Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce
Abstract
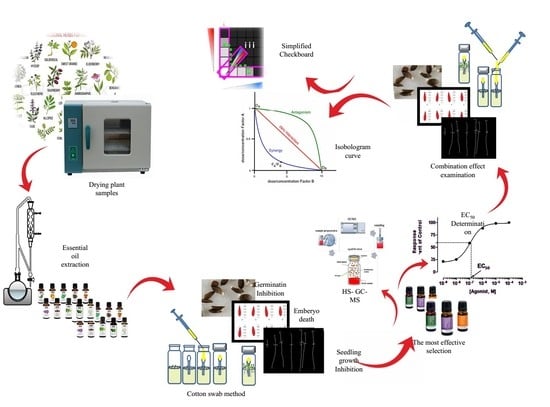
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Evaluation of Allelopathic Effect of EOs on Lettuce Seed and Germination Characteristics
2.2.1. Cotton Swab Method
2.2.2. Germination and Seed Traits
2.3. Evaluation of Allelopathic Effect of EOs on Lettuce Seedling Growth
2.4. Statistical Analysis
2.5. Headspace Gas Chromatography-Mass Spectrometry (HS-GC-MS)
2.6. Allelopathic Interaction Effects of EOs
2.6.1. Determination of IC50 and LC50
2.6.2. Essential oils Combinations
3. Results
3.1. Allelopathic Effects of Essential Oils on Lettuce Seed and Germination Characteristics
3.1.1. Germination Percentage (G%)
3.1.2. Mean Germination Time (MGT)
3.1.3. Lethal Percentage of Seed Embryo (L%)
3.1.4. Seed Dormancy Induction (D%)
3.2. Allelopathic Effects of Essential Oils on Lettuce Seedling Growth
3.2.1. Hypocotyl Growth (H%)
3.2.2. Radicle Growth (R%)
3.2.3. Vigor Index (VI)
3.3. Headspace Gas Chromatography-Mass Spectrometry (HS-GC-MS)
3.4. Determination of Effective Concentration of EOs
3.5. Allelopathic Interaction Effect of EOs
3.5.1. Simplified Modified Dilution Check-Board Technique (SMCT)
3.5.2. Isobologram Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Batish, D.R.; Arora, K.; Singh, H.P.; Kohli, R.K. Potential Utilization of Dried Powder of Tagetes minuta as a Natural Herbicide for Managing Rice Weeds. Crop Prot. 2007, 26, 566–571. [Google Scholar] [CrossRef]
- Batish, D.R.; Kaur, M.; Singh, H.P.; Kohli, R.K. Phytotoxicity of a Medicinal Plant, Anisomeles indica, against Phalaris minor and Its Potential Use as Natural Herbicide in Wheat Fields. Crop Prot. 2007, 26, 948–952. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Akcin, T.A.; Mete, E.; Akcin, A.; Aydin, T.; Kilic, H. Antifungal and Herbicidal Properties of Essential Oils and N-Hexane Extracts of Achillea gypsicola Hub-Mor. and Achillea biebersteinii Afan. (Asteraceae). Ind. Crops Prod. 2009, 29, 562–570. [Google Scholar] [CrossRef]
- Heap, I.; Peterson, M.; Horak, M. International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 16 September 2019).
- Jabran, K. Allelopathy: Introduction and Concepts; Springer: Cham, Switzerland, 2017; pp. 1–12. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Zimdahl, R.L. Fundamentals of Weed Science, 5th ed.; Academic Press: San Diego, CA, USA, 2018. [Google Scholar]
- Journet, A.R.P.; Etherington, J.R. Plant Physiological Ecology. Bryologist 2006, 82, 508. [Google Scholar] [CrossRef]
- Azizi, M.; Fujii, Y. Allelopathic Effect of Some Medicinal Plant Substances on Seed Germination of Amaranthus retroflexus and Portulaca oleraceae. Acta Hortic. 2006, 699, 61–67. [Google Scholar] [CrossRef]
- Azizi, M.; Amini, S.; Joharchi, M.R.; Oroojalian, F.; Baghestani, Z. Genetic Resources for Allelopathic and Medicinal Plants from Traditional Persian Experience. In Proceedings of the MARCO Symposium (Challenges for Agro-Environmental Research in Monsoon Asia), Tsukuba, Japan, 5–7 October 2009. [Google Scholar]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of Allelopathic Potential in Some Medicinal and Wild Plant Species of Iran by Dish Pack Method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Inderijt. Soil Microorganisms: An Important Determinant of Allelopathic Activity. Plant Soil 2005, 274, 227–236. [Google Scholar] [CrossRef]
- Azizi, M.; Farzad, S.; Jafarpour, B.; Rastegar, M.F.; Jahanbakhsh, V. Inhibitory Effect of Some Medicinal Plants’ Essential Oils on Postharvest Fungal Disease of Citrus Fruits. Acta Hortic. 2008, 768, 279–286. [Google Scholar] [CrossRef]
- Oroojalian, F.; Kasra-Kermanshahi, R.; Azizi, M.; Bassami, M.R. Phytochemical Composition of the Essential Oils from Three Apiaceae Species and Their Antibacterial Effects on Food-Borne Pathogens. Food Chem. 2010, 120, 765–770. [Google Scholar] [CrossRef]
- Mardani, H.; Maninang, J.; Appiah, K.S.; Oikawa, Y.; Azizi, M.; Fujii, Y. Evaluation of Biological Response of Lettuce (Lactuca sativa l.) and Weeds to Safranal Allelochemical of Saffron (Crocus sativus) by Using Static Exposure Method. Molecules 2019, 24, 1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oroojalian, F.; Orafaee, H.; Azizi, M. Synergistic Antibaterial Activity of Medicinal Plants Essential Oils with Biogenic Silver Nanoparticles. Nanomed. J. 2017, 4, 237–244. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic Antifungal, Allelopatic and Anti-Proliferative Potential of Salvia officinalis L., and Thymus vulgaris L. Essential Oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foy, C.L. Understanding the Role of Allelopathy in Weed Interference and Declining Plant Diversity 1. Weed Technol. 2001, 15, 873–878. [Google Scholar] [CrossRef]
