Telomerase Activity is Downregulated Early During Human Brain Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Brain Tissue
2.2. Cells and Cell Lines
2.3. Human Neural Precursor Stem Cells (hNPSCs)
2.4. TRAP Assay
2.5. Quantitative PCR
2.6. qPCR Data Processing and Analysis
2.7. Statistical Analysis
3. Results
3.1. Telomerase During Human Brain Development and in Adult Cortex Tissues
3.2. In Vitro Differentiation of Neural Precursor Stem Cells (NPSCs)
4. Discussion
NSPCs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GW | gestational week |
| h | human |
| NPSC | neural precursor stem cells |
| pcw | post conception weeks |
| ROS | reactive oxygen species |
| TA | telomerase activity |
| TR | RNA component of telomerase |
| TERT | telomerase reverse transcriptase |
| WT | wild type |
References
- Saretzki, G. Extra-telomeric functions of human telomerase: Cancer, mitochondria and oxidative stress. Curr. Pharm. Des. 2014, 20, 6386–6403. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Tichon, A.; Gazit, A.; Gitler, D.; Slavin, S.; Priel, E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol. Med. 2012, 4, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Spilsbury, A.; Miwa, S.; Attems, J.; Saretzki, G. The role of telomerase protein TERT in Alzheimer’s disease and in tau-related pathology in vitro. J. Neurosci. 2015, 35, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.; Zubov, D.; Hagen, G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT). Gene 1999, 232, 97–106. [Google Scholar] [CrossRef]
- Colgin, L.M.; Wilkinson, C.; Englezou, A.; Kilian, A.; Robinson, M.O.; Reddel, R.R. The hTERTα splice variant is a dominant negative inhibitor of telomerase activity. Neoplasia 2000, 2, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Hrdlickova, R.; Nehyba, J.; Bose, H.R., Jr. Alternatively spliced telomerase reverse transcriptase variants lacking telomerase activity stimulate cell proliferation. Mol. Cell. Biol. 2012, 32, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Shay, J.W.; Wright, W.E. Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells. Nucleic Acids Res. 2001, 29, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Giudice, L.C. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol. Hum. Reprod. 1997, 3, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Hu, J.F.; Vu, T.H.; Giudice, L.C.; Hoffman, A.R. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998, 58, 4168–4172. [Google Scholar] [PubMed]
- Liu, Y.; Wu, B.Q.; Zhong, H.H.; Tian, X.X.; Fang, W.G. Quantification of alternative splicing variants of human telomerase reverse transcriptase and correlations with telomerase activity in lung cancer. PLoS ONE 2012, 7, e38868. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Hoare, S.F.; Ashcroft, M.; Bilsland, A.E.; Keith, W.N. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene 2006, 25, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Radan, L.; Hughes, C.S.; Teichroeb, J.H.; Vieira Zamora, F.M.; Jewer, M.; Postovit, L.M.; Betts, D.H. Microenvironmental regulation of telomerase isoforms in human embryonic stem cells. Stem Cells Dev. 2014, 23, 2046–2066. [Google Scholar] [CrossRef] [PubMed]
- Kilian, A.; Bowtell, D.D.; Abud, H.E.; Hime, G.R.; Venter, D.J.; Keese, P.K.; Duncan, E.L.; Reddel, R.R.; Jefferson, R.A. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum. Mol. Genet. 1997, 6, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Krams, M.; Claviez, A.; Heidorn, K.; Krupp, G.; Parwaresch, R.; Harms, D.; Rudolph, P. Regulation of telomerase activity by alternate splicing of human telomerase reverse transcriptase mRNA in a subset of neuroblastomas. Am. J. Pathol. 2001, 159, 1925–1932. [Google Scholar] [CrossRef]
