Enhancement of Activated Sludge Dewaterability by Using Filamentous Fungi as Bioadditives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Activated Sludge
2.2. Preparation of Fungal Spores Solutions
2.3. Experimental Device
2.4. Evaluation of Sludge Dewaterability
2.5. Extraction and Analysis of EPS
2.6. Interaction between Fungi and Microbial Communities
3. Results and Discussion
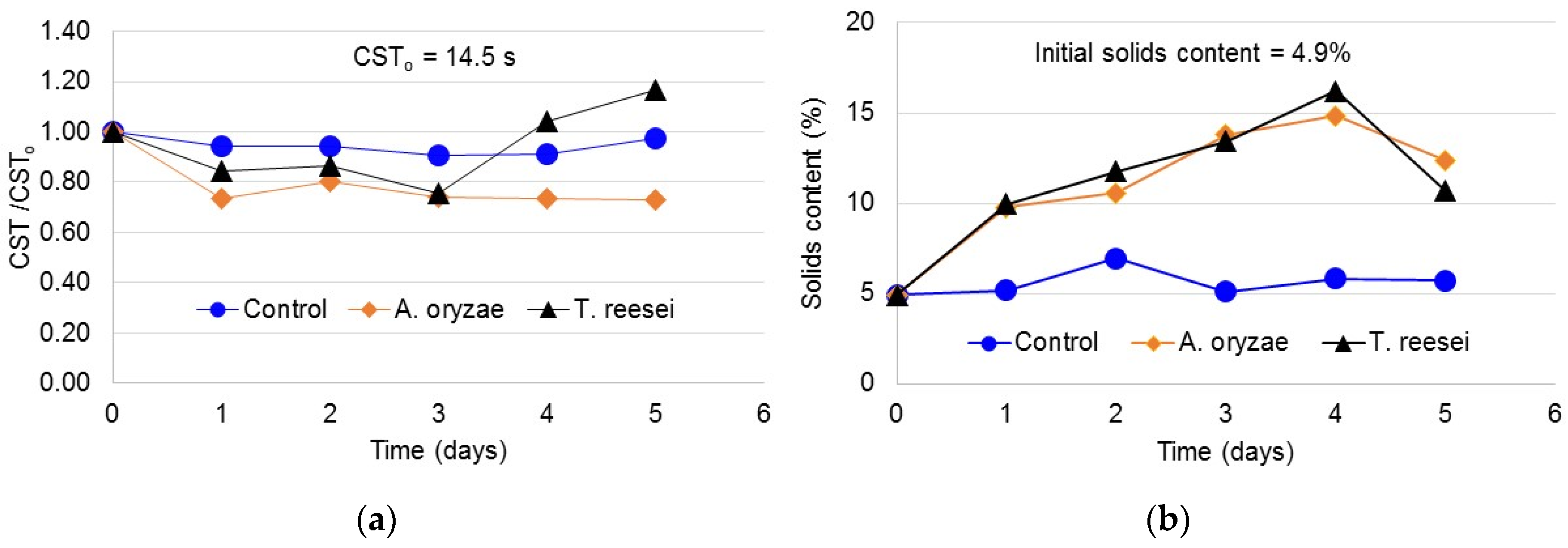
3.1. Capillary Suction Time (CST)
3.2. Sludge Solids Content
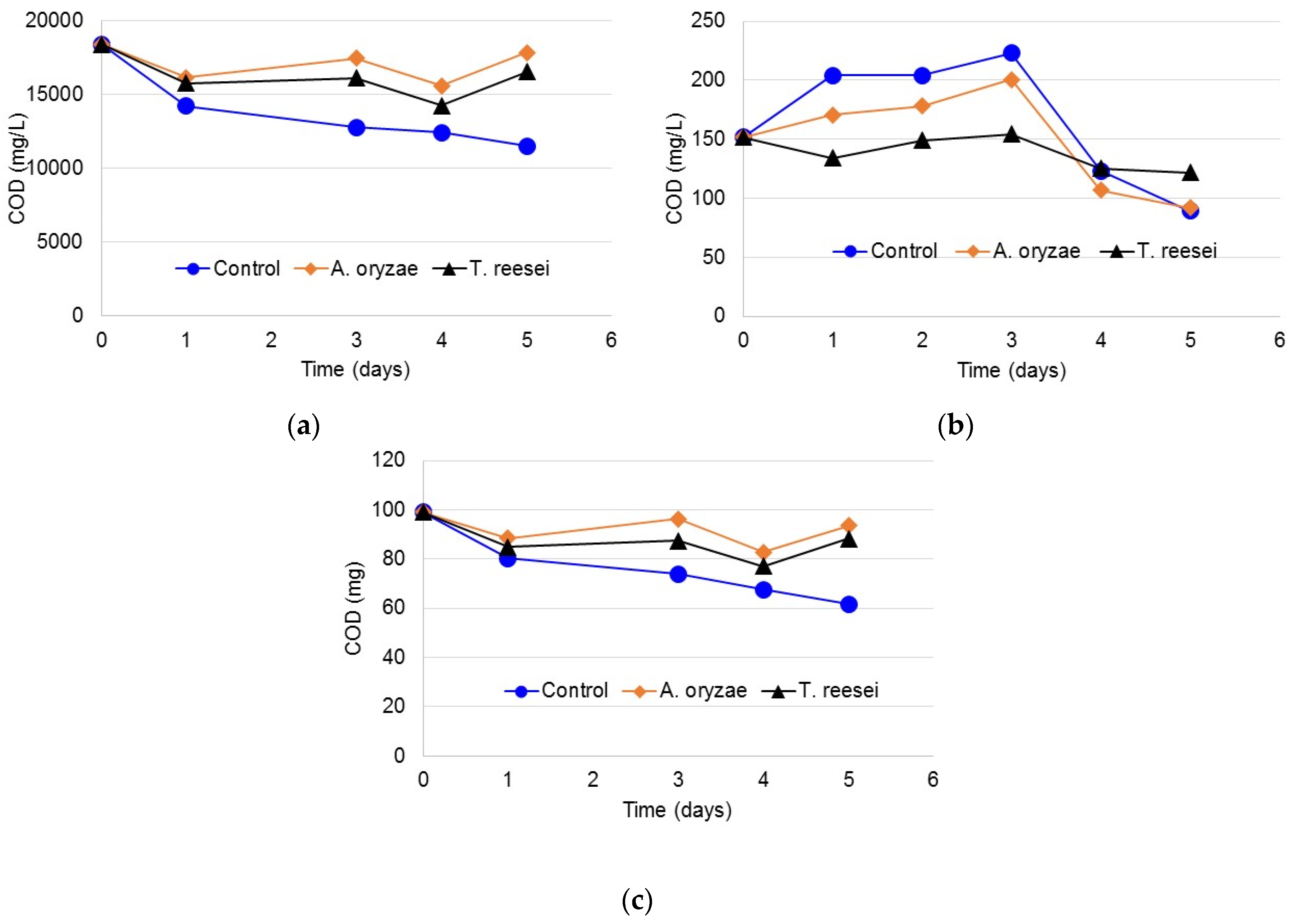
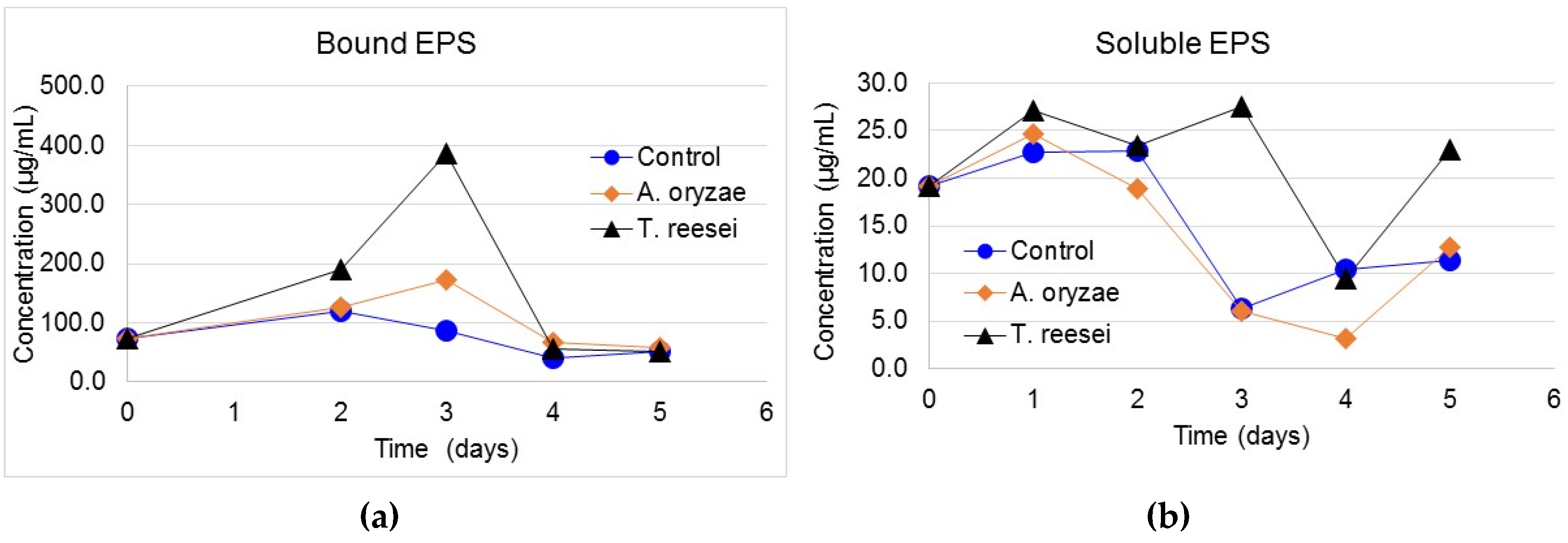
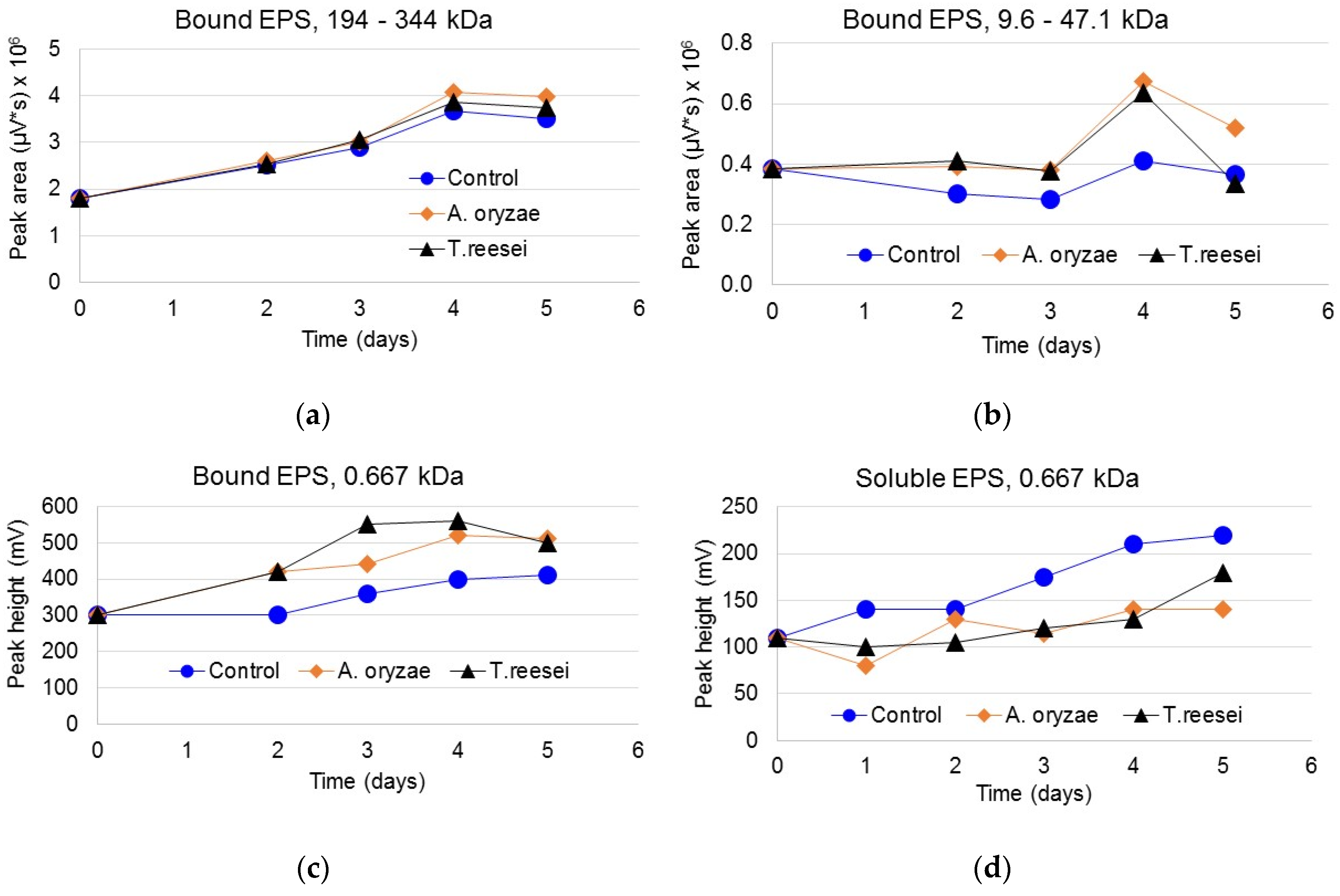
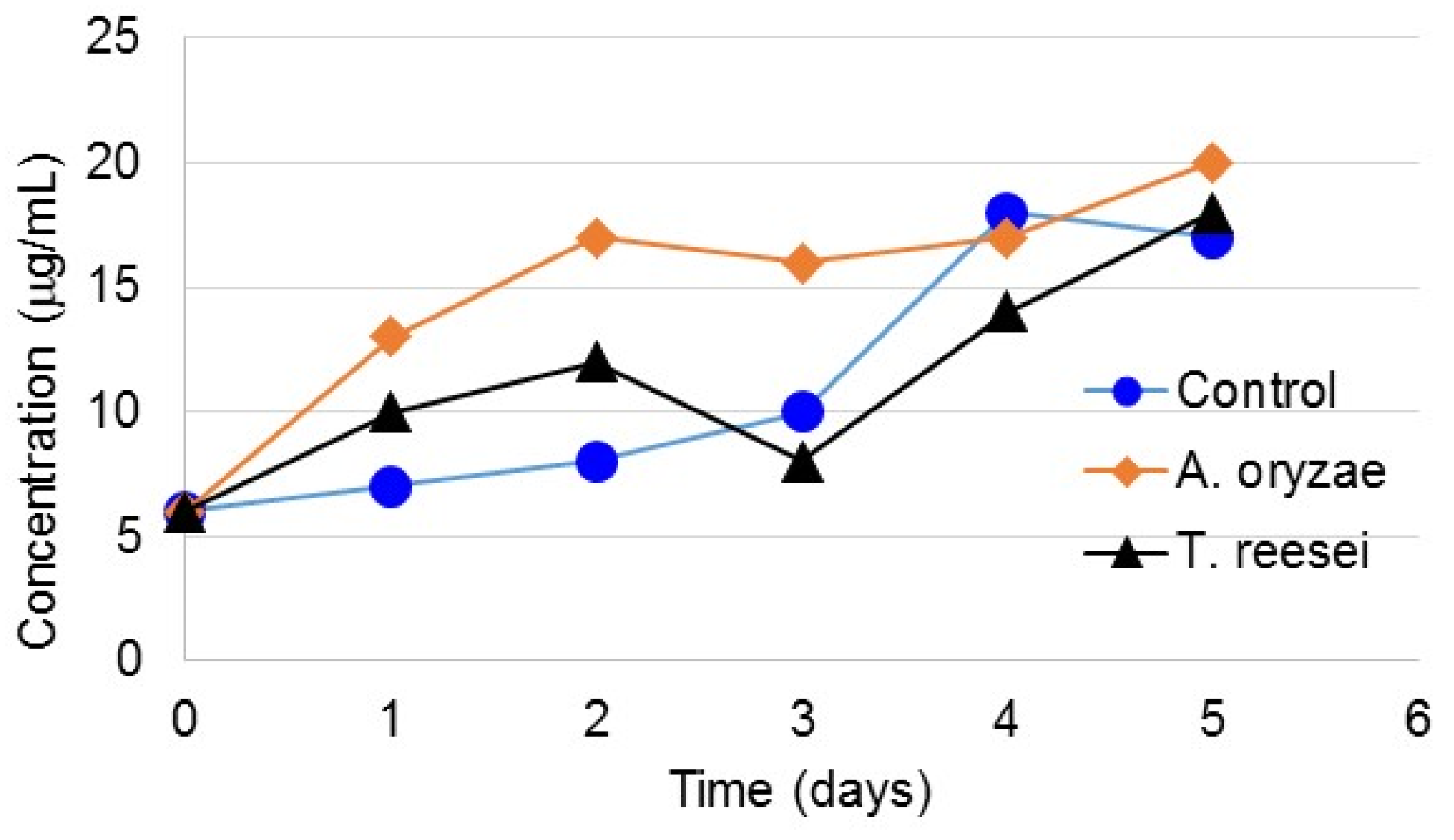
3.3. Protein Content of Bound and Soluble EPS
3.4. Carbohydrate Content in Bound and Soluble EPS
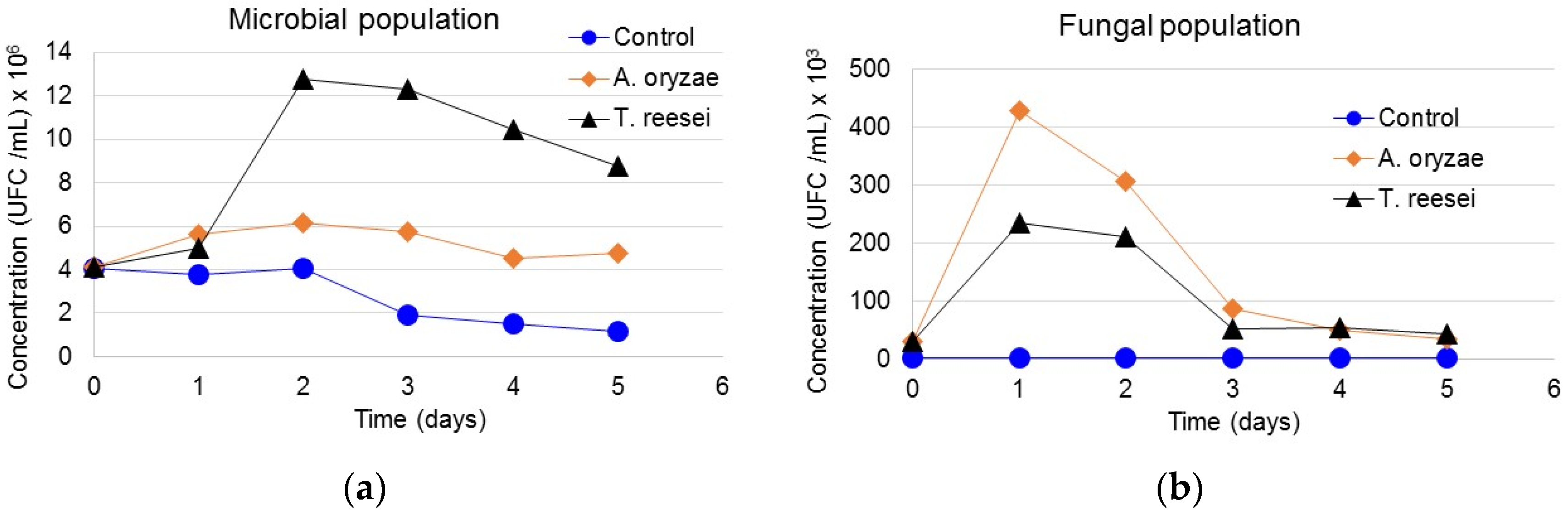
3.5. Interaction of Microbial and Fungi Communities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neyens, E.; Baeyens, J.; Dewil, R.; De Heyder, B. Advanced sludge treatment affects extracellular polymeric substances to improve activated sludge dewatering. J. Hazard. Mater. 2004, 106, 83–92. [Google Scholar] [CrossRef]
