per plant was between 0.6 and 0.85 in 2008 and up to 0.63 in 2009, respectively. The only exception was on April 28, 2011 when 1.36 eggs/plant were recorded (
Figure 3). Egg numbers were low compared to those of Easter
et al. [
30] who reported 13 or more eggs per plant. Bligaard [
31] noted that an artificial infestation with 100 eggs per plant resulted in a 5% reduction in plant growth. Bligaard [
18] considered an average infestation of 21 eggs/infested plant, with 30% of plants infested, as an economic threshold (ET). If the same infestation is recalculated into the average number of eggs per plant, the economic threshold is 6.2. The highest average number of eggs/plant in our trial was 1.36, less than the ET discussed by Bligaard [
18]. In the guidelines for cabbage production published by Ministry of Agriculture Republic of Croatia [
17] an ET of one to two eggs or larva/plant is recommended, much lower than the ET suggested by Bligaard [
18]. The difference is probably related to sampling method; Bligaard [
18] presented results obtained by absolute sampling in which 99% of eggs could be obtained, whereas the guidelines for cabbage production in Croatia recommend a nondestructive method,
i.e., a visual survey of the plant base, which underestimates the number of eggs present. Although eggs are largely deposited in the upper soil layer by the stem base or on the lower petioles, under natural conditions, the female fly pushes its ovipositor into the soil, leaving only a few eggs exposed on the soil surface and others only partly visible or buried. Therefore this method is less precise than an absolute sampling method.
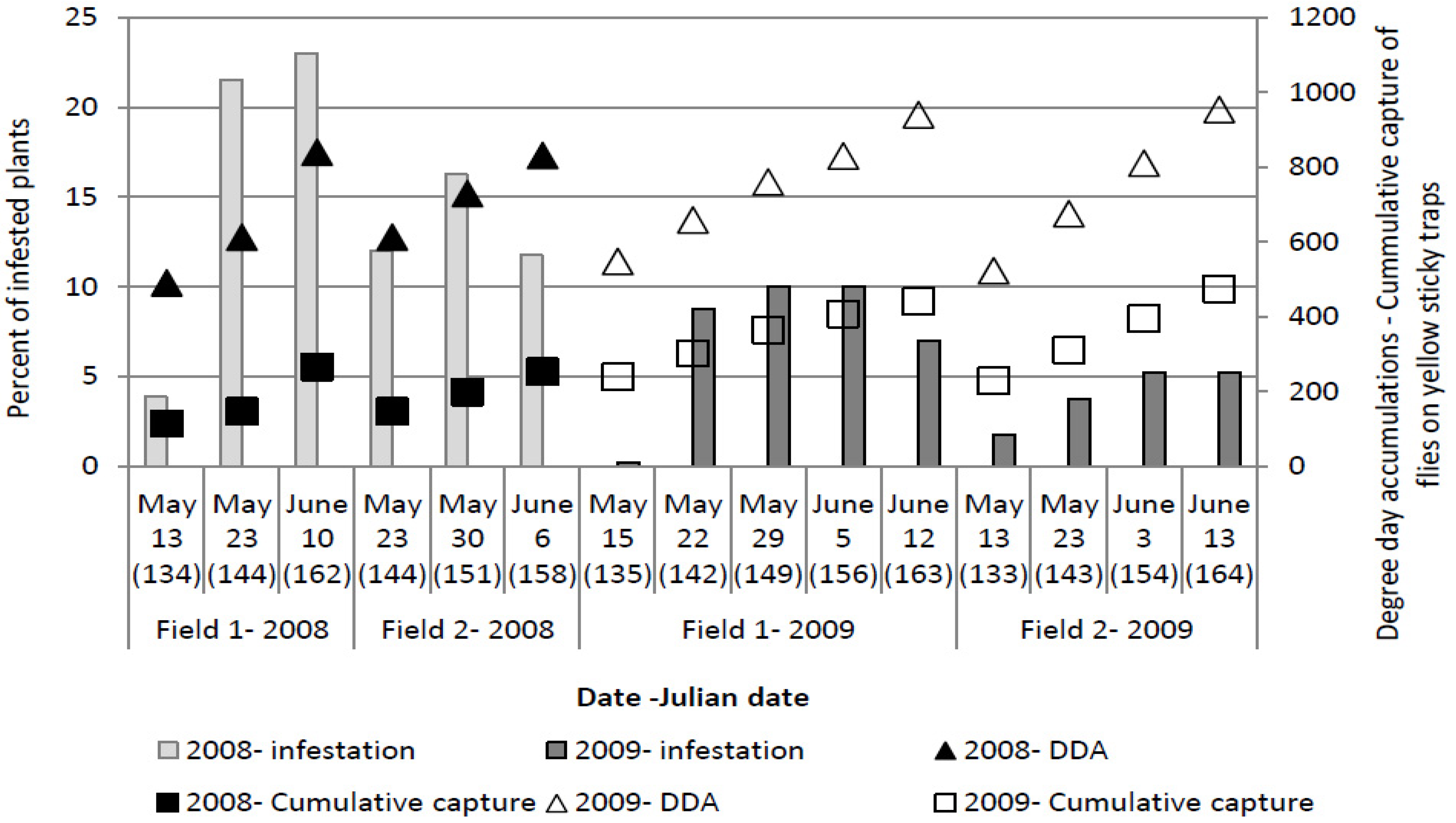
The highest number of eggs per plant (
Figure 3) was recorded at 123 ± 6 Julian days over the three years, which corresponds with the end of April and beginning of May. At this time, the cumulative captures were 124.11 ± 48.6 flies per yellow sticky trap and DDAs had reached 412.31 ± 47.34 °C.
