Impact of Rural Domestic Wastewater Irrigation on the Physicochemical and Microbiological Properties of Pakchoi and Soil
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Design
| Parameters | Soil | ||
|---|---|---|---|
| Soil particle proportion (%) | >0.05 mm | 31.25 | sand |
| 0.05–0.02 mm | 22.20 | ||
| 0.02–0.005 mm | 13.40 | lime | |
| 0.005–0.002 mm | 6.30 | ||
| <0.002 mm | 26.85 | clay | |
| pH | 7.96 | ||
| EC (μs/cm) | 571 | ||
| Organic matter (g/kg) | 1.58 | ||
| NO3-N (mg/kg) | 8.56 | ||
| NH3-N (mg/kg) | 0.063 | ||
| Available P (mg/kg) | 3.1 | ||
| Available K (mg/kg) | 107.7 | ||
| Cu (mg/kg) | 20.3 | ||
| Zn (mg/kg) | 67.3 | ||
| Cd (mg/kg) | 0.023 | ||
| Pb (mg/kg) | 36.3 | ||
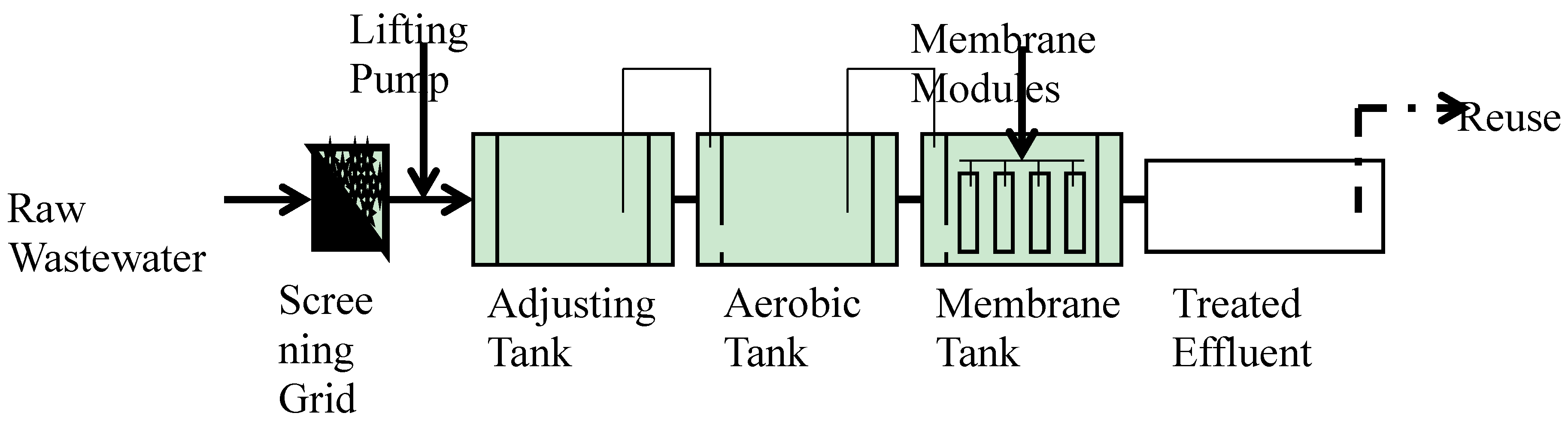
| Parameters | Raw Wastewater | Treated Wastewater | Criteria of Tap Water [21] | Criteria of Irrigation Water [22] |
|---|---|---|---|---|
| pH | 7.60 | 7.59 | 6.5–8.5 | 5.5–8.5 |
| EC (μs/cm) | 754 | 376 | -- | ≤1,000 |
| Salinity (mg/L) | 415–566 | 207–282 | ≤1,000 (TDS) | - |
| Temperature (°C) | 12.2 | 12.1 | -- | ≤35 |
| COD (mg/L) | 346 | 35 | -- | ≤100 a or 60 b |
| SS (mg/L) | 26 | 21 | -- | ≤60 a or 15 b |
| anionic surfactant (mg/L) | 2.00 | no detectable (<0.05) | ≤0.3 | ≤5 |
| TN (mg/L) | 54.6 | 26.2 | -- | -- |
| TP (mg/L) | 4.97 | 7.66 | -- | -- |
| Zn (mg/L) | 0.153 | no detectable (<0.006) | ≤1.0 | ≤2 |
| Cu (mg/L) | no detectable (<0.01) | no detectable (<0.01) | ≤1.0 | ≤1 |
| Pb (mg/L) | 0.003 | no detectable (<0.001) | ≤0.01 | ≤0.2 |
| Cd (mg/L) | 0.0002 | 0.0002 | ≤0.005 | ≤0.01 |
| Cl− (mg/L) | 44.2 | 56.1 | ≤250 | ≤350 |
| NH3 -N (mg/L) | 45.7 | 8.59 | ≤0.5 | -- |
| NO3- N (mg/L) | no detectable (<0.2) | 16.0 | ≤20 | -- |