- Council of Europe. European Directorate for the Quality of Medicines & HealthCare. In European Pharmacopoeia, 6th ed.; Council of Europe (COE): Strasbourg, France, 2007. [Google Scholar]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of Octanal as Plant Growth Inhibitory Volatile Compound Released from Heracleum sosnowskyi Fruit. NPC Nat. Prod. Commun. 2015, 10, 771–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetouh, M.I.; Hassan, F.A. Seed Germination Criteria and Seedling Characteristics of Magnolia grandiflora L. Trees after Cold Stratification Treatments. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 235–241. [Google Scholar]
- Leist, N.; Krämer, S.; Jonitz, A. ISTA Working Sheets on Tetrazolium Testing; The International Seed Testing Association (ISTA): Basserdorf, Switzerland, 2003. [Google Scholar]
- Dudai, N.; Larkov, O.; Putievsky, E.; Lerner, H.R.; Ravid, U.; Lewinsohn, E.; Mayer, A.M. Biotransformation of Constituents of Essential Oils by Germinating Wheat Seed. Phytochemistry 2000, 55, 375–382. [Google Scholar] [CrossRef]
- Sihoglu Tepe, A.; Tepe, B. Traditional Use, Biological Activity Potential and Toxicity of Pimpinella Species. Ind. Crops Prod. 2015, 69, 153–166. [Google Scholar] [CrossRef]
- Khalil, N.; Ashour, M.; Fikry, S.; Singab, A.N.; Salama, O. Chemical Composition and Antimicrobial Activity of the Essential Oils of Selected Apiaceous Fruits. Future J. Pharm. Sci. 2018, 4, 88–92. [Google Scholar] [CrossRef]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; Mcclements, D.J. Superior Antibacterial Activity of Nanoemulsion of Thymus daenensis Essential Oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Raina, A.P.; Dureja, P. Evaluation of the Antifungal and Phytotoxic Effects of Various Essential Oils against Sclerotium rolfsii (Sacc) and Rhizoctonia bataticola (Taub). Arch. Phytopathol. Plant Prot. 2009, 42, 65–72. [Google Scholar] [CrossRef]
- Kashkooli, A.B.; Saharkhiz, M.J. Essential Oil Compositions and Natural Herbicide Activity of Four Denaei Thyme (Thymus daenensis Celak.) Ecotypes. J. Essent. Oil-Bear. Plants 2014, 17, 859–874. [Google Scholar] [CrossRef]
- Azirak, S.; Karaman, S. Allelopathic Effect of Some Essential Oils and Components on Germination of Weed Species. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, Repellent and Oviposition-Deterrent Activity of Selected Essential Oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute Larvicidal Toxicity of Five Essential Oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the Filariasis Vector Culex quinquefasciatus: Synergistic and Antagonistic Effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Park, I.K.; Choi, K.S.; Kim, D.H.; Choi, I.H.; Kim, L.S.; Bak, W.C.; Choi, J.W.; Shin, S.C. Fumigant Activity of Plant Essential Oils and Components from Horseradish (Armoracia rusticana), Anise (Pimpinella anisum) and Garlic (Allium sativum) Oils against Lycoriella ingenua (Diptera: Sciaridae). Pest Manag. Sci. 2006, 62, 723–728. [Google Scholar] [CrossRef]
- Tu, X.F.; Hu, F.; Thakur, K.; Li, X.L.; Zhang, Y.S.; Wei, Z.J. Comparison of Antibacterial Effects and Fumigant Toxicity of Essential Oils Extracted from Different Plants. Ind. Crops Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Gatsis, T.D.; Panou-Pholotheou, E.; Eleftherohorinos, I.G. Effects of aromatic plants incorporated as green manure on weed and maize development. Field Crops Res. 2009, 110, 235–241. [Google Scholar] [CrossRef]
- Mander, L.; Liu, H. Comprehensive Natural Products II: Chemistry and Biology Development Modification of Bioactivity; Elsevier: Oxford, UK, 2010; Volume 1. [Google Scholar]