Figure 3.
Numbers of Delia radicum (L.) eggs per plant on early cabbage, compared with cumulative numbers of D. radicum adults on yellow sticky traps and DDA over three field seasons (2008, 2009, 2011).
Figure 3.
Numbers of Delia radicum (L.) eggs per plant on early cabbage, compared with cumulative numbers of D. radicum adults on yellow sticky traps and DDA over three field seasons (2008, 2009, 2011).
Although in 2011, the number of eggs was similar to or higher than that observed in the other two years, no plants showing symptoms of larval infestation were recorded in the visual survey. A higher percentage of plants showing symptoms of larval infestation (wilting) was observed in 2008, compared to 2009 (
Figure 4). The experimental field in 2011 was not rich with organic matter, so it is likely that the eggs were
D. radicum rather than a related species like
Delia platura, which lays eggs similar in appearance to those of
D. radicum, and which feeds on organic matter in the soil. There are several possible causes for the absence of symptoms of wilting in 2011: egg and larval mortality, presence of natural enemies, or ability of the plants to tolerate a certain number of larvae. Bligaard [
31] reported that the percentage of mortality of eggs and larvae is very high (between 47% and 61%). Eggs of
D. radicum are resistant to low soil moisture and high temperature conditions, while larval survival tends to increase with an increase in soil temperature up to 33 °C and in the and moisture [
32]. Climatic conditions during the period of egg laying and larval development (April and May) in 2011 were similar to those observed in the other three years, which supports the idea that the absence of wilting symptoms was due to other factors. In May of 2011 the amount of precipitation was the highest among all experimental years, and this may have influenced larval survival [
32]. Several authors [
8,
33,
34] have suggested that some of the 60–100 species of carabid and staphylinid beetles found commonly in cultivated soils are important predators or parasitoids of the cabbage maggot and that their activity is related to crop rotation and cultivation practices. In fact, over the three years of investigation the highest number of larvae per plant was found in 2011. It is possible that the low percent of the plants showing symptoms of larval infestation was due to the very good growing conditions in 2011, which enabled plants to develop very quickly and successfully even in the presence of larvae.
Figure 4.
The average percent of plants showing symptoms of larval infestation by Delia radicum (L.) over two field seasons (2008, 2009).
Figure 4.
The average percent of plants showing symptoms of larval infestation by Delia radicum (L.) over two field seasons (2008, 2009).
The maximum number of plants which showed symptoms of larval infestation was recorded on 153 ± 6 Julian days, which corresponds with the end of May and beginning of June. At the time when the highest number of plants showing symptoms of larval infestation was observed, cumulative captures were 299.07 ± 109.07 flies per yellow sticky trap and DDAs had reached 764.1 ± 85.15 °C.