- Lincz, L.F.; Mudge, L.M.; Scorgie, F.E.; Sakoff, J.A.; Hamilton, C.S.; Seldon, M. Quantification of hTERT splice variants in melanoma by SYBR green real-time polymerase chain reaction indicates a negative regulatory role for the beta deletion variant. Neoplasia 2008, 10, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Listerman, I.; Sun, J.; Gazzaniga, F.S.; Lukas, J.L.; Blackburn, E.H. The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. J. Neurosci. 2013, 73, 2817–2828. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Drose, S.; Buchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Singhapol, C.; Pal, D.; Czapiewski, R.; Porika, M.; Nelson, G.; Saretzki, G. Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS ONE 2013, 8, e52989. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Killen, M.; Culmsee, C.; Dhar, S.; Pandita, T.K.; Mattson, M.P. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J. Mol. Neurosci. 2000, 14, 3–15. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, W.; Mattson, M.P. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J. Neurochem. 2000, 75, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Choi, Y.S.; Hong, S.B.; Kim, K.W.; Woo, R.S.; Won, S.J.; Kim, E.J.; Jeon, H.K.; Jo, S.Y.; Kim, T.K.; et al. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. J. Neurosci. 2004, 24, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Haendeler, J.; Hoffmann, J.; Brandes, R.P.; Zeiher, A.M.; Dimmeler, S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol. Cell. Biol. 2003, 23, 4598–4610. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.H.; Meyer, J.N.; Skorvaga, M.; Annab, L.A.; Van Houten, B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 2004, 3, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Reyes, A.; Green, P.; Caron, M.J.; Bonini, M.G.; Gordon, D.M.; Holt, I.J.; Santos, J.H. Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res. 2012, 40, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Klapper, W.; Shin, T.; Mattson, M.P. Differential regulation of telomerase activity and TERT expression during brain development in mice. J. Neurosci. Res. 2001, 64, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Tichon, A.; Daniel, G.; Priel, E. Telomerase expression in adult and old mouse Purkinje neurons. Rejuvenation Res. 2012, 15, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Braverman, C.; Tichon, A.; Gitler, D.; Hutchison, E.R.; Mattson, M.P.; Priel, E. Excitotoxic and radiation stress increase TERT levels in the mitochondria and cytosol of cerebellar Purkinje neurons. Cerebellum 2015. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, R.; Esumi, S.; Yagi, T.; Hirabayashi, T. Predominant expression of rTERTb, an inactive TERT variant, in the adult rat brain. Protein Pept. Lett. 2006, 13, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Mirra, S.S.; Heyman, A.; McKeel, D.; Sumi, S.M.; Crain, B.J.; Brownlee, L.M.; Vogel, F.S.; Hughes, J.P.; van Belle, G.; Berg, L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991, 41, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Madgwick, A.; Fort, P.; Hanson, P.S.; Thibault, P.; Gaudreau, M.C.; Lutfalla, G.; Moroy, T.; Abou Elela, S.; Chaudhry, B.; Elliott, D.J.; et al. Neural differentiation modulates the vertebrate brain specific splicing program. PLoS ONE 2015, 10, e0125998. [Google Scholar] [CrossRef] [PubMed]
- Nisar, R.; Hanson, P.S.; He, L.; Taylor, R.W.; Blain, P.G.; Morris, C.M. Diquat causes caspase-independent cell death in SH-SY5Y cells by production of ROS independently of mitochondria. Arch. Toxicol. 2015, 89, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Mahlakoiv, T.; Mordstein, M.; Duerr, C.U.; Michiels, T.; Stockinger, S.; Staeheli, P.; Hornef, M.W. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc. Natl. Acad. Sci. USA 2011, 108, 7944–7949. [Google Scholar] [CrossRef] [PubMed]
