Hemocyte Changes During Immune Melanization in Bombyx Mori Infected with Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Silkworm Rearing and Hemolymph Collection
2.2. Pathogen Preparation
2.3. Silkworm Injection
2.4. Hemocyte Profiles Observation In Vivo
2.5. Melanization Level Measurement
2.6. Hemocyte Numbers Calculation
2.7. Hemocytes Classification and Count
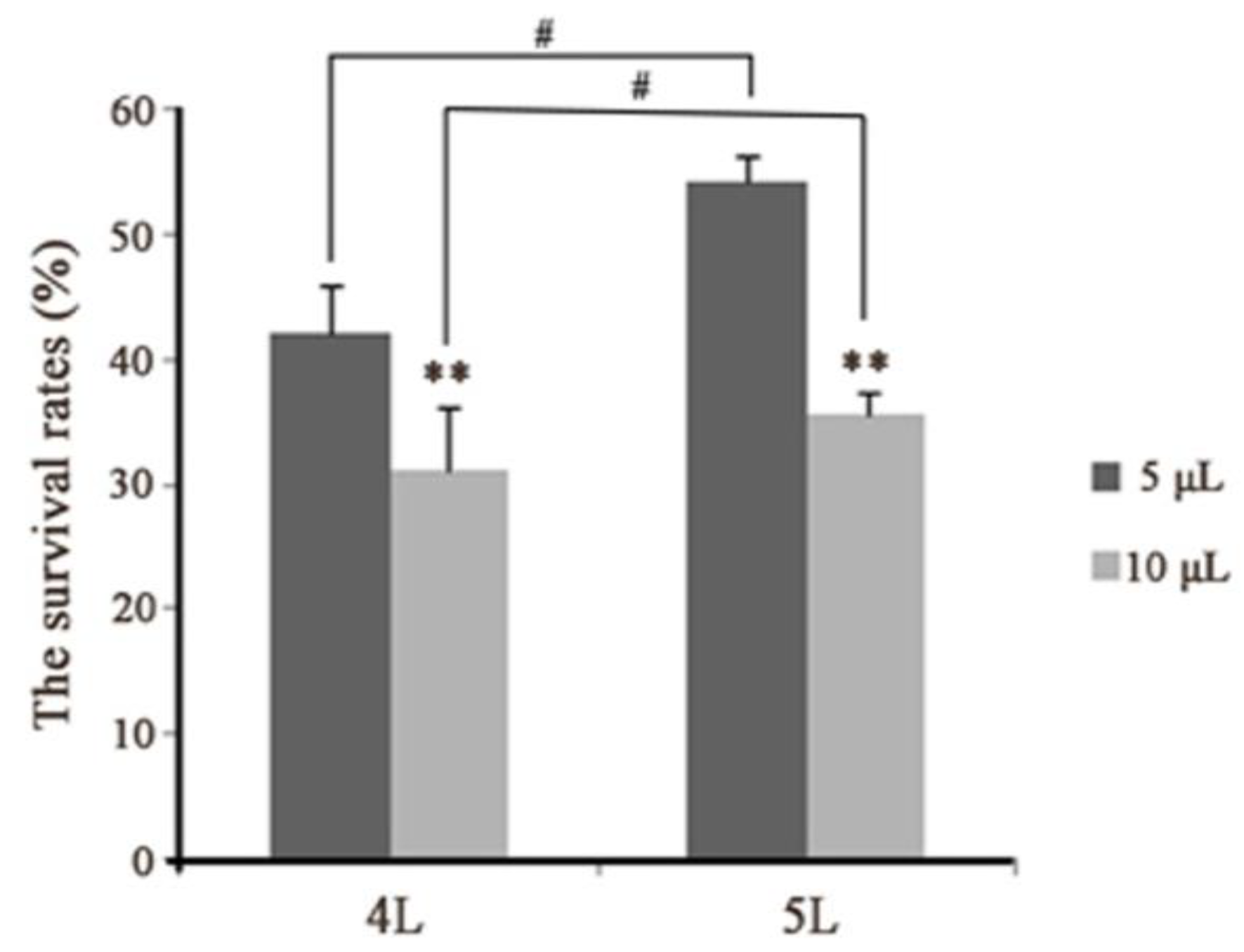
2.8. Survival Statistics
2.9. Hemolymph Melanization and Hemocyte Profiles Observation In Vitro
2.10. Hemocytes Immune Process Observation In Vitro
2.11. Antibacterial Activity Detection
3. Results
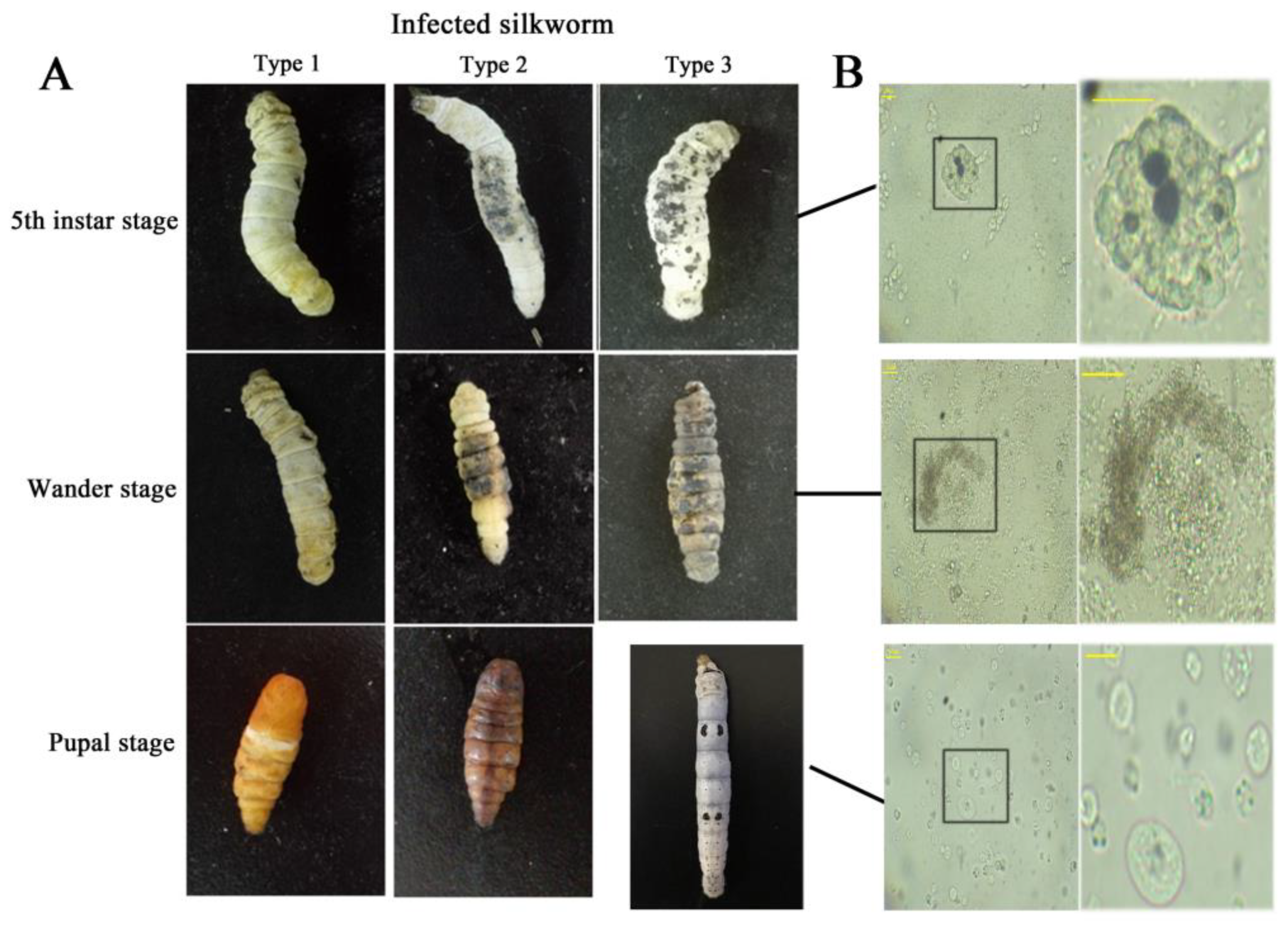
3.1. Cytological Morphology in Larval Hemocoel After Infection
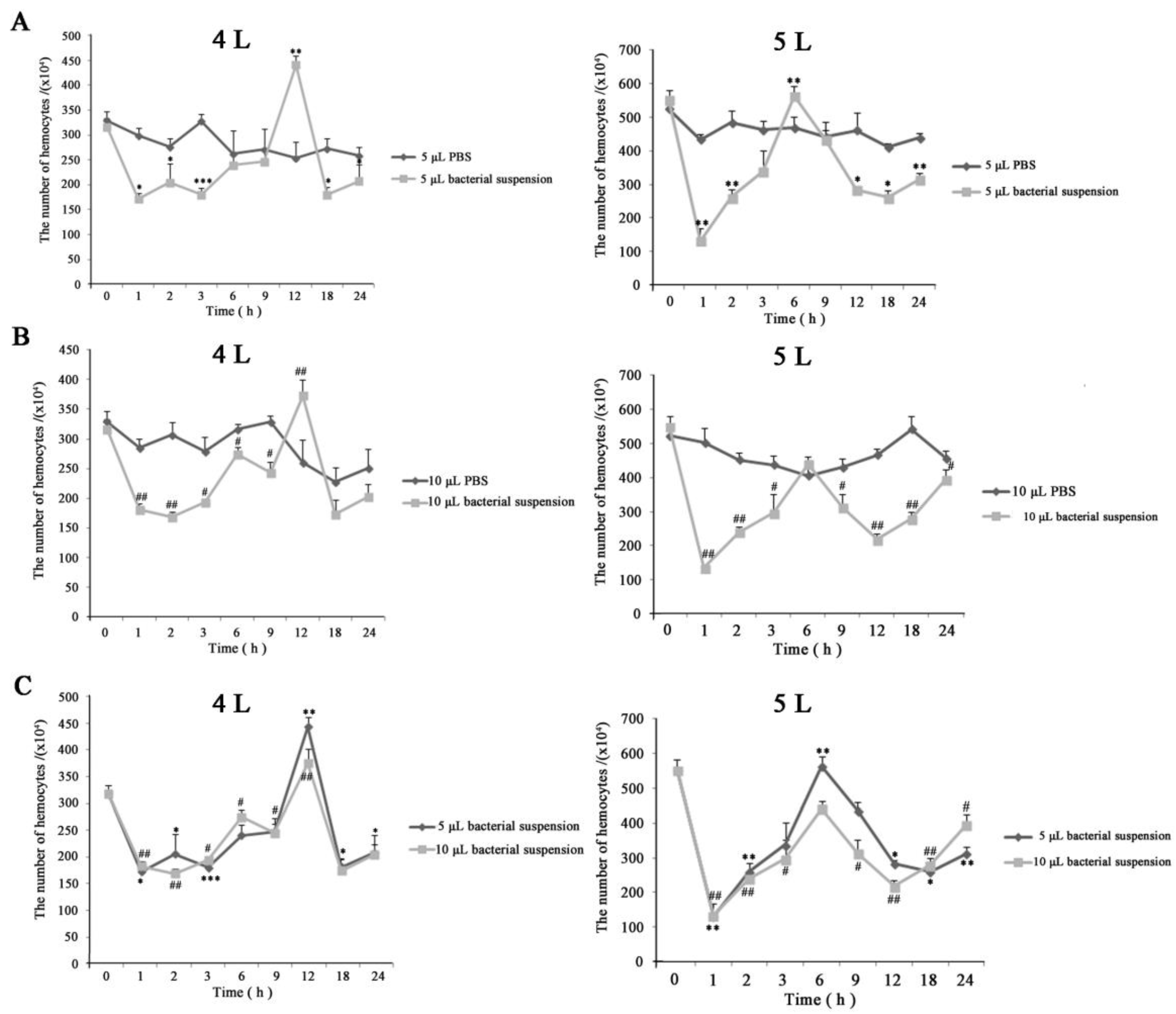
3.2. Hemocyte Numbers in Larval Hemocoel After Infection
3.3. Hemocyte Types in Larval Hemocoel After Infection
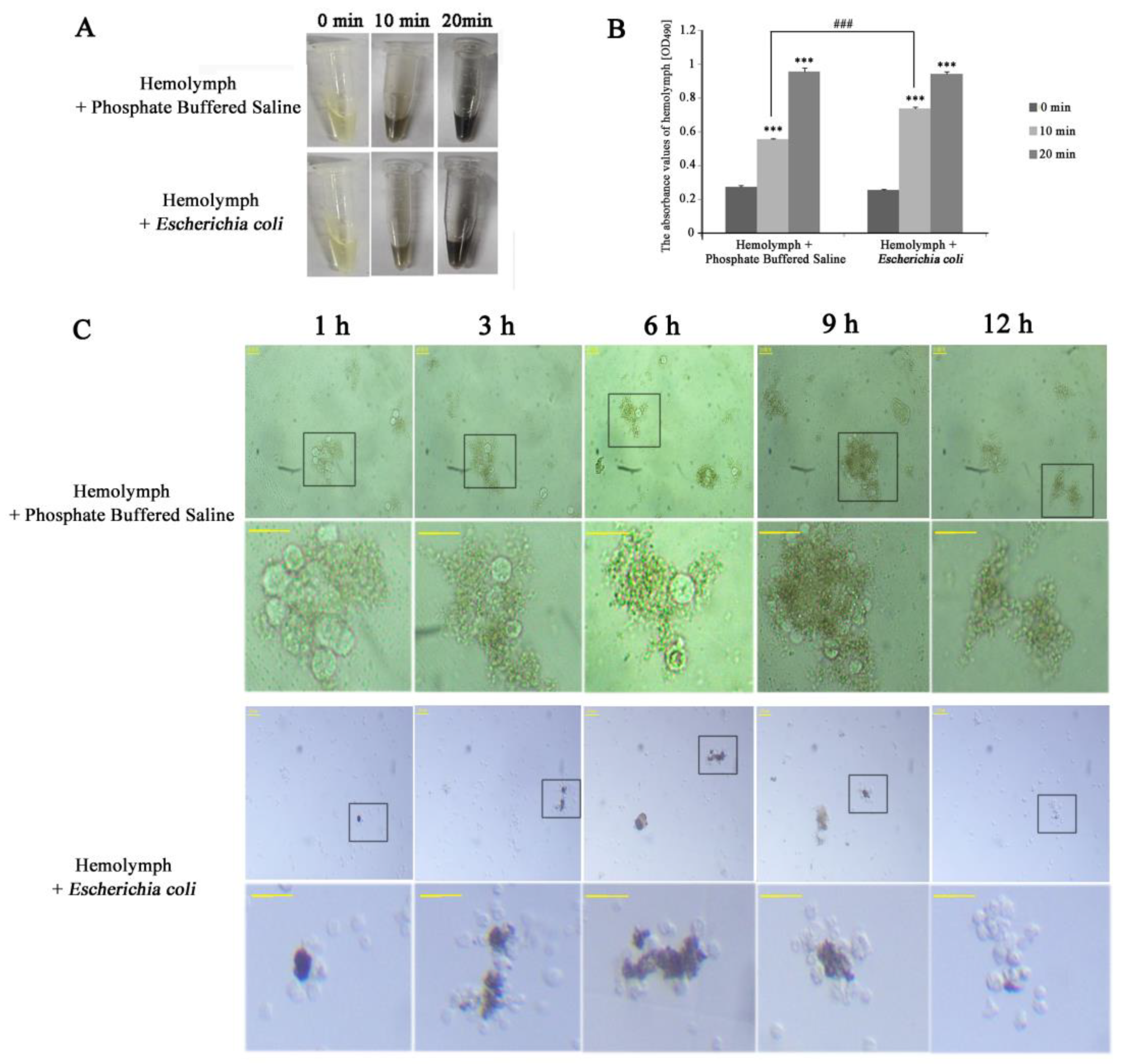
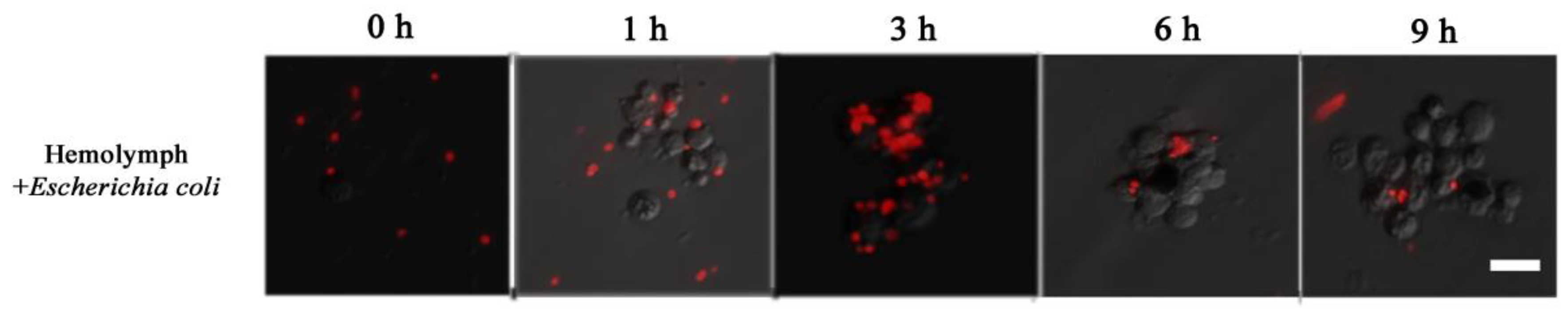
3.4. Hemolymph Melanization and Immunity In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.; Li, L.; Wang, J.Y.; Li, H.Y.; Li, Y.; Lv, Y.; Lu, C. BmPAH catalyzes the initial melanin biosynthetic step in Bombyx mori. PLoS ONE 2013, 8, e71984. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, J.Y.; Li, H.Y.; Li, Y.; Chen, P.; Li, T.; Chen, X.; Xiao, J.J.; Zhang, L. Role of GTP-CHI links PAH and TH in melanin synthesisin silkworm, Bombyx mori. Gene 2015, 567, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Barreaux, A.M.G.; Barreaux, P.; Koella, J.C. Overloading the immunity of the mosquito Anopheles gambiae with multiple immune challenges. Parasites Vector 2016, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, X.; Christensen, B. Dopachrome conversion activity in Aedes aegypti: Significance during melanotic encapsulation of parasites and cuticular tanning. Insect Biochem. Mol. Biol. 1994, 24, 1043–1049. [Google Scholar] [CrossRef]