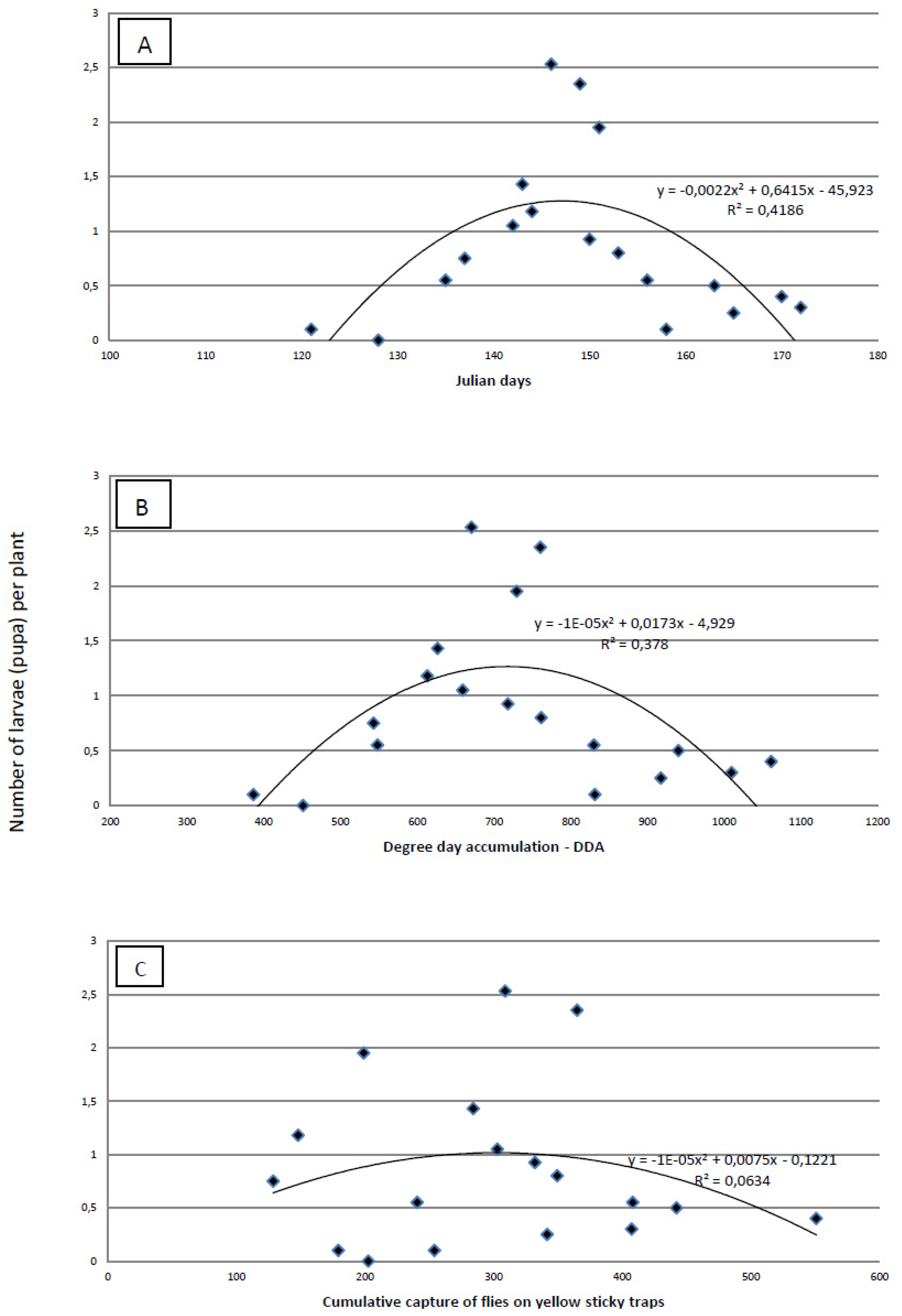
The proof that a visual survey of the plants for eggs is less precise than absolute sampling is obvious from the results of plant dissections. Using dissection, we found the highest infestation was 1–2.5 larvae per plant (
Figure 5), but the highest number of eggs found by visual survey was 1.36 eggs/plant. The maximum number of larvae per plant was recorded on 146 ± 3 Julian days, which corresponds to the end of May. At the time when the highest number of larvae per plant was established, cumulative captures were 267.99 ± 79.64 flies per yellow sticky trap and DDAs had reached 676.73 ± 57.80 °C. The most accurate tool for predicting the maximum number of larvae/plant is Julian days. The amount of variability was measured using the coefficient of determination (R
2) (
Figure 5). This indicates that the maximum number of larvae per plant was better predicted by Julian days (R
2 = 0.4186) and by the DDA (R
2 = 0.378) than by the cumulative capture of flies on yellow sticky traps (R
2 = 0.0634).
Figure 5.
Regression analysis of Julian days (A), degree-day accumulation (base threshold 4.3 °C)—DDA (B) and cumulative capture of cabbage maggot flies on yellow sticky traps (b) versus the average number of cabbage maggot larvae)/plant over three field seasons.
Figure 5.
Regression analysis of Julian days (A), degree-day accumulation (base threshold 4.3 °C)—DDA (B) and cumulative capture of cabbage maggot flies on yellow sticky traps (b) versus the average number of cabbage maggot larvae)/plant over three field seasons.
The mean larval damage occurred between the period when the maximum number of eggs were observed and the period when maximum symptoms of larval infestation were visible. If cabbage maggot is controlled by foliar application of insecticides, the application should be conducted a few days after the maximal number of eggs is established and before the maximal number of larvae is established. The data obtained in our investigation could serve as the parameters to help to determine the optimal timing of foliar insecticide application.
3.4. Cabbage Maggot Control
The trial in 2007 was carried out with the late variety Ogulinec which was planted in beginning of May, approximately 40 days after the peak flight of the overwintering generation was recorded. Late planting resulted with a low attack of cabbage maggot (
Table 5) because the most vulnerable stage of the plants corresponds with the period between two generations of cabbage maggot flies. Although the peak flight of the first generation was recorded between 17 and 22 June (
Figure 1), we did not establish high larval infestation in the second part of the trial. Larval infestation was not high enough to cause significant damages on the plants. At the beginning of July, when larvae from this generation started to infest the plants, plants reached the phenological phase of copping [
15],
i.e., they began to form a head and thus probably became more tolerant to larval infestation as it was stated by Bligaard [
31]. Established infestation confirmed the statements of some authors [
2,
5] that the early cabbage varieties are more endangered from cabbage maggot infestation.