- Ducray, C.; Pommier, J.P.; Martins, L.; Boussin, F.D.; Sabatier, L. Telomere dynamics, end-to-end fusions and telomerase activation during the human fibroblast immortalization process. Oncogene 1999, 18, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nehorai, A.; Dougherty, J. Cell type specific analysis of human brain transcriptome data to predict alterations in cellular composition. Syst. Biomed. 2013, 1, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Martynoga, B.; Drechsel, D.; Guillemot, F. Molecular control of neurogenesis: A view from the mammalian cerebral cortex. Cold Spring Harb. Perspect. Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A.; Rizen, M.; Greider, C.W.; Hanahan, D. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat. Genet. 1996, 12, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kedde, M.; le Sage, C.; Duursma, A.; Zlotorynski, E.; van Leeuwen, B.; Nijkamp, W.; Beijersbergen, R.; Agami, R. Telomerase-independent regulation of ATR by human telomerase RNA. J. Biol. Chem. 2006, 281, 40503–40514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bilsland, A.; Jackson, K.; Keith, W.N. MDM2 negatively regulates the human telomerase RNA gene promoter. BMC Cancer 2005. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Collins, K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006, 20, 2848–2858. [Google Scholar] [CrossRef] [PubMed]
- Brault, M.E.; Lauzon, C.; Autexier, C. Dyskeratosis congenita mutations in dyskerin SUMOylation consensus sites lead to impaired telomerase RNA accumulation and telomere defects. Hum. Mol. Genet. 2013, 22, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Campbell, H.G.; Wiles, A.K.; Eccles, M.R.; Reddel, R.R.; Braithwaite, A.W.; Royds, J.A. PAX8 regulates telomerase reverse transcriptase and telomerase RNA component in glioma. Cancer Res. 2008, 68, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Alvarez-Bolado, G.; Dressler, G.; Urbanek, P.; Busslinger, M.; Gruss, P. Pax2/5 and Pax6 subdivide the early neural tube into three domains. Mech. Dev. 1999, 82, 29–39. [Google Scholar] [CrossRef]