- An, C.; Ishibashi, J.; Ragan, E.J.; Jiang, H.; Kanost, M.R. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem. 2009, 284, 19716–19726. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Kenneth, S. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.Y.; Xue, J.; Wu, W.J.; Wang, Y.; Lv, Z.Y.; Zhang, C.X. An immune-induced reeler protein is involved in the Bombyx mori melanization cascade. Insect Biochem. Mol. Biol. 2011, 41, 696–706. [Google Scholar] [CrossRef]
- Clark, K.D. Altered tyrosine metabolism and melanization complex formation underlie the developmental regulation of melanization in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 58, 66–75. [Google Scholar] [CrossRef]
- Kan, H.; Kim, C.H.; Kwon, H.M.; Park, J.W.; Roh, K.B.; Lee, H.; Park, J.W.; Roh, K.B.; Lee, H.; Park, B.J.; et al. Molecular control of phenoloxidase-induced melanin synthesis in an insect. J. Biol. Chem. 2008, 283, 25316–25323. [Google Scholar] [CrossRef] [PubMed]
- Maki, S.; Masayuki, O.; Asahi, S.; Hinako, T.; Yasutaka, Y.; Madoka, K.; Ryoichi, S. Localization of the serine protease homolog BmSPH-1 in nodules of E. coli-injected Bombyx mori larvae and functional analysis of its role in nodule melanization. Dev. Comp. Immunol. 2011, 35, 611–619. [Google Scholar]
- Tokura, A.; Fu, G.S.; Sakamoto, M.; Endo, H.; Tanaka, S.; Kikuta, S.; Tabunoki, H.; Sato, R. Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J. Insect Physiol. 2014, 60, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, X.; Tan, J.; Zhang, K.; Guan, X.; Patterson, L.H.; Ding, H.; Cui, H. A novel Lozenge gene in silkworm, Bombyx mori regulates the melanization response of hemolymph. Dev. Comp. Immunol. 