Table 5.
Percentage (±SEM) of cabbage plants showing symptoms of Delia radicum (L.)larval infestation (wilting) in 2007 following treatment with various insecticides, or untreated.
Table 5.
Percentage (±SEM) of cabbage plants showing symptoms of Delia radicum (L.)larval infestation (wilting) in 2007 following treatment with various insecticides, or untreated.
| Treatment | Dose g a.i./ha | Appl. method * | Date of evaluation |
|---|
| 22 June | 29 June | 6 July | 13 July |
|---|
| Untreated | | | 5.0 ± 4.08 | 7.5 ± 2.89 | 2.5 ± 2.89 ab** | 1.88 ± 3.75 |
| Dimethoate | 400 | SP | 6.25 ± 7.5 | 5.63 ± 4.73 | 5.0 ± 2.04 ab | 0 |
| Imidacloprid | 167 | DS | 10.0 ± 8.16 | 10.0 ± 8.16 | 2.5 ± 2.89 ab | 5.0 ± 4.08 |
| | 100 | SP | 1.88 ± 1.25 | 1.25 ± 1.44 | 3.75 ± 2.5 ab | 0 |
| Thiamethoxam | 200 | DS | 5.0 ± 5.77 | 10.0 ± 8.16 | 2.5 ± 2.89 ab | 5.0 ± 4.08 |
| | 100 | SP | 3.75 ± 5.95 | 5.0 ± 5.4 | 8.13 ± 5.15 a | 0.63 ± 1.25 |
| Acetamiprid | 46 | DS | 0 | 2.5 ± 2.89 | 0 b | 0 |
| LSD p = 0.05 | | | ns | ns | 6.61 | ns |
The highest percent of plants showing symptoms of wilting, among the all years was observed in 2008. Up to 20.75% of plants on untreated plots had been damaged by larvae (
Table 6).
Table 6.
Experimental results determined on the basis of visual survey of plants and observing percent of plants showing symptoms of Delia radicum(L.)larval infestation (±SEM) in 2008 following treatment with various insecticides, or untreated.
Table 6.
Experimental results determined on the basis of visual survey of plants and observing percent of plants showing symptoms of Delia radicum(L.)larval infestation (±SEM) in 2008 following treatment with various insecticides, or untreated.
| Treatment | Dose g a.i./ha | Appl. method * | Date of evaluation |
|---|
| 12 May | 23 May | 7 June | 17 June |
|---|
| Untreated | | | 3.75 ± 2.63 | 20.75 ± 2.06 a** | 19.5 ± 7.14 | 19.75 ± 10.14 |
| Dimethoate | 400 | SP | 2.25 ± 0.5 | 13.75 ± 2.06 b | 18.5 ± 3.7 | 20.0 ± 4.24 |
| Imidacloprid | 100 | SP | 3.0 ± 1.41 | 13.5 ± 2.06 b | 16.5 ± 6.56 | 15.25 ± 5.74 |
| Thiamethoxam | 200 | DS | 1.5 ± 0.58 | 13.5 ± 1.91 b | 16.75 ± 11.53 | 16.25 ± 10.37 |
| | 100 | SP | 3.5 ± 1.91 | 13.0 ± 1.26 b | 19.75 ± 4.35 | 19.5 ± 7.55 |
| Acetamiprid | 50 | DS | 1.5 ± 0.58 | 7.0 ± 4.11 c | 10.75 ± 4.92 | 10.5 ± 4.36 |
| LSD p = 0.05 | | | ns | 5.7 | ns | ns |
The percent of plants showing symptoms of wilting in 2009 was much lower than in 2008. A maximum of 5.25% of plants on untreated plots and 9.00% on acetamiprid applied as a seedling dip had been damaged by larvae (
Table 7).
Table 7.
Experimental results determined on the basis of visual survey of plants and observing percent of plants showing symptoms of Delia radicum (L.) larval infestation (±SEM) in 2009 following treatment with various insecticides, or untreated.
Table 7.