| Total bacteria count/mL | (1.24 ± 0.05) × 106 | (2.80 ± 0.20) × 105 | ≤100 | -- |
| Total coliforms/mL | 6.05 × 105 ± 1.75 × 105 | no detectable (<30) | no detectable | -- |
| Fecal coliforms/mL | 240,000 | no detectable (<3) | no detectable | ≤20 a or 10 b |
| Escherichia coli | 8.9 × 104 | no detectable (<30) | no detectable | -- |
| Ascaris Lumbricoides (eggs/L) | 34 | no detectable | no detectable | ≤2a or 1b |
2.2. Collection of Microbial Samples and DNA Extraction
2.3. Physical and Chemical Properties Detection of Plants and Soil
2.4. Molecular Assays
| Pathogenic Bacteria | Targeted Gene | Primer Sequences | Amplicon Size (bp) | Ref. |
|---|---|---|---|---|
| Aeromonas hydrophila | Cytolytic enterotoxin | AHCF1: GAGAAGGTGACCACCAAGAACA AHCR1: AACTGACATCGGCCTTGAACTC | 232 | [29] |
| Arcobacter spp. | 23S rRNA | ARCO1: GTCGTGCCAAGAAAAGCCA ARCO2: TTCGCTTGCGCTGACAT | 331 | [30] |
| Bacillus cereus | 16S rRNA | F: TCGAAATTGAAAGGCGGC R: GGTGCCAGCTTATTCAAC | 288 | [31] |
| Clostridium difficile | 16S rRNA | Clo-16F: TTGAGCGATTTACTTCGGTAAAGA Clo-16R: CCATCCTGTACTGGCTCACCT | 157 | [32] |
| Clostridium perfringens | 16S rRNA | Clp-F: ATGCAAGTCGAGCGA(G/T)G Clp-R: TATGCGGTATTAATCT(C/T)CCTTT | 120 | [32] |
| Escherichia coli | uidA | Eco-F: CTGCTGCTGTCGGCTTTA Eco-R: CCTTGCGGACGGGTAT | 205 | [33] |
| Legionella spp. | 16S rRNA | LEG448:GAGGGTTGATAGGTTAAGAGC LEG858:GTCAACTTATCGCGTTTGCT | 430 | [34] |
| Mycobacterium spp. | 16S rRNA | Myco F: ATGCACCACCTGCACACAGG Myco R: GGTGGTTTGTCGCGTTGTTC | 470 | [35] |
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effects of Sewage Irrigation on Soil Properties

3.2. Effects of Sewage Irrigation on Plant Properties