2015, 53, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Satoh, D.; Horii, A.; Ochiai, M.; Ashida, M. Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori. J. Biol. Chem. 1999, 274, 7441. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Mang, D.; Fu, G.S.; Tanaka, S.; Endo, H.; Kikuta, S.; Sato, R. Mechanisms of nodule-specific melanization in the hemocoel of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2016, 70, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Imai, Y.; Morozumi, A.; Imamura, M.; Kadotani, T.; Yaoi, K.; Iwahana, H.; Sato, R. Lipopolysaccharide-binding protein of Bombyx mori participates in a hemocyte-mediated defense reaction against gram-negative bacteria. J. Insect Physiol. 1999, 45, 853–859. [Google Scholar] [CrossRef]
- Hultmark, D. Drosophila immunity: Paths and patterns. Curr. Opin. Immunol. 2003, 15, 12–19. [Google Scholar] [CrossRef]
- Sumathipala, N.; Jiang, H. Involvement of Manduca sexta peptidoglycan recognition protein-1 in the recognition of bacteria and activation of prophenoloxidase system. Insect Biochem. Mol. Biol. 2010, 40, 487–495. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Gokçen, F.; Felfoldi, G.; Millichap, P.J.; Trenczek, T.E.; Ffrench-Constant, R.H.; Reynolds, S.E. The immunoglobulin family protein Hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cell. Microbiol. 2007, 9, e1137–e1147. [Google Scholar] [CrossRef]
- Marmaras, V.J.; Lampropoulou, M. Regulators and signalling in insect haemocyte immunity. Cell. Signal. 2009, 21, 186–195. [Google Scholar] [CrossRef]
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bioessays 2001, 23, 344–351. [Google Scholar] [CrossRef]