- Egemen, E.; Corpening, J.; Nirmalakhandan, N. Evaluation of an ozonation system for reduced waste sludge generation. Water Sci. Technol. 2001, 44, 445–452. [Google Scholar] [PubMed]
- Conagua. Diagnóstico del Programa U031 “Operación y Mantenimiento de Las Plantas de Tratamiento de Aguas Residuales”; Publication of the Ministry of Environment and Natural Resources (SEMARNAT): Mexico City, Mexico, 2014. (In Spanish) [Google Scholar]
- Guo, J.S.; Xu, Y.F. Review of Enzymatic Sludge Hydrolysis. J. Bioremed. Biodegrad. 2011, 2. [Google Scholar] [CrossRef]
- American Society of Civil Engineers (ASCE); Environmental and Water Resources Institute (EWRI); Water Environment Federation (WEF). Vol. 3: Solids processing and management. In Design of Municipal Wastewater Treatment Plants, 5th ed.; McGraw Hill: New York, NY, USA, 2009. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering, Treatment and Reuse, 4th ed.; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Chang, G.R.; Liu, J.C.; Lee, D.J. Co-conditioning and dewatering of chemical sludge and waste activated sludge. Water Res. 2001, 35, 786–794. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, Z.; Wang, D.; Zhou, L. Enhancement of sludge dewaterability by sequential inoculation of filamentous fungus Mucor. circinelloides ZG-3 and Acidithiobacillus. ferrooxidans LX5. Chem. Eng. J. 2016, 284, 216–223. [Google Scholar] [CrossRef]
- Murugesan, K.; Selvam, A.; Wong, J.W.C. Flocculation and dewaterability of chemically enhanced primary treatment sludge by bioaugmentation with filamentous fungi. Bioresource Technol. 2014, 168, 198–203. [Google Scholar]
- Molla, A.H.; Fakhru’l-Razi, A. Mycoremediation—A prospective environmental friendly technique of bioseparation and dewatering of domestic wastewater sludge. Environ. Sci. Pollut. Res. 2012, 19, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, C.; D’Souza-Ticlo, D.; Verma, A. Treatment of colored effluents with lignin-degrading enzymes: An emerging role of marine-derived fungi. Crit. Rev. Microbiol. 2008, 34, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Fakhru’l-Razi, A.; Molla, A.H. Biosolids accumulation and biodegradation of domestic wastewater treatment plant sludge by developed liquid state bioconversion process using batch fermenter. Water Res. 2003, 37, 3569–3578. [Google Scholar] [CrossRef]