- Ibáñez, M.; Blázquez, M. Phytotoxicity of Essential Oils on Selected Weeds: Potential Hazard on Food Crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Wogiatzi, E.; Gougoulias, N.; Papachatzis, A.; Vagelas, I.; Chouliaras, N. Greek Oregano Essential Oils Production, Phytotoxicity and Antifungal Activity. Biotechnol. Biotechnol. Equip. 2009, 23, 1150–1152. [Google Scholar] [CrossRef] [Green Version]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, Phytotoxic and Insecticidal Properties of Essential Oil Isolated from Turkish Origanum acutidens and Its Three Components, Carvacrol, Thymol and p-Cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic Activity of Origanum vulgare L. on Hepatocellular carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saharkhiz, M.J.; Smaeili, S.; Merikhi, M. Essential Oil Analysis and Phytotoxic Activity of Two Ecotypes of Zataria multiflora Boiss. Growing in Iran. Nat. Prod. Res. 2010, 24, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Moradinezhad, F. Evaluation of Allelopathic Activity of 68 Medicinal and Wild Plant Species of Iran by Sandwich Method. Int. J. Hortic. Sci. Technol. 2016, 3, 243–253. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Comparison of Antifungal Activities of Various Essential Oils on the Phytophthora drechsleri, the Causal Agent of Fruit Decay. Iran. J. Microbiol. 2015, 7, 31–37. [Google Scholar]
- Vivanco, J.M.; Paschke, M.W.; Callaway, R. Allelochemical Control of Non-Indigenous Invasive Plant Species Affecting Military Testing and Training Activities, Final Report, U.S. Department of Defense Strategic Environmental Research and Development Program (SERDP) Project RC-1388. 2010. [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [Green Version]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxic Effects of β-Pinene on Early Growth and Associated Biochemical Changes in Rice. Acta Physiol. Plant. 2011, 33, 2369–2376. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Kainulainen, P.; Aflatuni, A.; Tiilikkala, K.; Holopainen, J.K. Insecticidal, Repellent, Antimicrobial Activity and Phytotoxicity of Essential Oils: With Special Reference to Limonene and Its Suitability for Control of Insect Pests. Agric. Food Sci. Finland 2001, 10, 243–259. [Google Scholar] [CrossRef]
- Sfara, V.; Zerba, E.N.; Alzogaray, R.A. Fumigant Insecticidal Activity and Repellent Effect of Five Essential Oils and Seven Monoterpenes on First-Instar Nymphs of Rhodnius prolixus. J. Med. Entomol. 2009, 46, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Klocke, J.A.; Darlington, M.V.; Balandrin, M.F. 1,8-Cineole (Eucalyptol), a Mosquito Feeding and Ovipositional Repellent from Volatile Oil of Hemizonia fitchii (Asteraceae). J. Chem. Ecol. 1987, 13, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Natsume, M. Allelochemicals for Plant–Plant and Plant–Microbe Interactions; Elsevier Inc.: Philadelphia, PA, USA, 2010. [Google Scholar] [CrossRef]
- Alonso-Amelot, M.E. Multitargeted Bioactive Materials of Plants in the Curcuma Genus and Related Compounds: Recent Advances. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 47, pp. 111–200. [Google Scholar] [CrossRef]
- Fischer, N.H.; Williamson, G.B.; Tanrisever, N.; de la Pena, A.; Weidenhamer, J.D.; Jordan, E.D.; Richardson, D.R. Allelopathic Actions in the Florida Scrub Community. Biol. Plant. 1989, 31, 471–478. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Dang Vu, K.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for Synergistic Activity of Plant-Derived Essential Oils against Fungal Pathogens of Food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| No. | Plant Scientific Name | Plant Family | Part of Use a | G% b | MGT (Day) b | D% b | L% b | H% b | R% b | VI% b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 μL | 3 μL | 1 μL | 3 μL | 1 μL | 3 μL | 1 μL | 3 μL | 1 μL | 3 μL | 1 μL | 3 μL | 1 μL | 3 μL | ||||
| 1 | Abies alba | Pinaceae | L | 100 | 100 | 3.3 | 2.5 | 0 | 0 | 0 | 0 | 27.8 | 43.2 | 42.7 | 63.9 | 36.9 | 56 |
| 2 | Achillea filipendulina | Asteraceae | Ap | 60.7 | 96.5 | 4.3 *** | 2.5 | 0 | 0 | 3.6 | 0 | 10.8 ** | 25.8 ** | 5.7 ** | 16.6 *** | 4.7 ** | 19.4 ** |
| 3 | Achillea wilhelmsii | Asteraceae | Ap | 60.7 | 93 | 4.8 **** | 3.7 ** | 0 | 0 | 3.6 | 0 | 12.1 ** | 21.2 ** | 4.8 ** | 18.2 ** | 4.6 ** | 18.0 ** |
| 4 | Allium hirtifolium | Liliaceae | C | 0.0 **** | 3.5 ***** | 5 | 0 | 0 | 100 ***** | 96.4 ***** | 6.9 ** | 10.4 *** | 1.2 ** | 5.2 *** | 0.0 ** | 0.3 *** | |
| 5 | Amomum subulatum | Zingiberaceae | Fr | 0.0 **** | 28.5 **** | 4.8 ***** | 3.6 | 0 | 96.4 ***** | 0 | 0.8 *** | 12.2 *** | 1.0 ** | 10.8 *** | 0.0 ** | 3.2 *** | |
| 6 | Anethum graveolens | Apiaceae | Fr | 21.4 *** | 92.8 | 4.7 **** | 3.8 ** | 57.1 ***** | 0 | 0 | 0 | 4.4 *** | 14.6 *** | 0.5 ** | 18.0 ** | 0.4 ** | 15.5 ** |
| 7 | Apium graveolens | Apiaceae | Fr | 100 | 89.3 | 2.2 | 2.3 | 0 | 0 | 0 | 3.6 | 22.2 | 29.2 * | 59.9 | 61.2 | 45.4 | 43.7 |