- Sigle, L.T.; Hillyer, J.F. Mosquito hemocytes preferentially aggregate and phagocytose pathogens in the periostial regions of the heart that experience the most hemolymph flow. Dev. Comp. Immunol. 2016, 55, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, Y.; Shimura, S.; Ueno, C.; Kanamori, Y.; Mita, K.; Kiuchi, M.; Kamimura, M. Purification and characterization of silkworm hemocytes by flow cytometry. Dev. Comp. Immunol. 2009, 33, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Carlos, R.; Michel, B. Insect hemocytes: What type of cell is that? J. Insect Physiol. 2006, 52, 417–429. [Google Scholar]
- Wago, H. Humoral factors promoting the adhesive properties of the granular cells and plasmatocytes of the silkworm, Bombyx mori, and their possible role in the initial cellular reactions to foreignness. Cell. Immunol. 1980, 54, 155–169. [Google Scholar] [CrossRef]
- Ling, E.; Yu, X.Q. Prophenoloxidase binds to the surface of hemocytes and is involved in hemocyte melanization in Manduca sexta. Insect Biochem. Mol. Biol. 2005, 35, 1356–1366. [Google Scholar] [CrossRef]
- Tan, J.; Xu, M.; Zhang, K.; Wang, X.; Chen, S.; Li, T.; Xiang, Z.H.; Cui, H.J. Characterization of hemocytes proliferation in larval silkworm, Bombyx mori. J. Insect Physiol. 2013, 6, 595–603. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, Q.; Tang, L.; Chen, L.; Liu, X.S.; Wang, Y.F. Involvement of a pattern recognition receptor C-type lectin 7 in enhancing cellular encapsulation and melanization due to its carboxyl-terminal CRD domain in the cotton bollworm, Helicoverpa armigera. Dev. Comp. Immunol. 2013, 44, 21–29. [Google Scholar] [CrossRef]
- Goldsmith, M.R.; Shimada, T.; Abe, H. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 2005, 50, 71–100. [Google Scholar] [CrossRef]