Experimental results determined on the basis of visual survey of plants and observing percent of plants showing symptoms of Delia radicum (L.) larval infestation (±SEM) in 2009 following treatment with various insecticides, or untreated.
| Treatment | Dose g a.i./ha | Appl. method * | Date of evaluation |
|---|
| 13 May | 23 May | 3 June | 14 June |
|---|
| Untreated | | | 1.75 ± 1.5 | 3.75 ± 2.22 ab** | 5.25 ± 1.89 | 5.25 ± 1.26 |
| Dimethoate | 400 | DS | 0.75 ± 0.96 | 4.5 ± 3.7 ab | 5.25 ± 4.19 | 3.75 ± 3.59 |
| | 400 | SP | 0.25 ± 0.5 | 2.5 ± 1.0 b | 3.0 ± 1.41 | 3.0 ± 1.15 |
| Imidacloprid | 200 | DS | 0.25 ± 0.5 | 3.0 ± 3.56 ab | 2.75 ± 3.59 | 2.25 ± 2.63 |
| | 100 | SP | 0.5 ± 1.0 | 2.25 ± 1.5 b | 2.75 ± 3.1 | 2.0 ± 1.41 |
| Thiamethoxam | 200 | DS | 0.75 ± 1.5 | 2.5 ± 2.65 b | 2.0 ± 3.57 | 1.5 ± 2.38 |
| | 100 | SP | 1.5 ± 1.29 | 4.0 ± 1.41 ab | 6.75 ± 2.5 | 2.25 ± 2.22 |
| Acetamiprid | 50 | DS | 3.5 ± 2.65 | 9.0 ± 5.03 a | 6.0 ± 4.9 | 5.25 ± 4.11 |
| | 50 | SP | 0.25 ± 0.5 | 1.75 ± 2.36 b | 2.5 ± 2.08 | 2.0 ± 1.26 |
| LSD p = 0.05 | | | ns | 6.05 | ns | ns |
In visual surveys conducted on May 2, 17, 23, and 30, 2011, very few plants showed symptoms of wilting (up to 1.5%). Therefore the results were not processed by ANOVA.
The data on the number of larvae per plant (
Table 8) show the opposite with regards to larval feeding, compared to the data from visual surveys. This indicates that observing plant damage without establishing the number of larvae per plant could result in an erroneous conclusion. Plants which had symptoms of wilting and had changed color were infested with different numbers of larvae. Other factors such as temperature, humidity and agronomic conditions may influence the final effect of the same larval infestation on plant development and survival. Researching insecticide efficacy and damage thresholds, other workers [
30,
32] used the establishment of the following three things as criteria: the number of larvae and pupae/plant; the number of dead plants; and, the dry matter content in the roots. However, only by determining the dry matter content could the difference between plants which were infested but which were able to compensate larval damage, be established.
Among all years, the highest number of larvae per plant was observed in 2011. The fact that high adult populations also were observed in 2011, but with only a few plants with symptoms of wilting, supports our conclusion that in 2011, good cultivation conditions such as adequate precipitation and favorable temperatures enabled plants to tolerate the larval infestation. Ester
et al. [
30] also reported differences between years in the response of cauliflower plants to similar larval infestations. They suggested that when it is wet, plants develop new roots permitting rapid regrowth following insect feeding. In our investigation, the difference in amount of precipitation between 2008 and 2011 in May when main larval damage occurred (