- Alam, M.Z.; Fakhru’l-Razi, A. Enhanced settleability and dewaterability of fungal treated domestic wastewater sludge by liquid state bioconversion process. Water Res. 2003, 37, 1118–1124. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Molla, A.H. Enhancement of bioseparation and dewaterability of domestic wastewater sludge by fungal treated dewatered sludge. J. Hazard. Mater. 2007, 147, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Chancharoonpong, C.; Hsieh, P.C.; Sheu, S.C. Enzyme production and growth of Aspergillus oryzae S. on Soybean Koji Fermentation. APCBEE Procedia 2012, 2, 57–61. [Google Scholar] [CrossRef]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.I.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 43, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Berka, R.M.; Henrissat, B.; Saloheimo, M.; et al. Genome sequencing and analysis of the biomass-degrading fungus Trichoderma. reesei (syn. Hypocrea jecorina). Nat. Biotechnol. 2008, 26, 553–560. [Google Scholar] [CrossRef] [PubMed]
- American Type Culture Collection: Manassas (ATCC). Mycology Culture Guide Tips and Techniques for Culturing Yeasts and Filamentous Fungi; ATCC: Manassas, VA, USA, 2013. [Google Scholar]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water & Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of Extracellular Polymers from Activated Sludge Using a Cation Exchange Resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Scholz, M. Review of recent trends in capillary suction time (CST) dewaterability testing research. Ind. Eng. Chem. Res. 2005, 44, 8157–8163. [Google Scholar] [CrossRef]
- Sawalha, O.; Scholz, M. Modeling the relationship between capillary suction time and specific resistance to filtration. J. Environ. Eng. ASCE 2010, 136, 983–991. [Google Scholar] [CrossRef]
- Chen, G.W.; Lin, W.W.; Lee, D.J. Capillary Suction Time (CST) as a Measure of Sludge Dewaterability. Water Sci. Technol. 1996, 34, 443–448. [Google Scholar]