- Yamashita, M.; Iwabuchi, K. Bombyx mori prohemocyte division and differentiation in individual microcultures. J. Insect Physiol. 2001, 47, 325–331. [Google Scholar] [CrossRef]
- Ling, E.; Shirai, K.; Kanekatsu, R.; Kiguchi, K. Classification of larval circulating hemocytes of the silkworm, Bombyx mori, by acridine orange and propidium iodide staining. Histochem. Cell Biol. 2003, 120, 505–511. [Google Scholar] [CrossRef]
- Ling, E.; Shirai, K.; Kanehatsu, R.; Kiguchi, K. Reexamination of phenoloxidase in larval circulating hemocytes of the silkworm, Bombyx mori. Tissue Cell 2005, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Jiravanichpaisal, P.; Lee, B.L.; Soderhall, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- King, J.G.; Hillyer, J.F. Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: Hemocyte mitosis following infection. BMC Biol. 2013, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003, 313, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.M.; Li, J.Y.; Chen, C.C.; Nappi, A.J. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Hamamoto, H.; Imamura, K.; Adachi, T.; Shoji, M.; Nakayama, K.; Sekimizu, K. Porohyromonas gingivalis peptidoglycans induce excessive activation of the innate immune system in silkworm larvae. J. Biol. Chem. 2010, 285, 33338–33347. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Kanamori, Y.; Kiuchi, M.; Kamimura, M. In vitro studies of hematopoiesis in the silkworm: Cell proliferation in and hemocyte discharge from the hematopoietic organ. J. Insect Physiol. 2003, 49, 907–916. [Google Scholar] [CrossRef]