Table 3) was not very high. However, the amount of precipitation was somewhat higher in 2011 and this, together with better plant conditions and very good fertilization, contributed to better plant regrowth in 2011 compared to 2008. It should be noted that white cabbage plants have a stronger capacity for plant regrowth compared to cauliflower plants [
30].
Table 8.
Experimental results determined on the basis of dissection of plants and establishing average number of Delia radicum (L.) larvae/plant (±SEM) in three year trials with insecticides, or untreated.
Table 8.
Experimental results determined on the basis of dissection of plants and establishing average number of Delia radicum (L.) larvae/plant (±SEM) in three year trials with insecticides, or untreated.
| Treatment | Dose g a.i./ha | Application method * | Average number of larvae/plant ±SEM |
|---|
| 2008 | 2009 | 2011 |
|---|
| Untreated | | | 1.33 ± 0.83 ab** | 1.8 ± 0.2 bc | 2.2 ± 0.36 ab |
| Dimethoate | 400 | DS | | 0.5 ± 0.12 f | 1.38 ± 0.62 bc |
| 400 | SP | 1.48 ± 0.56 ab | 1.53 ± 0.29 cd | 1.08 ± 0.25 cd |
| Imidacloprid | 200 | DS | | 2.9 ± 0.73 a | 0.25 ± 0.13 d |
| 100 | SP | 1.23 ± 0.86 ab | 0.75 ± 0.93 ef | 0.58 ± 0.17cd |
| 200 | SP | | | 2.85 ± 0.79 a |
| Thiamethoxam | 200 | DS | 0.38 ± 0.29 b | 1.3 ± 0.08 cde | |
| 100 | SP | 0.43 ± 0.53 b | 0.93 ± 0.05 def | |
| Acetamiprid | 50 | DS | | 2.29 ± 0.24 ab | |
| 50 | SP | 1.92 ± 0.9 a | 0.88 ± 0.17 def | |
| LSD p = 0.05 | | | 1.43 | 0.73 | 0.95 |
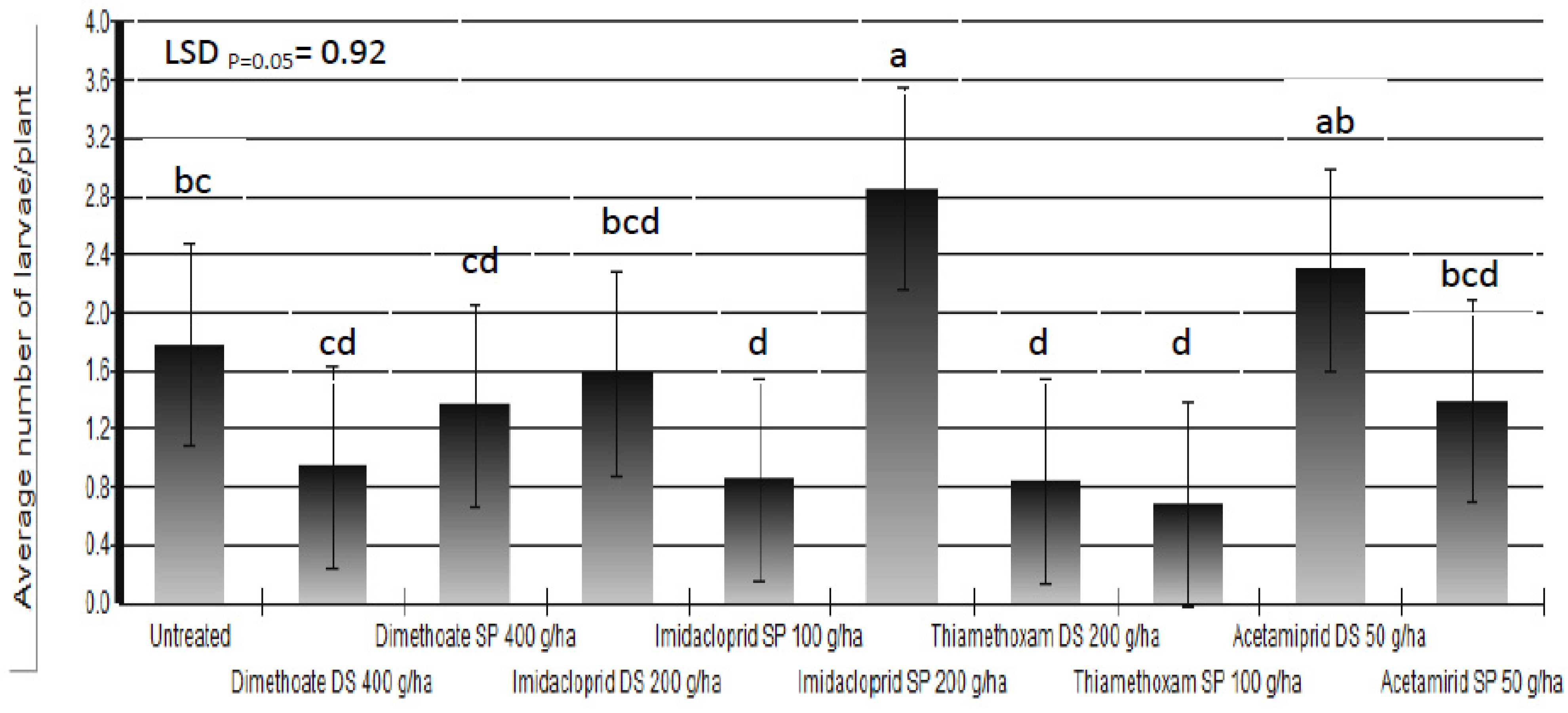
Based on the results of the three-year trials we could not state that any of the applied insecticides protected white cabbage plants completely against cabbage maggot larvae. The percent of plants which showed symptoms of larval infestation was reduced up to 67% in insecticide-treated plots compared to untreated control plots. Beside the larval infestation, the final damage was determined by additional factors which influenced plant regrowth. Somewhat better results were achieved in the reduction of average number of larvae/plant (
Figure 6). There are three possible causes of poor insecticide efficacy: leaching or degradation of insecticides; incorrect timing of foliar application of insecticides; and, lack of efficacy of applied insecticides.
Figure 6.
Average number of Delia radicum (L.) larvae/plant following different insecticide treatments (DS = dipping of the seedlings into insecticide solution before transplanting; SP = spraying)over three field seasons (means followed by the same letter are not significantly different according to Tukey's HSD test (LSDp = 0.05 = 0.92).
Figure 6.
Average number of Delia radicum (L.) larvae/plant following different insecticide treatments (DS = dipping of the seedlings into insecticide solution before transplanting; SP = spraying)over three field seasons (means followed by the same letter are not significantly different according to Tukey's HSD test (LSDp = 0.05 = 0.92).