- Merrylin, J.; Kaliappan, S.; Adish Kumar, S.; Yeom, I.T.; Rajesh Banu, J. Enhancing aerobic digestion potential of municipal waste-activated sludge through removal of extracellular polymeric substance. Environ. Sci. Pollut. Res. 2014, 21, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.; Chao, L.; Sze, S. Quantitative secretomic analysis of Trichoderma reesei strains reveals enzymatic composition for lignocellulosic biomass degradation. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef]
- Chutmanop, J.; Chuihulcherm, S.; Chisti, Y.; Srinophakun, P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Zavala, M.Á.; Ramos Patlán, J.I. Enhancement of Activated Sludge Dewaterability by Using Filamentous Fungi as Bioadditives. Water 2016, 8, 531. https://doi.org/10.3390/w8110531
López Zavala MÁ, Ramos Patlán JI. Enhancement of Activated Sludge Dewaterability by Using Filamentous Fungi as Bioadditives. Water. 2016; 8(11):531. https://doi.org/10.3390/w8110531
Chicago/Turabian StyleLópez Zavala, Miguel Ángel, and Jorge Isidro Ramos Patlán. 2016. "Enhancement of Activated Sludge Dewaterability by Using Filamentous Fungi as Bioadditives" Water 8, no. 11: 531. https://doi.org/10.3390/w8110531