| 8 | Artemisia absinthium | Asteraceae | L | 96.4 | 100 | 3.2 | 2.5 | 0 | 0 | 0 | 0 | 28.7 | 42.2 | 44.9 | 49.9 | 37.3 | 46.9 |
| 9 | Artemisia aucheri | Asteraceae | Ap | 82.1 | 100 | 4.7 **** | 3.8 ** | 0 | 0 | 0 | 0 | 6.9 ** | 23.0 ** | 0.0 *** | 13.8 *** | 2.2 ** | 17.3 ** |
| 10 | Artemisia deserti | Asteraceae | Ap | 89.3 | 100 | 3.9 ** | 3.3 * | 0 | 0 | 0 | 0 | 7.1 ** | 21.0 ** | 0.0 *** | 16.7 ** | 2.4 ** | 18.4 ** |
| 11 | Artemisia dracunculus | Asteraceae | L | 100 | 100 | 2.5 | 2.1 | 0 | 0 | 0 | 0 | 20.8 | 39.9 | 17.5 * | 43.6 | 18.8 | 42.2 |
| 12 | Artemisia ludoviciana | Asteraceae | L | 10.7 *** | 96.5 | 4.8 | 3.9 *** | 7.2 | 0 | 7.2 | 0 | 4.7 *** | 14.1 *** | 1.3 ** | 3.7 *** | 0.3 ** | 7.4 *** |
| 13 | Artemisia scoparia | Asteraceae | L | 25.0 ** | 82.3 | 4.8 **** | 4.5 **** | 3.6 | 3.6 | 0 | 7.2 | 10.8 | 19.9 ** | 2.3 ** | 16.9 ** | 1.4 ** | 14.9 ** |
| 14 | Artemisia turanies | Asteraceae | Ap | 82.1 | 100 | 4.3 *** | 3.6 ** | 0 | 0 | 3.6 | 0 | 19.1 * | 35.8 | 20.2 | 48.6 | 16.2 | 43.7 |
| 15 | Artemisia vulgaris | Asteraceae | L | 85.7 | 100 | 3.4 | 2.6 | 0 | 0 | 3.6 | 0 | 24.7 | 45.1 | 14.8 * | 33.8 * | 15.9 | 38.1 |
| 16 | Artemisia vulgaris | Asteraceae | F | 89.5 | 100 | 4.5 *** | 3.9 *** | 0 | 0 | 7.2 | 0 | 12.2 ** | 18.4 ** | 5.4 ** | 7.3 *** | 7.2 ** | 11.6 *** |
| 17 | Artemisia sieberi | Asteraceae | L,S | 0.0 **** | 50.0 *** | 4.7 **** | 7.2 | 0 | 17.9 | 7.2 | 11.3 ** | 20.7 ** | 0.6 ** | 7.6 *** | 0.0 ** | 6.3 *** | |
| 18 | Ballota nigra | Lamiaceae | L,F | 100 | 92.8 | 2.8 | 3 | 0 | 0 | 0 | 0 | 83.2 | 93.5 | 78.9 | 90.1 | 80.6 | 84.9 |
| 19 | Bunium persicum | Apiaceae | Fr | 0.0 **** | 25.3 **** | 4.5 **** | 0 | 0 | 89.3 ***** | 10.7 | 11.2 ** | 30.6 * | 4.3 ** | 33.1 * | 0.0 ** | 8.0 *** | |
| 20 | Cedrus atlantica | Pinaceae | L | 96.4 | 96.5 | 3.1 | 2.7 | 3.6 | 0 | 0 | 0 | 40.8 | 44.3 | 63.9 | 67.6 | 53.1 | 56.5 |
| 21 | Chamaecyparis lawsoniana | Cupressaceae | L | 57.1 | 93 | 4.8 **** | 3.7 ** | 0 | 0 | 3.6 | 0 | 20.9 | 42.8 | 27.4 | 37.5 | 14.2* | 36.7 |
| 22 | Chamaecyparis lawsoniana | Cupressaceae | L | 96.4 | 100 | 2 | 2 | 0 | 0 | 0 | 0 | 23.5 | 33.8 | 30.7 | 51.2 | 27 | 44.5 |
| 23 | Chamaecyparis sp. | Cupressaceae | L | 96.4 | 96.5 | 3 | 2.8 | 0 | 0 | 0 | 0 | 18.6 * | 34 | 9.3 ** | 24.3 ** | 12.4 * | 27.0 * |
| 24 | Chenopodium botrys | Chenopodiaceae | L | 100 | 93 | 3.1 | 3.1 | 0 | 0 | 0 | 3.6 | 46 | 50 | 66.1 | 66.7 | 58.4 | 56 |
| 25 | Chrysanthemum morifolium | Asteraceae | L | 92.9 | 96.5 | 3.8 ** | 3.3 * | 3.6 | 0 | 0 | 0 | 18.4 * | 35.3 | 4.2 ** | 33.3 * | 9.0 ** | 32.8 |
| 26 | Citrus × limon | Rutaceae | Fp | 92.9 | 96.5 | 3.7 * | 2.4 | 0 | 3.6 | 0 | 0 | 40.2 | 67.6 | 59.7 | 90.5 | 48.4 | 78.8 |
| 27 | Citrus × paradisi | Rutaceae | Fp | 92.9 | 100 | 2.4 | 2.3 | 3.6 | 0 | 0 | 0 | 26.7 | 46.1 | 24.5 | 57.8 | 23.5 | 53.3 |
| 28 | Citrus × sinensis | Rutaceae | Fp | 100 | 96.5 | 2.7 | 2.3 | 0 | 0 | 0 | 0 | 29.4 | 71.1 | 37.4 | 85.9 | 34.3 | 77.3 |
| 29 | Citrus aurantifolia | Rutaceae | Fp | 100 | 100 | 2.5 | 2.2 | 0 | 0 | 0 | 0 | 28.8 | 64.3 | 15.6 * | 53.4 | 20.7 | 57.6 |
| 30 | Citrus aurantifolia | Rutaceae | L | 0.0 **** | 29.0 **** | 4.5 *** | 25.0 **** | 3.6 | 39.3 ** | 3.6 | 23.1 | 30.6 * | 13.3 * | 45.2 | 0.0 ** | 11.3 *** | |
| 31 | Citrus aurantium | Rutaceae | Fp | 100 | 100 | 2.2 | 2 | 0 | 0 | 0 | 0 | 54.6 | 70.9 | 72.6 | 74.3 | 65.7 | 73 |
| 32 | Citrus japonica | Rutaceae | Fp | 92.9 | 96.5 | 2.2 | 2.3 | 3.6 | 3.6 | 0 | 0 | 32.8 | 56.2 | 54.1 | 87 | 42.6 | 72.5 |
| 33 | Citrus medica | Rutaceae | Fp | 92.9 | 96.5 | 3 | 2.6 | 7.2 | 3.6 | 0 | 0 | 45.2 | 58.5 | 77.5 | 76.8 | 60.4 | 67.2 |
| 34 | Citrus reticulata | Rutaceae | Fp | 100 | 93 | 2.4 | 2.3 | 0 | 7.2 * | 0 | 0 | 35.3 | 60.6 | 35.5 | 61.2 | 35.4 | 56.6 |
| 35 | Citrus × tangelo | Rutaceae | Fp | 100 | 100 | 2.1 | 2.1 | 0 | 0 | 0 | 0 | 48.6 | 71.4 | 57.5 | 85 | 54.1 | 79.7 |
| 36 | Citrus × latifolia | Rutaceae | Fp | 89.3 | 100 | 3.3 | 2.8 | 0 | 0 | 0 | 0 | 15.6 * | 28.2 * | 14.3 * | 34.1 * | 13.2 * | 31.8 |
| 37 | Coffea arabica | Rubiaceae | Fr | 89.3 | 100 | 2.5 | 2.1 | 0 | 0 | 10.7 | 0 | 85.4 | 94.9 | 94.2 | 106.9 | 81.1 | 102.3 |
| 38 | Coriandrum sativum | Apiaceae | Fr | 50.0 * | 93 | 4.2 ** | 3.1 | 0 | 0 | 0 | 0 | 6.0 ** | 18.8 ** | 1.9 ** | 24.9 ** | 1.7 ** | 20.9 ** |
| 39 | Cuminum cyminum | Apiaceae | Fr | 3.6 **** | 86 | 5 | 3.8 ** | 3.6 | 0 | 14.3 | 3.6 | 11.6 ** | 23.2 ** | 6.0 ** | 21.2 ** | 0.3 ** | 18.9 ** |