- Bauer, E.; Trenczek, T.; Dorn, S. Instar-dependent hemocyte changes in Pieris brassicae after parasitization by Cotesia glomerata. Entomol. Exp. Appl. 1998, 88, 49–58. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Baldwin, H.; Constant, R.H.; Reynolds, S.E. Developmental modulation of immunity: Changes within the feeding period of the fifth larval stage in the defence reactions of Manduca sexta to infection by Photorhabdus. J. Insect Physiol. 2008, 54, 309–318. [Google Scholar] [CrossRef]
- Wu, G.Q.; Liu, Y.; Ding, Y.; Yi, Y.H. Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell 2016, 48, 297–304. [Google Scholar] [CrossRef]
- Manachini, B.; Arizza, V.; Parrinello, D.; Parrinello, N. Hemocytes of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) and their response to Saccharomyces cerevisiae and Bacillus thuringiensis. J. Invertebr. Pathol. 2011, 106, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. The antibacterial innate immune response by the mosquito Aedes aegypti is mediated by hemocytes and independent of Gram type and pathogenicity. Microbes Infect. 2004, 6, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Amaral, I.M.R.; Neto, J.F.M.; Pereira, G.B.; Franco, M.B.; Beletti, M.E.; Kerr, W.E.; Bonetti, A.M.; Carlos, U.V. Circulating hemocytes from larvae of Melipona scutellaris (Hymenoptera, Apidae, Meliponini): Cell types and their role in phagocytosis. Micron 2010, 41, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y.; Kanamori, Y.; Kiuchi, M.; Kamimura, M. Two hemocyte lineages exist in silkworm larval hematopoietic organ. PLoS ONE 2010, 8, e11816. [Google Scholar] [CrossRef] [PubMed]