- Burger, A.M.; Bibby, M.C.; Double, J.A. Telomerase activity in normal and malignant mammalian tissues: Feasibility of telomerase as a target for cancer chemotherapy. Br. J. Cancer 1997, 75, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiong, X.; Zhou, H.; Zhou, Q. Expression of telomerase activity, telomerase RNA component and telomerase catalytic subunit gene in lung cancer. Chin. Med. J. 2002, 115, 290–292. [Google Scholar] [PubMed]
- Zhang, H.; Wang, Y.; Zhao, Y.; Yin, Y.; Xu, Q.; Xu, Q. Immortalized human neural progenitor cells from the ventral telencephalon with the potential to differentiate into GABAergic neurons. J. Neurosci. Res. 2008, 86, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G.; Walter, T.; Atkinson, S.; Passos, J.F.; Bareth, B.; Keith, W.N.; Stewart, R.; Hoare, S.; Stojkovic, M.; Armstrong, L.; et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells 2008, 26, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Chen, S.M.; Cong, Y.S.; Nicholls, C.; Zhou, S.F.; Tao, Z.Z.; Li, H. Regulation of telomerase activity by apparently opposing elements. Ageing Res. Rev. 2010, 9, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Schepers, A.G.; Vries, R.; van den Born, M.; van de Wetering, M.; Clevers, H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011, 30, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
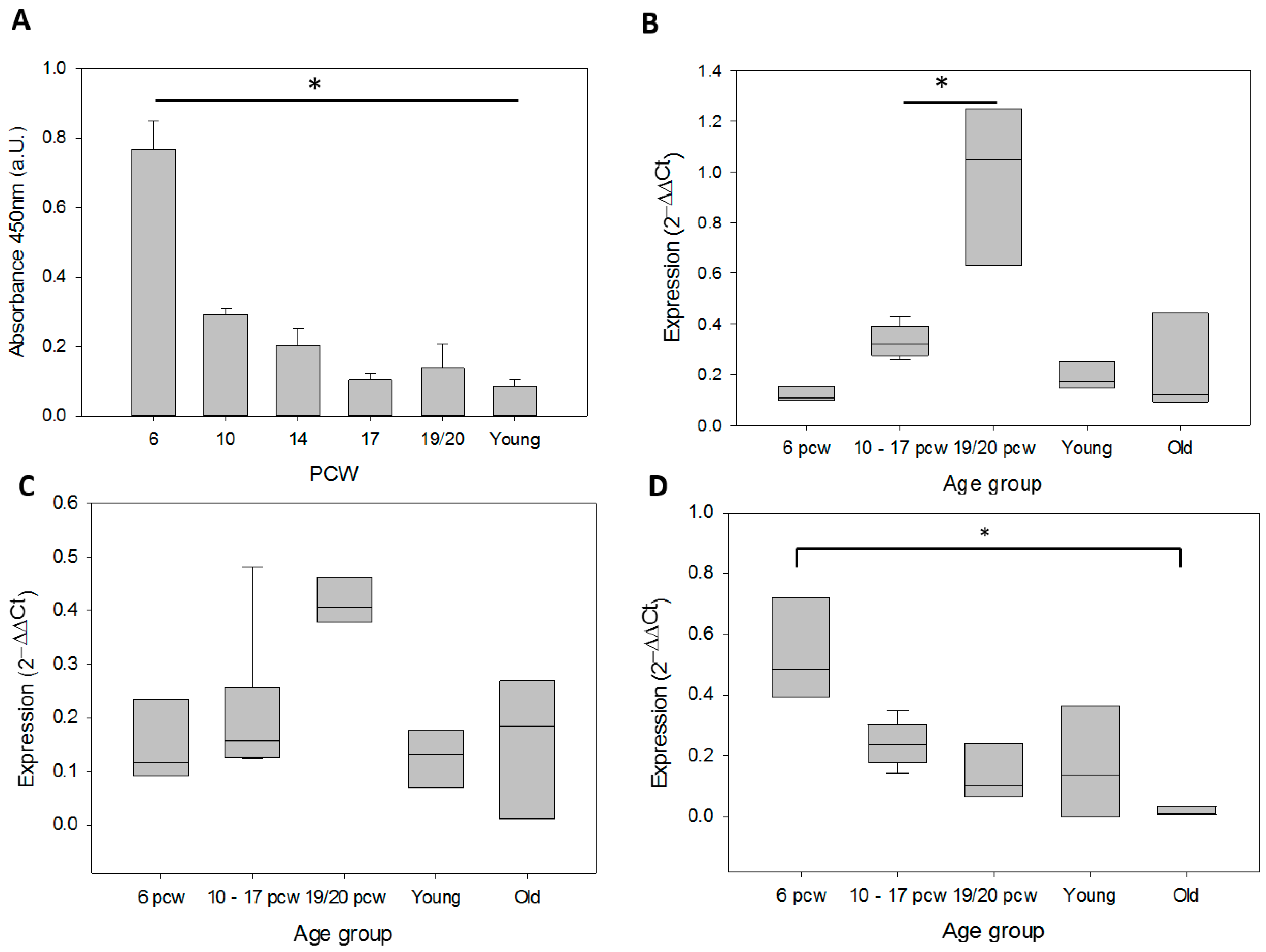
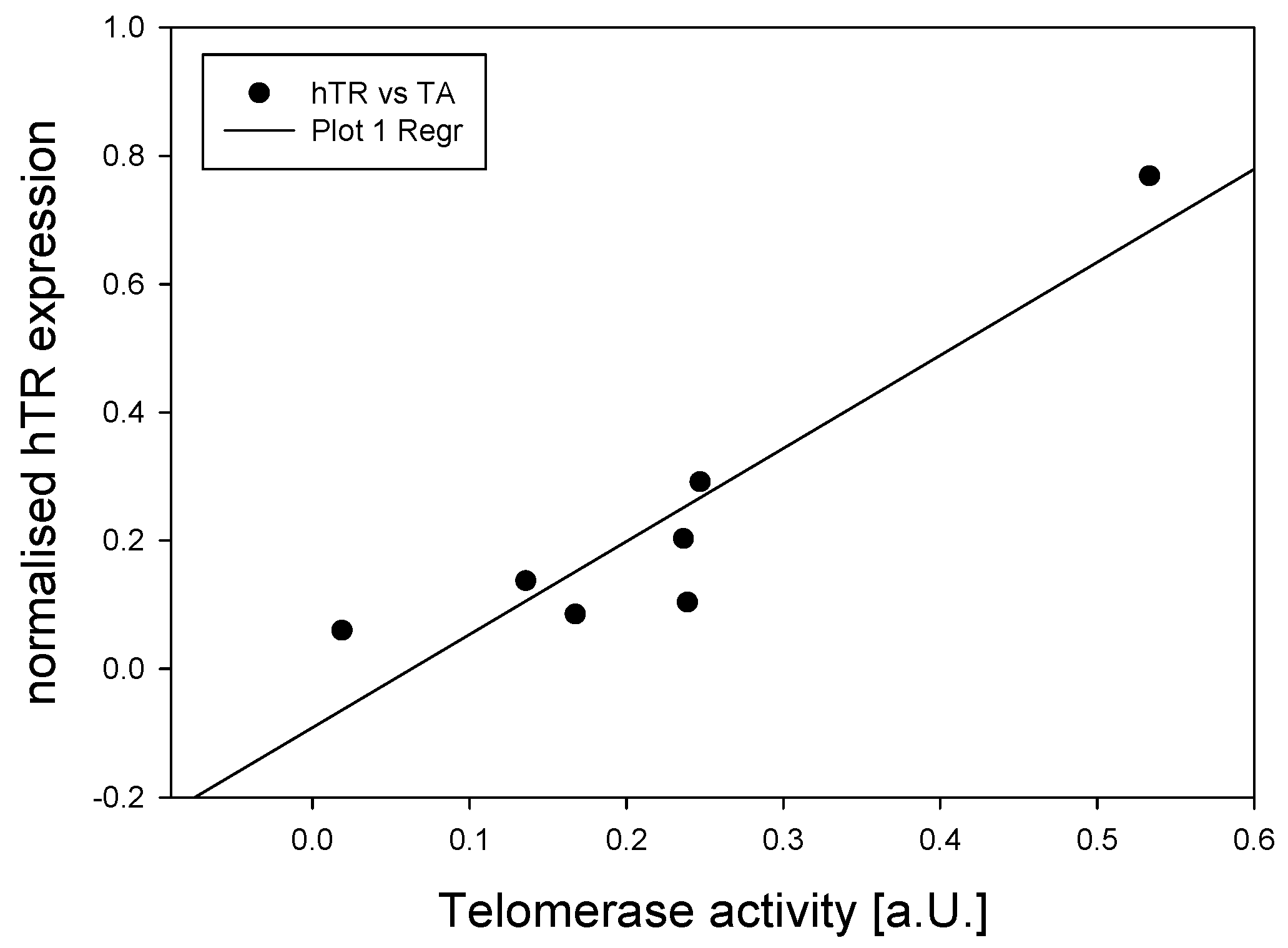

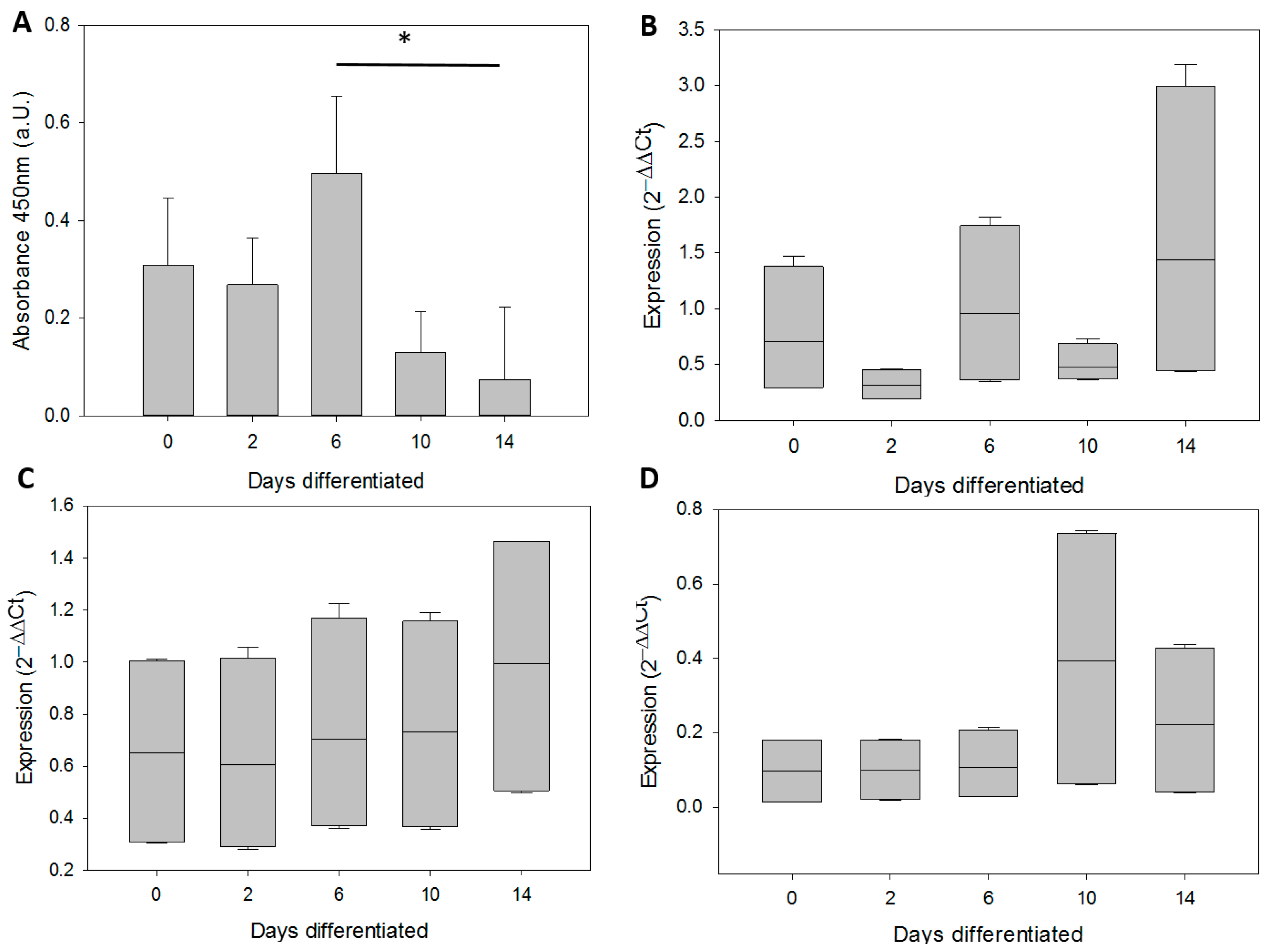
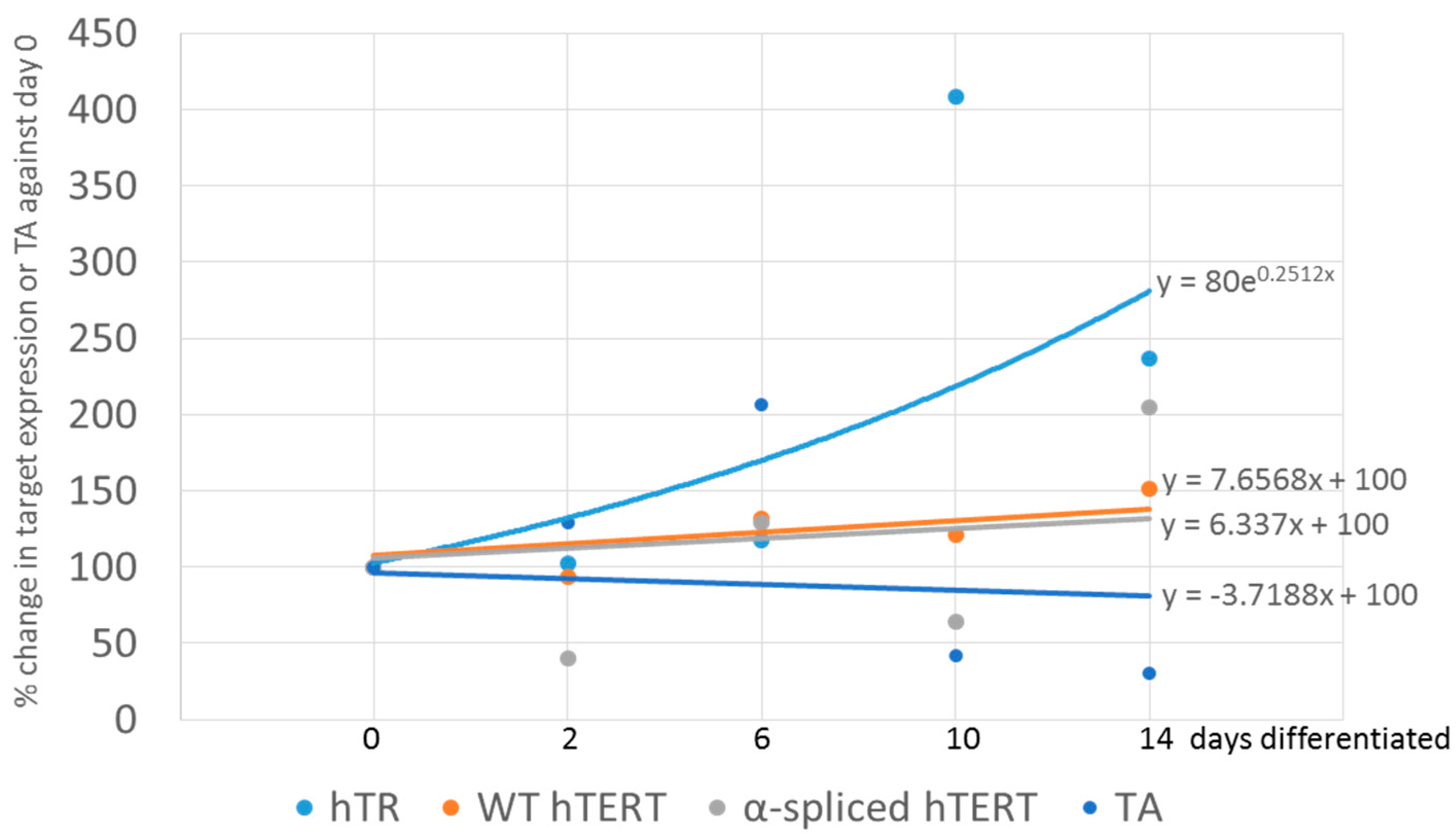
| Age | Age group | Source |
|---|---|---|
| Carnegie Stage 17 | 6 pcw | HDBR |
| Carnegie Stage 17 | 6 pcw | HDBR |
| Carnegie Stage 17 | 6 pcw | HDBR |
| 10 pcw | 10–17 pcw | HDBR |