| Samples | Height (cm) | Fresh Weight (g) | Soluble Sugar (%) | Soluble Protein (mg/g) | Nitrate (g/kg) | Vc (mg/g) |
|---|---|---|---|---|---|---|
| DW | 15.25 ± 0.76 a | 5.06 ± 0.57 a | 0.18 ± 0.04 a | 9.74 ± 0.33 a | 1.19 ± 0.10 a | 1.02 ± 0.11 a |
| WW | 19.18 ± 0.76 b | 10.83 ± 1.22 c | 0.16 ± 0.01 a | 10.16 ± 0.42 a | 1.53 ± 0.09 a,b | 1.06 ± 0.19 a |
| TW | 17.75 ± 0.89 a,b | 7.31 ± 1.05 a,b | 0.15 ± 0.01 a | 9.26 ± 0.11 a | 1.58 ± 0.07 b | 0.89 ± 0.08 a |
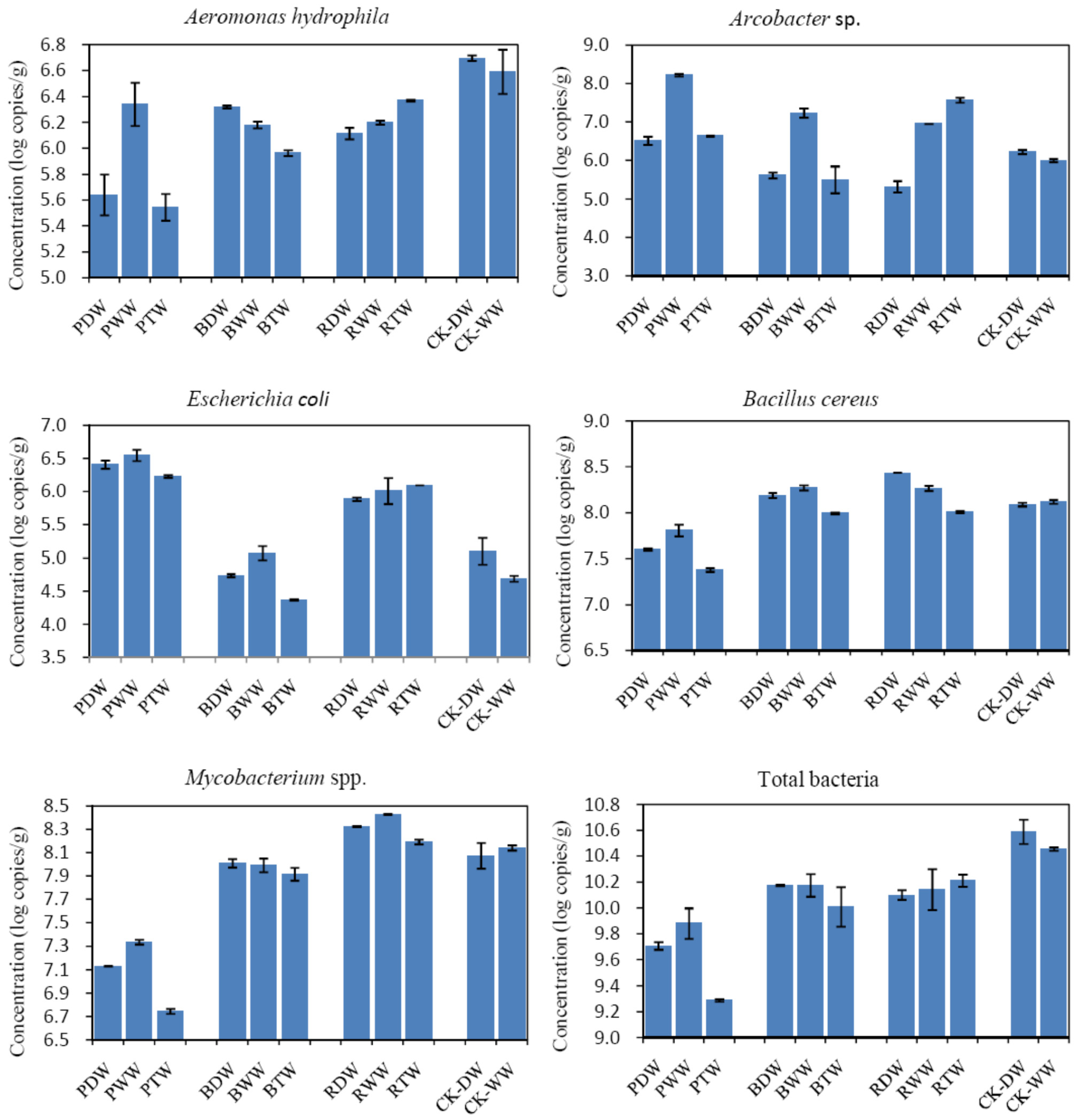
3.3. Quantitative PCR Detection of Pathogenic Bacteria
| Pathogens | WW (Copies/L) a | TW (Copies/L) b |
|---|---|---|
| Aeromonas hydrophila | (5.48 ± 0.22) × 1010 | (1.96 ± 0.02) × 107 |
| Arcobacter spp. | (1.01 ± 0.04) × 1011 | (1.45 ± 0.01) × 108 |
| Bacillus cereus | (2.01 ± 0.10) × 108 | (2.02 ± 0.06) × 107 |
| Clostridium difficile | (4.20 ± 0.10) × 106 | (6.35 ± 0.11) × 104 |
| Clostridium perfringens | (2.85 ± 0.09) × 109 | (5.00 ± 0.25) × 104 |
| E. coli | (1.02 ± 0.06) × 109 | (2.40 ± 0.36) × 105 |
| Legionella spp. | (1.27 ± 0.09) × 106 | (6.10 ± 0.77) × 105 |
| Mycobacterium spp. | (5.71 ± 0.40) × 107 | (3.17 ± 0.35) × 107 |
| Total bacteria | (3.13 ± 0.38) × 1011 | (1.56 ± 0.08) × 1010 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, Y. China’s water scarcity. J. Environ. Manag. 2009, 90, 3185–3196. [Google Scholar] [CrossRef]
- Li, L.J.; Li, B.Q. The problems of water resources in north China and the strategy. Chin. Geogr. Sci. 1992, 2, 183–196. [Google Scholar] [CrossRef]
- Zhang, J.Y.; He, R.M.; Qi, J.; Liu, C.S.; Wang, G.Q.; Jin, J.L. A new perspective on water issues in North China. Adv. Water Sci. 2013, 24, 303–310. [Google Scholar]