- Hemandez, S.; Lanz, H.; Rodriguez, M.H.; Torres, J.A.; Martinez-Palomo, A.; Tsutsumi, V. Morphological and cytochemical characterization of female Anopheles albimanus (Diptera: Culicidae) hemocytes. J. Med. Entomol. 1999, 36, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Kiuchi, T.; Shimada, T.; Nagata, M.; Katsuma, S. Silkworm plasmatocytes are more resistant than other hemocyte morphotypes to Bombyx mori nucleopolyhedrovirus infection. J. Invertebr. Pathol. 2013, 112, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Beaulaton, J. Hemocytes and hemocytopoiesis in silkworms. Biochimie 1979, 61, 157–164. [Google Scholar] [CrossRef]
- Clark, K.D.; Strand, M.R. Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J. Biol. Chem. 2013, 288, 14476–14487. [Google Scholar] [CrossRef] [PubMed]
- Sideri, M.; Tsakas, S.; Markoutsa, E.; Lampropoulou, M.; Marmaras, V.J. Innate immunity in insects: Surface-associated dopa decarboxylase-dependent pathways regulate phagocytosis, nodulation and melanization in medfly haemocytes. Immunology 2007, 123, 528–537. [Google Scholar] [CrossRef]
- Li, T.; Jiang, G.B.; Lv, Y.; Li, L.; Zhang, L.; Fan, X.L.; Chen, P. In vitro induction of Bombyx mori haemolymph melanization by three types of microbes and their cell wall ingredients. Sci. Seric. 2015, 41, 0278–0285. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Yan, D.; Wang, X.; Zhang, L.; Chen, P. Hemocyte Changes During Immune Melanization in Bombyx Mori Infected with Escherichia coli. Insects 2019, 10, 301. https://doi.org/10.3390/insects10090301
Li T, Yan D, Wang X, Zhang L, Chen P. Hemocyte Changes During Immune Melanization in Bombyx Mori Infected with Escherichia coli. Insects. 2019; 10(9):301. https://doi.org/10.3390/insects10090301
Chicago/Turabian StyleLi, Tian, Dengfeng Yan, Xiaohui Wang, Liang Zhang, and Ping Chen. 2019. "Hemocyte Changes During Immune Melanization in Bombyx Mori Infected with Escherichia coli" Insects 10, no. 9: 301. https://doi.org/10.3390/insects10090301