| 10 pcw | 10–17 pcw | HDBR |
| 14 pcw | 10–17 pcw | HDBR |
| 14 pcw | 10–17 pcw | HDBR |
| 17 pcw | 10–17 pcw | HDBR |
| 17 pcw | 10–17 pcw | HDBR |
| 19 pcw | 19–20 pcw | HDBR |
| 19 pcw | 19–20 pcw | HDBR |
| 20 pcw | 19–20 pcw | HDBR |
| 15 years old | Young | NBTR |
| 20 years old | Young | NBTR |
| 20 years old | Young | NBTR |
| 68 years old | Old | NBTR |
| 73 years old | Old | NBTR |
| 78 years old | Old | NBTR |
| Primer | Sequence | Annealing Temperature (°C) | Ref. |
|---|---|---|---|
| hGAPDH fw | TGCACCACCAACTGCTTAGC | 60 | [37] |
| hGAPDH rev | GGCATGGACTGTGGTCATGA | 60 | [37] |
| hTR fw | GCCTTCCACCGTTCATTCTA | 60 | [38] |
| hTR rev | CCTGAAAGGCCTGAACCTC | 60 | [38] |
| WT hTERT fw | TGTACTTTGTCAAGGTGGATGTG | 60 | [14] |
| α-hTERT fw | CTGAGCTGTACTTTGTCAAGGAC | 53 | [15] |
| hTERT rev | GTACGGCTGGAGGTCTGTCAA | 60/53 * | [14,15] |
| Tissue | Decrease of TA (PCW) | hTR expression (PCW) | WT hTERT expression (PCW) | α-spliced hTERT expression (PCW) |
|---|---|---|---|---|
| Heart | 11 | maintained | 11 | Not detected |
| Kidney | 15 | maintained | 16 | 9 |
| Liver, Lung, Spleen | maintained | maintained | maintained | maintained |
| Brain | 17 | Downregulated from 6 towards 19/20 | 19/20 | Increased from 6 towards 19/20 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishaq, A.; Hanson, P.S.; Morris, C.M.; Saretzki, G. Telomerase Activity is Downregulated Early During Human Brain Development. Genes 2016, 7, 27. https://doi.org/10.3390/genes7060027
Ishaq A, Hanson PS, Morris CM, Saretzki G. Telomerase Activity is Downregulated Early During Human Brain Development. Genes. 2016; 7(6):27. https://doi.org/10.3390/genes7060027
Chicago/Turabian StyleIshaq, Abbas, Peter S. Hanson, Christopher M. Morris, and Gabriele Saretzki. 2016. "Telomerase Activity is Downregulated Early During Human Brain Development" Genes 7, no. 6: 27. https://doi.org/10.3390/genes7060027