- Srinivasan, J.T.; Reddy, V.R. Impact of irrigation water quality on human health: A case study in India. Ecol. Econ. 2009, 68, 2800–2807. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agarwal, M.; Marshall, F. Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull. Environ. Contam. Toxicol. 2006, 77, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Lado, M.; Bar-Tal, A.; Azenkot, A.; Assouline, S.; Ravina, I.; Erner, Y.; Fine, P.; Dasberg, S.; Ben-Hur, M. Changes in Chemical Properties of Semiarid Soils under Long-Term Secondary Treated Wastewater Irrigation. Soil Sci. Soc. Am. J. 2012, 76, 1358–1369. [Google Scholar] [CrossRef]
- Toze, S. Reuse of effluent water- benefits and risks. Agric. Water Manag. 2006, 80, 147–159. [Google Scholar] [CrossRef]
- Al-Sa’ed, R. Pathogens assessment in reclaimed effluent used for industrial crops irrigation. Int. J. Environ. Res. Public Health 2007, 4, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Ashraf, S.; Rashid, U.; Ibrahim, M.; Hina, S.; Iftikhar, T.; Ramzan, S. Comparison of proximate and heavy metal contents of vegetables grown with fresh and wastewater. Pak. J. Bot. 2013, 45, 391–400. [Google Scholar]
- Pereira, L.S.; Oweis, T.; Zairi, A. Irrigation management under water scarcity. Agric. Water Manag. 2002, 57, 175–206. [Google Scholar] [CrossRef]
- Murtaza, G.; Ghafoor, A.; Qadir, M.; Owens, G.; Aziz, M.A.; Zia, M.H.; Saifullah. Disposal and use of sewage on agricultural lands in Pakistan: A review. Pedosphere 2010, 20, 23–34. [Google Scholar]
- Peasey, A.; Blumenthal, U.; Mara, D.; Ruiz-palacios, P.G. A Review of Policy and Standards for Wastewater reuse in Agriculture: A Latin American Perspective. Well Study, Task No. 68 Part II. 2000. Available online: http://www.lboro.ac.uk/well (accessed on 13 January 2014).