| 40 | Cupressus arizonica | Cupressaceae | L | 89.3 | 96.5 | 4.3 *** | 3.7 ** | 0 | 3.6 | 3.6 | 0 | 21.8 | 39.6 | 19.7 * | 47.5 | 18.3 | 42.8 |
| 41 | Cupressus arizonica | Cupressaceae | Fr | 67.9 | 100 | 3.4 | 3.1 | 0 | 0 | 0 | 0 | 37.5 | 47 | 74.7 | 86.1 | 41 | 71.1 |
| 42 | Cupressus sempervirens | Cupressaceae | L | 100 | 89.3 | 3.6 * | 2.7 | 0 | 0 | 0 | 7.2 | 38.2 | 51.1 | 73.2 | 91.8 | 59.7 | 68 |
| 43 | Curcuma longa | Zingiberaceae | Rh | 60.7 | 86 | 3.8 ** | 3.8 ** | 3.6 | 0 | 0 | 0 | 47.1 | 49.2 | 91.8 | 99.5 | 45.3 | 68.7 |
| 44 | Datura stramonium | Solanaceae | L | 100 | 96.5 | 3 | 3.3 * | 0 | 0 | 0 | 0 | 73.1 | 72.7 | 65.4 | 76.6 | 68.3 | 72.4 |
| 45 | Daucus carota | Apiaceae | Fr | 92.9 | 96.5 | 2.9 | 2.6 | 0 | 0 | 3.6 | 0 | 19.6 | 27.1 * | 12.8 ** | 28.7 ** | 14.3 * | 27.1 * |
| 46 | Dracocephalum moldavica | Lamiaceae | Ap | 0.0 **** | 39.5 *** | 4.7 ***** | 0 | 0 | 92.9 **** | 7.2 | 4.8 *** | 24.2 ** | 0.7 ** | 23.8 ** | 0.0 ** | 9.4 *** | |
| 47 | Durema ammonicum | Apiaceae | O | 92.9 | 100 | 2.4 | 2.4 | 0 | 0 | 0 | 0 | 37 | 49.6 | 41.5 | 71.8 | 36.9 | 63.3 |
| 48 | Elettaria cardamomum | Zingiberaceae | Fr | 100 | 96.5 | 3.3 | 2.4 | 0 | 3.6 | 0 | 0 | 12.9 ** | 27.1 * | 11.0 ** | 38.2 | 11.8 * | 32.7 |
| 49 | Eucalptus globulus | Myrtaceae | L3 | 100 | 89.3 | 3.4 | 2.6 | 0 | 0 | 0 | 7.2 | 20.6 | 32.2 | 24.2 | 46.1 | 22.8 | 36.4 |
| 50 | Ferula alliacea | Apiaceae | Ap | 96.4 | 100 | 3.1 | 2.6 | 0 | 0 | 3.6 | 0 | 24 | 43 | 23.6 | 51.9 | 22.9 | 48.5 |
| 51 | Ferula foetida | Apiaceae | O | 0.0 **** | 93 | 3.2 | 0 | 3.6 | 100 ***** | 0 | 9.3 ** | 38.5 | 4.7 ** | 48.1 | 0.0 ** | 41.3 | |
| 52 | Ferula gumosa | Apiaceae | O | 100 | 100 | 2.6 | 2.6 | 0 | 0 | 0 | 0 | 42.2 | 76 | 54.8 | 88.3 | 50 | 83.6 |
| 53 | Ferula lutensis | Apiaceae | L | 89.3 | 100 | 2.9 | 2.3 | 0 | 0 | 3.6 | 0 | 17.8 * | 32 | 10.9 ** | 30.7 * | 12.1 * | 31.2 |
| 54 | Foeniculum vulgare | Apiaceae | Fr | 100 | 100 | 3.2 | 2.5 | 0 | 0 | 0 | 0 | 9.0 ** | 29.8 * | 1.8 ** | 32.2 * | 4.6 ** | 31.3 |
| 55 | Foeniculum vulgare | Apiaceae | L | 78.6 | 100 | 3.9 ** | 3.1 | 0 | 0 | 3.6 | 0 | 25.6 | 42.2 | 29.1 | 48.4 | 21.8 | 46.1 |
| 56 | Grindelia robusta | Asteraceae | L,F | 92.9 | 93 | 2.8 | 2.4 | 3.6 | 0 | 0 | 0 | 26.6 | 51.7 | 31 | 72.4 | 27.2 | 59.8 |
| 57 | Helianthus annuus | Asteraceae | L | 100 | 100 | 2.8 | 2.4 | 0 | 0 | 0 | 0 | 26.9 | 47.5 | 17.4 * | 63.1 | 21.1 | 57.1 |
| 58 | Helichrysum italicum | Asteraceae | Ap | 53.6 * | 96.5 | 4.1 ** | 3.6 ** | 0 | 0 | 3.6 | 0 | 19.4 | 31.1 * | 27.4 | 42.4 | 13.0 * | 36.7 |
| 59 | Heracleum persicum | Apiaceae | Fr | 25.0 ** | 100 | 3.3 | 2.4 | 14.3 ** | 0 | 3.6 | 0 | 7.5 ** | 29.6 * | 3.5 | 30.1 * | 1.3 ** | 29.9 * |
| 60 | Inula paecockianum | Asteraceae | R | 100 | 93 | 2 | 1.7 | 0 | 0 | 0 | 0 | 71.4 | 70.7 | 75.1 | 74 | 73.7 | 67.5 |
| 61 | Juniperus chinensis | Cupressaceae | L | 92.9 | 96.5 | 3.3 | 2.9 | 0 | 3.6 | 0 | 0 | 19.4 | 27.7 * | 23.4 | 39.2 | 20.3 | 33.5 |
| 62 | Juniperus excelsa | Cupressaceae | L | 92.9 | 100 | 2 | 2.2 | 7.2 | 0 | 0 | 0 | 42.9 | 61.5 | 73.8 | 91.9 | 57.5 | 80.2 |
| 63 | Juniperus horizontalis | Cupressaceae | Fr | 96.5 | 100 | 2.4 | 2.6 | 0 | 0 | 0 | 0 | 45.9 | 56.5 | 59.7 | 69.5 | 52.5 | 64.5 |
| 64 | Juniperus horizontalis | Cupressaceae | L | 96.4 | 92.8 | 2.1 | 2 | 3.6 | 3.6 | 0 | 0 | 15.6 * | 31.2 * | 22.7 | 53.3 | 19.2 | 41.6 |
| 65 | Juniperus sp. | Cupressaceae | L,F | 100 | 96.5 | 2.8 | 2.4 | 0 | 3.6 | 0 | 0 | 33.3 | 38.3 | 37 | 36.2 * | 35.6 | 35.7 |
| 66 | Lantana camara | Verbenaceae | L,F | 92.9 | 93 | 2.9 | 2.4 | 3.6 | 0 | 0 | 0 | 32.4 | 39.1 | 53.7 | 47.5 | 42.2 | 41.1 |
| 67 | Lavandula angustifolia | Lamiaceae | L | 71.4 | 93 | 3.9 ** | 3.5 ** | 0 | 0 | 3.6 | 3.6 | 15.4 * | 27.5 * | 9.3 ** | 45.6 | 8.3 ** | 35.9 |
| 68 | Lavandula angustifolia | Lamiaceae | F | 21.4 *** | 96.5 | 4.1 ** | 3.8 ** | 0 | 0 | 3.6 | 0.0 | 2.0 *** | 13.1 *** | 0.2 *** | 4.1 *** | 0.2 ** | 7.3 *** |
| 69 | Lippia citriodora | Verbenaceae | L | 60.7 | 96.5 | 4.4 *** | 3.3 * | 0 | 0 | 10.7 | 0 | 7.3 ** | 33 | 5.8 ** | 24.3 ** | 3.9 ** | 26.6 * |
| 70 | Magnolia virginiana | Magnoliaceae | L | 96.4 | 85.8 | 2.7 | 3.1 | 3.6 | 0 | 0 | 10.7 | 63.6 | 62.1 | 70.2 | 70.7 | 65.2 | 57.8 |
| 71 | Melissa officinalis | Lamiaceae | L | 0.0 **** | 3.5 ***** | 4 | 0 | 0 | 100 ***** | 21.5 ** | 15.0 * | 21.1 ** | 6.3 ** | 18.8 ** | 0.0 ** | 0.7 *** | |