By applying insecticides as seedling dips, we apply them before larval infestation occurs. Insecticides enter the potting mix first and, if they are systemic (as it was the case in our trials), they are immediately taken from the plants. The amount of insecticides that is taken from the plants depends on various factors (plant size, plant water intake, etc.). However, a portion of the active ingredient could be retained in the potting mix and be taken from the plant later. Leaching of insecticides from the potting mix and degradation of insecticides in both, potting mix and plants, could occur in the period between transplanting and larval infestation. For good activity, the amount of insecticide in the plant tissues has to remain at the toxic level up to the time when larval infestation occurs. The time span between transplanting (i.e., application) and the time when the maximum larval attack occurs influences the insecticide efficacy. The important factor is probably the amount of rainfall in this period. Rainfall could increase leaching of insecticides from the upper soil level or could influence the speed of breakdown of applied insecticides. In 2008, there was a time span of 40 to 50 days between insecticide application and peak larval infestation and the amount of precipitation in April and May was very high. This could have led to relatively poor results of insecticides applied as a dipping of the seedlings. Compared to 2008, the time span between transplanting and larval infestation was longer in 2009 but the amount of rainfall in this period was somewhat lower (especially in May). In 2011, a high amount of rainfall after transplanting was recorded, similar to 2008.
Dimethoate applied as a seedling dip showed better results in 2009 compared to 2011, probably because it is highly soluble in water, it adsorbs only very weakly to soil particles and thus it may be subject to considerable leaching [
35]. It is known that dimethoate breaks down faster in moist than in dry soils and is rapidly broken down by most soil microorganisms. On the other hand, imidacloprid breaks down within one year [
36]. It is immobile in the soil and, according to Lee
et al. [
37], after it is applied it stays in upper soil levels (root zone). When applied as a seedling dip, imidacloprid showed better efficacy in 2011 than in 2009.
Thiamethoxam has no bioaccumulation potential in the soil [
38]. It degrades into other neonicotinoid insecticide, chlothianidin [
39].
However, the main portion of insecticides at the time of transplanting was already taken from the plants and is thus not exposed to leaching. The residual activity of the various applied insecticides is similar [
38,
39,
40,
41].
At the time of foliar application, plants were small and insecticides were applied on both the soil surface and the plants. The insecticide that was applied to the soil surface could have been exposed to leaching or photodegradation. In 2008, in the second week following foliar application of insecticides, a large amount of rainfall was recorded (69.00 mm/m
2). Similar climatic conditions were recorded in 2011; in the two weeks following foliar application in 2011, 35.0 mm rain/m
2 was recorded. In 2009, no rain was recorded in the two-week period that followed foliar application. However, it could not be concluded that amount of rainfall after foliar application of any of applied insecticides influenced their activity. After application, dimethoate is rapidly absorbed and decomposed both on the surface and inside the foliage [
35], and, on the soil surface, imidacloprid degrades very quickly [
37]. Thiamethoxam is moderately mobile in soil and degrades at fast to moderate rates under field conditions [
38]. The quick photodegradation of acetamiprid was noted by Gupta
et al. [
42].
The one reason for poor insecticide efficacy could be the timing of the application. The foliar application of insecticides corresponded with a maximum number of eggs (
Figure 3). If we assume that larval emergence starts three to nine days after oviposition [
9], it seems likely that foliar sprays were applied at the correct time and that timing was not the reason for poor efficacy.
The poor results of insecticides might be caused by the lack of insecticide efficacy against the cabbage maggot. Cabbage maggot control in Croatia is conducted using two organophosphorous insecticides, chlorpyrifos and dimethoate, and one neonicotinoid insecticide, imidacloprid [
7]. Chlorpyrifos is widely used for the control of cabbage maggot larvae in brassica crops and could be considered as a standard treatment in numerous countries [
19,
30]. However, chlorpyrifos is not frequently used in Croatia because cabbage growers in Croatia usually do not have the necessary equipment to apply granular formulations of chlorpyrifos. After many years of use, it is suspected that cabbage maggot resistance to chlorpyrifos has developed in British Columbia (BC, Canada) [
19], although this is yet to be confirmed by the Insecticide Resistance Action Committee (IRAC) [
43]. The use of dimethoate either by spraying or by dipping of seedlings could be considered as the standard treatment against the cabbage maggot in Croatia. We did not find in the literature any supportive data on the strong efficacy of dimethoate, but growers in Croatia have used it for years with apparently acceptable control. Our results show some reduction in the average number of larvae/plant when dimethoate was applied either as a seedling dip or as a foliar spray but, the reduction was not significantly different from untreated plots (
Figure 6). It is possible that the cabbage maggot adult, over years of dimethoate use in the area, has developed a level of resistance. If we take into account that 37 insect species already developed resistance to dimethoate [
44] and that there are 60 reported cases of resistance development in the cabbage maggot to various other insecticides [
44], it is a plausible scenario. However, until now, no resistance development to dimethoate has been reported in the cabbage maggot. Therefore, the possibility that the cabbage maggot has developed resistance to dimethoate in the region of Ogulin should be considered and investigated in the future.