- Elifantz, H.; Kautsky, L.; Mor-Yosef, M.; Tarchitzky, J.; Bar-Tal, A.; Chen, Y.N.; Minz, D. Microbial activity and organic matter dynamics during 4 years of irrigation with treated wastewater. Microb. Ecol. 2011, 62, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Meli, S.; Porto, M.; Belligno, A.; Bufo, S.A.; Mazzatura, A.; Scopa, A. Influence of irrigation with lagooned urban wastewater on chemical and microbiological soil parameters in a citrus orchard under Mediterranean condition. Sci. Total. Environ. 2002, 285, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Speir, T.W. Soil biochemical properties as indices of performance and sustainability of effluent irrigation systems in New Zealand—A review. J. Roy. Soc. N. Z. 2002, 32, 535–553. [Google Scholar] [CrossRef]
- Lv, M.C.; Cai, H.J.; Chen, X.M. Effects of sewage irrigation on physiological characteristics of potato and soil environment. J. Irrig. Drain. 2007, 26, 26–29. [Google Scholar]
- Al-Lahham, O.; el Assi, N.M.; Fayyad, M. Impact of treated wastewater irrigation on quality attributes and contamination of tomato fruit. Agric. Water Manag. 2003, 61, 51–62. [Google Scholar] [CrossRef]
- Singh, P.K.; Deshbhratar, P.B.; Ramteke, D.S. Effect of sewage wastewater irrigation on soil properties, crop yield and environment. Agric. Water Manag. 2012, 103, 100–104. [Google Scholar] [CrossRef]
- Maldonado, V.M.; Rubio Arias, H.O.; Quintana, R.; Saucedo, R.A.; Gutierrez, M.; Ortega, J.A.; Nevarez, G.V. Heavy Metal Content in Soils under Different Wastewater Irrigation Patterns in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2008, 5, 441–449. [Google Scholar]
- Tsado, E.K.; Adesina, O.A.; Oyeleke, S.B. A Survey on the Bacterial Load of Selected Fruits and Leafy Vegetables in Minna Metropolis of Niger State. Niger. J. Anim. Prod. Adv. 2013, 3, 6–11. [Google Scholar]
- Ministry of Health of P.R. China; Standardization Administration of P.R. China. Standards for Drinking Water Quality; GB 5749–2006; The National Standard of the People’s Republic of China; Ministry of Health of P.R.: Beijing, China, 2006.
- Ministry of Environmental Protection of P.R. China. Standards for Irrigation Water Quality; GB 5084–92; The National Standard of the People’s Republic of China; Ministry of Environmental Protection of P.R. China: Beijing, China, 1992.
- Morugán-Coronado, A.; García-Orenes, F.; Mataix-Solera, J.; Arcenegui, V.; Mataix-Beneyto, J. Short-term effects of treated waste water irrigation on Mediterranean calcareous soil. Soil Tillage Res. 2011, 112, 18–26. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Chemical and microbiological properties. In Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 1982; pp. 903–947. [Google Scholar]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Young, V.L. Rapid colorimetric determination of nitrate in plant tissues by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Tee, E.S.; Young, S.; Young, S.I.; Ho, S.K.; Mizura, S.S. Determination of vitamin C in fresh fruits and vegetables using the dye-titration and microfluorometric methods. Pertanika 1988, 11, 39–44. [Google Scholar]
- Kingombe, C.I.; Huys, G.; Tonolla, M.; Albert, M.J.; Swings, J.; Peduzzi, R.; Jemmi, T. PCR detection, characterization, and distribution of virulence genes in Aeromonas spp. Appl. Environ. Microbiol. 1999, 65, 5293–5302. [Google Scholar]
- Bastyns, K.; Cartuyvels, D.; Chapelle, S.; Vandamme, P.; Goossens, H.; DeWachter, R. A variable 23S rDNA region is a useful discriminating target for genus-specific and species-specific PCR amplification in Arcobacter species. Syst. Appl. Microbiol. 1995, 18, 353–356. [Google Scholar] [CrossRef]
- Priha, O.; Hallamaa, K.; Saarela, M.; Raaska, L. Detection of Bacillus cereus group bacteria from cardboard and paper with real-time PCR. J. Ind. Microbiol. Biot. 2004, 31, 161–169. [Google Scholar] [CrossRef]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Kaushik, R.; Balasubramanian, R.; de la Cruz, A.A. Influence of air quality on the composition of microbial pathogens in fresh rainwater. Appl. Environ. Microbiol. 2012, 78, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yamamoto, H.; Arima, K.; Fujii, J.; Maruta, K.; Izu, K.; Shiomori, T.; Yoshida, S. Development of a new seminested PCR method for detection of Legionella species and its application to surveillance of legionellae in hospital cooling tower water. Appl. Environ. Microbiol. 1997, 63, 2489–2494. [Google Scholar] [PubMed]
- Mendum, T.A.; Chilima, B.Z.; Hirsch, P.R. The PCR amplification of non-tuberculous mycobacterial 16S rRNA Sequences from soil. FEMS Microbiol. Lett. 2000, 185, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 7 December 2013).