| 72 | Mentha longifolia | Lamiaceae | L | 53.6 * | 96.5 | 4.3 *** | 3.6 ** | 0 | 0 | 0 | 0 | 11.8 ** | 19.0 ** | 5.2 ** | 14.3 *** | 4.1 ** | 15.5 ** |
| 73 | Mentha piperita | Lamiaceae | L,F | 32.2 ** | 60.5 ** | 4.4 *** | 4.2 *** | 17.9 *** | 3.6 | 0 | 3.6 | 9.4 ** | 26.8 ** | 4.7 ** | 35.6 * | 2.1 ** | 19.6 ** |
| 74 | Mentha pulegium | Lamiaceae | L,F | 53.6 * | 96.5 | 4.4 *** | 3.7 | 7.2 | 0 | 0 | 0 | 7.3 ** | 21.3 ** | 3.3 ** | 20.2 ** | 2.6 ** | 19.9 ** |
| 75 | Mentha suaveolens | Lamiaceae | L | 0.0 **** | 10.8 ***** | 4.5 | 0 | 14.3 *** | 92.9 ***** | 3.6 | 12.2 ** | 15.8 *** | 6.5 ** | 7.6 *** | 0.0 ** | 1.2 *** | |
| 76 | Microcephala lamellata | Asteraceae | L,F | 89.3 | 100 | 2.7 | 2.3 | 0 | 0 | 3.6 | 0 | 39.5 | 46.4 | 43.5 | 75.4 | 37.5 | 64.2 |
| 77 | Myristica fragrans | Myrtaceae | Fr | 96.4 | 100 | 3.1 | 2.5 | 0 | 0 | 0 | 0 | 25.3 | 41.8 | 38.2 | 54.5 | 32 | 49.6 |
| 78 | Nepeta binaludensis | Lamiaceae | Ap | 10.7 *** | 78.5 | 5 | 4.6 **** | 3.6 | 3.6 | 3.6 | 0 | 26.9 | 57.4 | 38.2 | 66.6 | 3.6 ** | 49.5 |
| 79 | Nepeta cataria | Lamiaceae | L,F | 14.3 *** | 68.0 * | 4.3 | 4.4 **** | 42.9 ***** | 3.6 | 14.3 | 0 | 24.8 | 55.4 | 10.9 ** | 54.7 | 2.3 ** | 37.3 |
| 80 | Origanum vulgare subsp. viridi | Lamiaceae | F | 46.4 * | 89.5 | 4.5 *** | 3.3 * | 0 | 0 | 7.2 | 3.6 | 5.6 *** | 5.0 **** | 0.1 *** | 2.3 **** | 1.0 ** | 3.0 *** |
| 81 | Origanum vulgare subsp. vulgare | Lamiaceae | L | 0.0 **** | 7.0 ***** | 5 | 0 | 0 | 100 ***** | 85.7 ***** | 4.6 *** | 10.5 *** | 0.2 *** | 8.4 *** | 0.0 ** | 0.7 *** | |
| 82 | Origanum vulgare subsp. vulgare | Lamiaceae | F | 100 | 100 | 2.2 | 2.1 | 0 | 0 | 0 | 0 | 44.1 | 45.3 | 61 | 62.1 | 54.5 | 55.7 |
| 83 | Pelargonium graveolnes | Geraniaceae | L | 3.6 **** | 7.0 ***** | 5 | 3.5 | 25.0 **** | 57.1 ***** | 71.4 **** | 0 | 14.3 ** | 19.0 ** | 26.6 | 27.8 ** | 0.8 ** | 1.7 *** |
| 84 | Pelargonium graveolnes | Geraniaceae | L,F | 0.0 **** | 0.0 ***** | 14.3 ** | 32.2 ***** | 82.1 ***** | 32.2 *** | 16.4 * | 13.7 *** | 18.4 * | 25.6 ** | 0.0 ** | 0.0 *** | ||
| 85 | Perovskia abrotanoides | Lamiaceae | F | 53.6 * | 89.3 | 4.8 **** | 3.8 ** | 3.6 | 0 | 0 | 0 | 7.8 ** | 6.7 **** | 1.0 ** | 4.3 *** | 1.9 ** | 4.7 *** |
| 86 | Perovskia abrotanoides | Lamiaceae | L | 89.3 | 100 | 4.3 *** | 3.3 | 0 | 0 | 0 | 0 | 31.8 | 35.9 | 24.9 | 43.4 | 24.6 | 40.5 |
| 87 | Petroselinum sativum | Apiaceae | Fr | 96.4 | 96.5 | 2.5 | 2.4 | 0 | 0 | 3.6 | 3.6 | 19.4 | 31.2 | 36.5 | 85 | 28.8 | 62 |
| 88 | Pimpinella anisum | Apiaceae | Fr | 0.0 **** | 0.0 ***** | 0 | 0 | 10 0 ***** | 10 0 ***** | 1.9 *** | 5.8 **** | 0.5 ** | 4.2 *** | 0.0 ** | 0.0 *** | ||
| 89 | Pinus eldarica | Pinaceae | O | 100 | 100 | 2 | 2.3 | 0 | 0 | 0 | 0 | 23.1 | 58.7 | 13.6 * | 65.7 | 17.2 | 63 |
| 90 | Pinus eldarica | Pinaceae | L | 100 | 89.3 | 3 | 3 | 0 | 7.2 * | 0 | 0 | 66.7 | 71.8 | 81.4 | 79.7 | 75.8 | 68.5 |
| 91 | Pinus eldarica | Pinaceae | L | 96.4 | 96.5 | 3.4 | 3 | 0 | 0 | 0 | 0 | 44.6 | 32.2 | 53.6 | 25.6 ** | 48.4 | 27.1 * |
| 92 | Pistacia vera | Anacardiaceae | Fp | 100 | 100 | 3 | 2.4 | 0 | 0 | 0 | 0 | 47.3 | 51 | 60.2 | 69.2 | 55.2 | 62.2 |
| 93 | Rosmarinus officinalis | Lamiaceae | L | 35.7 ** | 89.3 | 5.0 **** | 4.5 **** | 10.7 * | 0 | 0 | 0 | 8.8 ** | 28.1 * | 0.8 ** | 12.8 *** | 1.4 ** | 16.7 ** |
| 94 | Rosmarinus officinalis | Lamiaceae | F | 17.9 *** | 89.5 | 5.0 **** | 4.1 *** | 10.7 * | 0 | 0 | 0 | 19.4 | 39.2 | 11.2 ** | 45.6 | 2.6 ** | 38.5 |
| 95 | Ruta graveolens | Rutaceae | L | 3.6 **** | 14.5 ***** | 4 | 2.5 | 42.9 ***** | 10.7 ** | 39.3 ** | 10.7 | 14.0 ** | 18.7 ** | 15.2 * | 23.0 ** | 0.5 ** | 3.1 *** |
| 96 | Salvia nemorosa | Lamiaceae | Ap | 67.9 | 100 | 4.1 ** | 3.2 | 0 | 0 | 7.2 | 0 | 11.7 ** | 18.7 ** | 0.3 *** | 11.7 *** | 3.2 ** | 14.4 ** |
| 97 | Salvia officinalis | Lamiaceae | Ap | 96.4 | 100 | 4.2 ** | 3.4 * | 0 | 0 | 0 | 0 | 5.6 *** | 14.3 *** | 0.5 ** | 6.7 *** | 2.4 ** | 9.6 *** |
| 98 | Salvia syriaca | Lamiaceae | Ap | 67.9 | 78.5 | 4.5 *** | 3 | 3.6 | 0 | 3.6 | 3.6 | 26 | 40.2 | 44.6 | 65.9 | 25.4 | 44 |
| 99 | Santolina chamaecyparissus | Asteraceae | L,F | 92.9 | 100 | 3 | 2.4 | 0 | 0 | 3.6 | 0 | 24.8 | 31.2 * | 27 | 38.8 | 24.3 | 35.8 |
| 100 | Syzygium aromaticum | Myrtaceae | F | 46.4 * | 93 | 2.1 | 2.4 | 0 | 3.6 | 21.5 | 0 | 10.6 ** | 20.9 ** | 16.0 * | 29.5 ** | 6.5 ** | 24.3 * |
| 101 | Tanacetum balsamita | Asteraceae | L,F | 3.6 **** | 100 | 5 | 3.3 | 0 | 0 | 25.0 * | 0 | 3.7 *** | 11.0 *** | 0.0 *** | 10.2 *** | 0.1 ** | 10.5 *** |