Research conducted by different authors showed that imidacloprid did not reduce the number of cabbage maggot per plant [
30,
45] and therefore should not be used for the control of this pest in the United Kingdom [
46]. However, imidaclopridbased products are officially allowed for cabbage maggot control in Croatia either by dipping of seedlings or by spraying. In some investigations, imidacloprid evidently resulted in an increase in the number of larvae per plant [
46], as observed in our investigation after the foliar application of imidacloprid at a dose of 200 g a.i./ha (
Figure 6). Finch and Edmonds [
45] reported that the presence of imidacloprid in treated plants extended the period over which the cabbage maggot larvae continued to feed. Neonicotinoid insecticides, such as imidacloprid, act on the nervous system. In the laboratory, contact exposure of several economic species of wireworms to neonicotinoids prolonged periods of morbidity up to 150 days, characterized by the loss of coordination and inability to feed. Following this period of morbidity, wireworms generally recovered fully [
47,
48,
49]. Although feeding by cabbage maggot larvae might be less intensive in the mentioned extended period due to loss of coordination, the final effect may be different. The effect of extended larval feeding on plant wilting might depend on climatic and agronomic conditions. If the conditions for plant regrowth are good, the final damage maybe will not be as visible as it would be in less favorable conditions. If larvae recover and finish their development, a population increase over a certain time period could be expected. A similar situation with wireworm populations in Canada was reported by Vernon
et al. [
50].
The best results were obtained by the use of thiamethoxam either through dipping of seedlings or as a foliar application. Acetamiprid had a certain effect on the percent of plants which showed symptoms of larval infestation, but it did not reduce the number of larvae per plant (
Figure 6). Whether or not thiamethoxam or acetamiprid cause similar symptoms in larval behavior as imidacloprid is not clear. Although some researchers indicated that thiamethoxam may have a slightly different mode of action than imidacloprid [
38], they agree that neonicotinoids act by binding to nicotinic acetylcholine receptors. In Croatia, thiamethoxam and acetamiprid are registered for foliar application for cabbage aphid control. Thiamethoxam is allowed for the dipping of seedlings for various vegetable species but it is not registered as a seedling dip for cabbage [
7]. Šubić [
6] reported that the efficacy of imidacloprid, thiamethoxam and acetamiprid-based insecticides applied by dipping seedlings is satisfactory for cabbage maggot control only if high doses of insecticides are applied. However, the application of high doses is not in accordance with IPM principles. Based on the fact that imidacloprid did not reduce the number of larvae per plant in all trials in this study, along with results obtained by other authors [
45,
46], we cannot support its use for cabbage maggot control. In the future, for cabbage maggot control in Croatia, the use of thiamethoxam should be considered, but further research is needed on possible application options. Siekmann and Hommes [
51] reported good efficacy of thiamethoxam + abamectin as a seed treatment on cabbage maggot larvae when infestation occurred 6 weeks after sowing. They observed a slight loss of efficacy when plants were infested 11 weeks after sowing.
Seed treatment by different insecticides has been advocated as an effective control strategy against cabbage maggot larvae [
21,
30,
45,
46,
51,
52,
53,
54]. For seed treatment, various insecticides are recommended: chlorpyrifos [
21,
52,
53], isofenphos [
52], spinosad [
30,
51], thiamethoxam, clothianidin and abamectin [
51], chlofenapyr, cyromazine and fipronil [
52]. No significant negative effect ongermination was observed in either of these studies. Siekman and Hommes [
51] and Jyoti
et al. [
52] reported that the activity of the seed treatment lasted 6 to 10 weeks after sowing, while Ester
et al. [
30] reported that the seed treatment could protect the plants from cabbage maggot larvae up to seven weeks after transplanting. In conditions that prevail in the region of Ogulin, cabbage maggot larvae start to infest cabbage plants at the earliest at the beginning of May, which is 5–7 weeks after transplanting (
i.e., 10 to 12 weeks after sowing). However, Šubić [
6] reported an earlier infestation in the Croatian region of Međimurje. Since seed treatment with insecticides is not allowed for cabbage seeds in Croatia, the efficacy of seed treatment was not investigated in this study.
Although white cabbage in Croatia is considered a major crop, the total area is relatively small. Therefore, the limited number of active ingredients allowed for cabbage maggot control in Croatia is a result of the lack of interest of manufacturers in obtaining registration for cabbage. Due to high pesticide registration costs, manufacturers are not interested in registration. Hopefully joining the EU and accepting uniform principles of pesticide registration will change this situation.
A similar topic related to insecticide seed treatments was discussed by Suett [
55], Finch and Collier [
8], and Jukes
et al. [
46] who pointed out that the film-coating technique for treating seeds should be stimulated by appropriate legislative and political pressures. In the meantime, new findings related to the use of neonicotinoids as seed treatments and the fact that neonicotinoids have become an important environmental issue due their possible hazardous effect on bees and their unknown fate in the ecosystem [
56,
57,
58,
59] put the new question mark on the future use of neonicotinoids for seed treatment [
60,
61].