- Whelan, J.A.; Russell, N.B.; Whelan, M.A. A Method for the absolute quantification of cDNA using real-time PCR. J. Immunol. Methods 2003, 278, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection of P.R. China. Environmental Quality Evaluation Standards for Farmland of Edible Agricultural Products; The National Standard of the People’s Republic of China; Ministry of Environmental Protection of P.R. China: Beijing, China, 2006.
- Song, C.-Y.; Zhang, X.-Y.; Liu, X.-B.; Gao, C.-S. Effect of soil organic matter on soil fertility and crop productivity. Syst. Sci. Comp. Stud. Agric. 2008, 24, 357–362. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soil, 13th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Ho, H.T.K.; Lipman, L.J.A.; Gaastra, W. Arcobacter, what is known and unknown about a potential foodborne zoonotic agent. Vet. Microbiol. 2006, 115, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.L.; Meng, H.C. Biological characteristics and diagnoses of pathogenic Arcobacter species. Mod. Food Sci. Technol. 2013, 29, 211–214. [Google Scholar]
- Quilliam, R.S.; Williams, A.P.; Jones, D.L. Lettuce cultivar mediates both phyllosphere and rhizosphere activity of Escherichia coli O157:H7. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Kroupitski, Y.; Golberg, D.; Belausov, E.; Pinto, R.; Swartzberg, D.; Granot, D.; Sela, S. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl. Environ. Microbial. 2009, 75, 6076–6086. [Google Scholar] [CrossRef]
- Solomon, E.B.; Yaron, S.; Matthews, K.R. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002, 68, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.; Jung, M.; Lee, E. Assessing environmental impacts of reclaimed wastewater irrigation in paddy fields using bioindicator. Irrig. Sci. 2013, 31, 1225–1236. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Kong, X.; Cui, B.; Jin, D.; Deng, Y.; Zhuang, X.; Zhuang, G.; Bai, Z. Impact of Rural Domestic Wastewater Irrigation on the Physicochemical and Microbiological Properties of Pakchoi and Soil. Water 2015, 7, 1825-1839. https://doi.org/10.3390/w7051825
Yang B, Kong X, Cui B, Jin D, Deng Y, Zhuang X, Zhuang G, Bai Z. Impact of Rural Domestic Wastewater Irrigation on the Physicochemical and Microbiological Properties of Pakchoi and Soil. Water. 2015; 7(5):1825-1839. https://doi.org/10.3390/w7051825
Chicago/Turabian StyleYang, Bo, Xiao Kong, Bingjian Cui, Decai Jin, Ye Deng, Xuliang Zhuang, Guoqiang Zhuang, and Zhihui Bai. 2015. "Impact of Rural Domestic Wastewater Irrigation on the Physicochemical and Microbiological Properties of Pakchoi and Soil" Water 7, no. 5: 1825-1839. https://doi.org/10.3390/w7051825
APA StyleYang, B., Kong, X., Cui, B., Jin, D., Deng, Y., Zhuang, X., Zhuang, G., & Bai, Z. (2015). Impact of Rural Domestic Wastewater Irrigation on the Physicochemical and Microbiological Properties of Pakchoi and Soil. Water, 7(5), 1825-1839. https://doi.org/10.3390/w7051825