| 102 | Thuja occidentalis | Cupressaceae | L | 71.4 | 92.8 | 4.7 **** | 4.0 *** | 0 | 0 | 3.6 | 3.6 | 14.3 ** | 24.7 ** | 3.1 ** | 26.2 ** | 5.3 ** | 23.8 ** |
| 103 | Thuja orientalis | Cupressaceae | L | 96.4 | 89.3 | 3.1 | 3.2 | 3.6 | 0 | 0 | 3.6 | 21.1 | 32.3 | 16.4 * | 30.5 * | 17.6 | 27.9 * |
| 104 | Thymus daenensis | Lamiaceae | L | 0.0 **** | 0.0 ***** | 0 | 0 | 100 ***** | 100 ***** | 0.8 *** | 5.8 **** | 0.0 *** | 0.5 **** | 0.0 ** | 0.0 *** | ||
| 105 | Thymus transcaspicus | Lamiaceae | L | 0.0 **** | 10.5 ***** | 5.0 ***** | 0 | 3.6 | 96.4 ***** | 57.1 ***** | 13.5 ** | 22.5 ** | 15.4 * | 26.7 ** | 0.0 ** | 2.7 *** | |
| 106 | Trachyspermum ammi | Apiaceae | Fr | 3.6 **** | 10.5 ***** | 4 | 3.7 ** | 67.8 ***** | 39.3 ***** | 14.3 | 28.6 *** | 13.5 ** | 35.8 | 13.6 * | 49.6 | 0.5 ** | 4.7 *** |
| 107 | Vitex agnus castus | Verbenaceae | Fr | 96.4 | 89.5 | 2.6 | 2.4 | 0 | 0 | 3.6 | 0 | 29.7 | 45.3 | 50.5 | 49.9 | 40.9 | 42.9 |
| 108 | Xanthium strumarium | Asteraceae | L | 64.3 | 96.5 | 4.0 ** | 3.3 * | 0 | 0 | 0 | 0 | 24.1 | 44.4 | 13.3 ** | 43.5 | 11.2 * | 42.3 |
| 109 | Zataria multiflora | Lamiaceae | L,F | 0.0 **** | 39.5 *** | 4.0 *** | 0 | 0 | 100 ***** | 53.6 ***** | 6.7 ** | 11.1 *** | 3.9 ** | 8.2 *** | 0.0 ** | 3.7 *** | |
| 110 | Zingiber officinale | Zingiberaceae | Rh | 64.3 | 100 | 3.5 | 2.8 | 7.2 | 0 | 3.6 | 0 | 19.4 | 25.7 ** | 17.2 * | 30.6 * | 11.6 * | 28.7 * |
| 111 | Ziziphora clinopodioides | Lamiaceae | L | 7.2 *** | 93 | 4.5 | 3.8 ** | 28.6 **** | 3.6 | 10.7 | 0 | 11.0 ** | 28.7 * | 8.2 ** | 40.5 | 0.7 ** | 33.4 |
| 112 | Ziziphora tenuior | Lamiaceae | L | 0.0 **** | 10.8 ***** | 3.5 | 71.4 ***** | 67. 8 ***** | 28. 6 * | 7.2 | 9.8 ** | 19.8 ** | 14.5 * | 21.9 ** | 0.0 ** | 2.3 *** | |
| Plant Scientific Name | Part of Use a | Main Components of EOs Based on HS-GC-MS | |||
|---|---|---|---|---|---|
| Amomum subulatum | Fr | Dihydrocarveol (32.1%) | β-Pinene (23.1%) | α-Pinene (18.4%) | |
| Anethum graveolens | Fr | 1,2-Diisopropenylcyclobutane (74.7%) | |||
| Artemisia ludoviciana | L | Borneol (44.2%) | Camphor (33.3%) | Eucalyptol (22.4%) | |
| Artemisia vulgaris | L,F | D-Limonene (33.7%) | Thujone (22.5%) | β-Pinene (14.9%) | |
| Citrus aurantifolia | L | β-Pinene (20.8%) | Linalyl anthranilate (20.8%) | Linalol (16.2%) | Limonene (11.2%) |
| Dracocephalum moldavica | Ap | Orthodene (32.6%) | Limonene (20.3%) | α-Pinene (17.4%) | 4-Carene (11.3%) |
| Mentha suaveolens | L | Limonene (47.9%) | α-Pinene (16.7%) | β-Pinene (14.0%) | |
| Origanum vulgare subsp. vulgare | L | Carvacrol (44.4%) | Thymol (23.0%) | o-Cymene (18.8%) | |
| Perovskia abrotanoides | F | Borneol (30.1%) | Eucalyptol (21.9%) | Camphor (11.1%) | |
| Pimpinella anisum | Fr | Trans- anethole (93.3%) | |||
| Ruta graveolens | L | β-Terpinyl acetate (40.8%) | β-Pinene (16.5%) | α-Pinene (10.7%) | |
| Tanacetum balsamita | L,F | Limonene (40.2%) | Thujone (21.0%) | ||
| Thymus daenensis | L | Carvacrol (90.9%) | |||
| Thymus transcaspicus | L | Camphene (23.0%) | α-Pinene (11.0%) | ||
| Zataria multiflora | L,F | o-Cymene (22.7%) | α-Pinene (22.1%) | Thymol (20.4%) | |
| Ziziphora tenuior | L | β-Pinene (24.2%) | α-Pinene (21.4%) | Limonene (20.6%) | |
| Plant Scientific Name | Part of Use a | Seed Germination Inhibition | Embryo Lethal Effect | ||||
|---|---|---|---|---|---|---|---|
| IC25 | IC50 | IC90 | LC25 | LC50 | LC90 | ||
| ppm | |||||||
| Amomum subulatum | Fr | 33.1 | 50.0 | 114 | 115 | 137 | 196 |
| Citrus aurantifolia | L | 9.0 | 20.9 | 114 | 23.2 | 64.9 | 510 |
| Dracocephalum moldavica | Ap | 13.3 | 21.0 | 52.3 | 69.4 | 126 | 414 |
| Ferula foetida | O | 88.6 | 115 | 193.0 | 119 | 138 | 183 |
| Melissa officinalis | L | 36.3 | 48.0 | 83.8 | 64.9 | 102 | 248 |
| Mentha suaveolens | L | 22.3 | 32.0 | 66.0 | 57.6 | 93.2 | 244 |
| Origanum vulgare subsp. vulgare | L | 8.0 | 11.4 | 23.4 | 12.2 | 16.2 | 28.5 |
| Pelargonium graveolnes | L | 15.8 | 26.3 | 73.1 | 34.8 | 64.9 | 226 |
| Pelargonium graveolnes | L,F | 20.8 | 30.0 | 63.0 | 66.7 | 116 | 351 |
| Pimpinella anisum | Fr | 7.0 | 7.5 | 8.5 | 9.4 | 22.9 | 136 |
| Ruta graveolens | L | 31.8 | 45.6 | 93.7 | 116 | 340 | 2916 |
| Thymus daenensis | L | 1.8 | 2.9 | 8.0 | 3.8 | 7.2 | 25.3 |
| Thymus transcaspicus | L | 12.9 | 17.3 | 30.8 | 18.3 | 23.1 | 36.7 |
| Trachyspermum ammi | Fr | 18.0 | 25.0 | 48.3 | 52.6 | 153 | 1294 |
| Zataria multiflora | L,F | 5.1 | 7.9 | 18.4 | 6.9 | 21.6 | 213 |
| Ziziphora tenuior | L | 8.6 | 13.7 | 35.4 | 30.3 | 49.2 | 130 |
| Plant Scientific Name | Part of Use a | Hypocotyl Inhibition | Radicle Inhibition | Seedling Inhibition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC25 | IC50 | IC90 | IC25 | IC50 | IC90 | IC25 | IC50 | IC90 | ||
| ppm | ||||||||||
| Allium hirtifolium | C | 3.4 | 18.1 | 512 | 34.9 | 62.2 | 197 | 18.9 | 46.2 | 278 |
| Amomum subulatum | Fr | 44.6 | 69.3 | 168 | 50.8 | 70.2 | 134 | 48.8 | 70.7 | 148 |
| Anethum graveolens | Fr | 20.6 | 46.7 | 238 | 30.4 | 64.4 | 289 | 24.8 | 56.2 | 287 |
| Artemisia aucheri | L | 39.7 | 79.7 | 321 | 63.8 | 73.0 | 95.6 | 50.8 | 72.3 | 147 |
| Artemisia ludoviciana | L | 15.4 | 37.8 | 227 | 10.5 | 18.3 | 54.8 | 11.6 | 24.9 | 116 |
| Artemisia vulgaris | L, F | 15.2 | 59.1 | 899 | 33.5 | 79.4 | 448 | 24.3 | 71.3 | 612 |
| Artemisia sieberi | L,S | 11.3 | 38.4 | 446 | 10.5 | 28.4 | 209 | 10.8 | 31.6 | 270 |
| Lavandula angustifolia | F | 9.9 | 27.8 | 221 | 17.6 | 29.3 | 80.5 | 14.5 | 28.7 | 113 |
| Mentha suaveolens | L | 16.0 | 31.5 | 122 | 19.0 | 32.8 | 97.0 | 17.9 | 32.2 | 105 |
| Origanum vulgare subsp. viridi | F | 5.4 | 12.9 | 73.9 | 6.2 | 9.6 | 23.2 | 6.0 | 10.5 | 31.6 |
| Origanum vulgare subsp. vulgar | L | 10.3 | 36.3 | 450 | 18.2 | 45.0 | 276 | 15.2 | 42.1 | 322 |
| Pelargonium graveolnes | L,F | 7.4 | 30.1 | 503 | 9.8 | 54.5 | 1693 | 8.3 | 42.2 | 1093 |
| Perovskia abrotanoides | F | 8.1 | 22.8 | 182 | 13.5 | 19.1 | 38.4 | 12.8 | 20.7 | 54.8 |
| Pimpinella anisum | Fr | 16.6 | 34.3 | 147 | 14.5 | 27.8 | 103 | 18.9 | 34.1 | 111 |
| Rosmarinus officinalis | L | 49.6 | 90.4 | 300 | 63.1 | 104 | 279 | 57.9 | 98.7 | 287 |
| Salvia nemorosa | Ap | 33.8 | 67.9 | 275 | 54.6 | 74.3 | 138 | 51.3 | 74.1 | 155 |
| Salvia officinalis | Ap | 16.1 | 44.6 | 342 | 10.2 | 23.4 | 124 | 11.2 | 29.1 | 196 |
| Tanacetum balsamita | L,F | 16.7 | 44.3 | 313 | 24.6 | 56.6 | 300 | 20.8 | 51.3 | 313 |
| Thymus daenensis | L | 15.1 | 30.7 | 127 | 25.1 | 45.9 | 153 | 22.1 | 40.9 | 140 |
| Zataria multiflora | L,F | 26.0 | 50.6 | 193 | 12.7 | 33.8 | 238 | 17.3 | 40.5 | 221 |
| Germination Interaction | Amomum subulatum -(Fr) | Citrus aurantifolia -(L) | Ruta graveolens -(L) | Dracocephalum moldavica -(Ap) | Mentha suaveolens -(L) | Thymus daenensis -(L) | Thymus transcaspicus -(L) | Ziziphora tenuior -(L) | |||||
| Amomum subulatum -(Fr) a | |||||||||||||
| Citrus aurantifolia -(L) | 1.09 | ||||||||||||
| Ruta graveolens- (L) | 0.76 A* | 0.84 A | |||||||||||
| Dracocephalum moldavica -(Ap) | 0.82 A | 1.06 | 0.56 A | ||||||||||
| Mentha suaveolens -(L) | 1.25 S | 0.85 A | 1.15 S | 0.96 | |||||||||
| Thymus daenensis -(L) | 0.84 A | 0.66 A | 0.81 A | 1.11 S | 0.86 A | ||||||||
| Thymus transcaspicus -(L) | 0.82 A | 0.72 A | 0.59 A | 0.84 A | 1.09 | 0.97 | |||||||
| Ziziphora tenuior -(L) | 0.88 A | 1.14 S | 1.02 | 0.79 A | 1.19 S | 0.94 | 0.80 A | ||||||
| Hypocotyl Growth Interaction | Amomum subulatum -(Fr) | Anethum graveolens -(Fr) | Artemisia ludoviciana -(L) | Tanacetum balsamita -(L,F) | Perovskia abrotanoides -(F) | Thymus daenensis -(L) | |||||||
| Amomum subulatum -(Fr) | |||||||||||||
| Anethum graveolens -(Fr) | 0.89 A* | ||||||||||||
| Artemisia ludoviciana -(L) | 0.79 A | 1.07 | |||||||||||
| Tanacetum balsamita -(L,F) | 0.72 A | 0.78 A | 0.94 | ||||||||||
| Perovskia abrotanoides -(F) | 0.96 | 1.19 S | 1.31 S | 1.17 S | |||||||||
| Thymus daenensis -(L) | 1.00 | 1.02 | 1.27 S | 1.13 S | 1.25 S | ||||||||
| Radicle Growth Interaction | Amomum subulatum -(Fr) | Artemisia ludoviciana -(L) | Artemisia vulgaris -(L,F) | Mentha suaveolens -(L) | Perovskia abrotanoides -(F) | Thymus daenensis -(L) | |||||||
| Amomum subulatum -(Fr) | |||||||||||||
| Artemisia ludoviciana -(L) | 0.84 A* | ||||||||||||
| Artemisia vulgaris -(L,F) | 0.94 | 1.01 | |||||||||||
| Mentha suaveolens -(L) | 0.98 | 1.17 S | 1.22 S | ||||||||||
| Perovskia abrotanoides -(F) | 0.68 A | 1.10 | 0.80 A | 0.82 A | |||||||||
| Thymus daenensis -(L) | 0.97 | 1.00 | 1.13 S | 1.16 S | 0.95 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. https://doi.org/10.3390/agronomy10020163
Mirmostafaee S, Azizi M, Fujii Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy. 2020; 10(2):163. https://doi.org/10.3390/agronomy10020163
Chicago/Turabian StyleMirmostafaee, Somayeh, Majid Azizi, and Yoshiharu Fujii. 2020. "Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce" Agronomy 10, no. 2: 163. https://doi.org/10.3390/agronomy10020163