1. Introduction
A wide range of qualitative, semi-quantitative and quantitative testing techniques are currently available, both at the laboratory scale and for commercial purposes, to evaluate the degradation and combustion behaviours of polymeric materials. They include, but not limited to, techniques such as: thermo-gravimetric analysis (TGA), oxygen bomb calorimetry, limiting oxygen index (LOI) measurements, Underwriters Laboratory 94 (UL-94) tests, cone calorimetry, etc. All of the above mentioned methods have their own advantages as well as limitations. For example, the LOI test determines the propensity of the material to undergo ignition and flaming combustion in a pre-set atmosphere of oxygen and nitrogen, under the influence of a pilot, and thus only reflects a low level flammability attribute of the material under consideration. Furthermore, this method does not provide other valuable information, for instance, the heat release rates. UL-94 test, which is commonly used as an industry standard, can be only considered as a qualitative flammability test method leading to a classification of the materials, and the main outcome of the test will be a pass or fail criteria, even though it can somewhat gauge the propensity of the material to melt and drip. Cone calorimetry, which is widely regarded as the standard method to evaluate the overall fire performance of a material, is based on the oxygen consumption calorimetry principle, and provides valuable parameters like: time to ignition, mass loss rate, heat release rates (peak and average), total heat released, effectiveness of combustion (through CO/CO2 ratios), smoke obscuration, etc. However, for typical measurements, often carried out in triplicate and under differing heat fluxes, cone calorimetry measurements require relatively high amounts of materials. Ideally, each test specimen should be of about 10 cm × 10 cm × 6 mm in dimension, thus requiring at least 50 g of material. In addition, there are also issues with varying sample thicknesses, and associated problems of heat losses which are also related to insulation material used to support the specimens in sample holders, and the actual material of the holder and/or its configurations.
Pyrolysis Combustion Flow Calorimetry (PCFC), also known as micro-scale combustion calorimetry, was shown to be a very valuable small-scale technique for screening flammability of different materials in recent years [
1]. We have chosen PCFC for our studies as a rapid screening technique as it only requires a few milligrams of a polymer for testing, and often provides a wealth of combustion-related data. PCFC works on a principle of oxygen depletion calorimetry, relating to Hugget’s principle that 1 kg of consumed oxygen corresponds to 13.1 MJ of released energy for any organic material [
2]. At first, a polymeric sample is rapidly heated to a state of controlled pyrolysis in an inert atmosphere of nitrogen (method A: anaerobic conditions) or in a mixture of nitrogen and oxygen (method B: aerobic conditions), followed by a rapid oxidation at high temperatures (
i.e., combustion) of the pyrolysate in an excess of oxygen [
3]. In the present study, the operational conditions were in conformance with the test method A. This method also is an established ASTM standard for testing flammability characteristics of solid materials [
1]. PCFC is capable to measure the following parameters: peak to heat release rate (PHRR); temperature at peak heat release rate (
TPHRR); total heat release (THR); heat release capacity (HRC) and a percentage of the char residues. The values of HRC (
i.e., maximum amount of heat released per unit mass per degree temperature) can serve as a reliable indicator of a polymer’s flammability [
3].
3. Results and Discussion
The aqueous slurry route was found to be a quite facile method for the preparation of polymeric products from AN, which provided typical yields of homo- and co-polymers between 55 and 88 wt% (
Table 1). It was previously shown that chemical modification of PAN through the incorporation of ADEPMAE groups significantly increased its flame retardance [
10]. In this case, we have also previously noticed strong correlation between the char residues obtained in nitrogen, air and in oxygen, and the wt% of P in AN-based polymers containing P/N-moieties. In addition, corresponding increases in LOI values coupled to decreases in heats of combustion were also found for these materials (see reference [
10] for detailed data). The above factors show that even with nominal amounts of P, there were substantial enhancements in flame retardance of the modified systems. Furthermore, as is expected, above certain levels (
ca. 3 wt%) of P incorporation there were indications of a diminished returns. It was also evident from the measured PCFC parameters that this effect is most pronounced for copolymers containing 1.18–1.41 wt% of P (corresponding mole fractions of ADEPMAE in the reaction feed ranged from 0.06 to 0.10).
In the current study, we intended to achieve similar contents of modifying groups (
i.e., mole fractions of P- or P/N-containing monomeric units) and comparable P loadings. Even though we have kept the same amount of the comonomer [co-M] in the feed (0.08–0.10 mole fraction), constant temperature (40 °C) and almost the same duration of polymerisation (16 h) with the same amount of the redox pair for all reactions carried out, the yields and the compositions of resultant copolymers did vary (
Table 5). The P contents in the prepared AN-based copolymers were within 1.0–1.2 wt% range with the exception of poly(AN-
co-ADEPAE), in which the level of P reached 1.9 wt%. This can be explained by different chemical environments for the penta-valent P-atom, which is expected to influence their reactivity ratios with AN. All the final polymeric products were white, powdery solids without any signs of discoloration. This indicates the absence of unwanted side reactions, such as intramolecular cyclization of nitrile groups in AN-based polymers, which could have led to extended conjugation and light absorption.
Table 5.
PCFC parameters for AN-based polymeric systems.
Table 5.
PCFC parameters for AN-based polymeric systems.
| Parameter | PAN | Poly(AN-co-DEAMP) | Poly(AN-co-DE1AEP) | Poly(AN-co-DEAEP) | Poly(AN-co-ADEPMAE) | Poly(AN-co-ADEPAE) |
|---|
| [M]/[co-M] (mole fraction) | 1.0/0.0 | 0.982/0.018 | 0.980/0.020 | 0.978/0.022 | 0.978/0.022 | 0.963/0.037 |
| P content (wt%) | 0 | 1.0 | 1.1 | 1.2 | 1.2 | 1.9 |
| TPHRR (°C) | 289 | 429 | 432 | 426 | 426 | 410 |
| PHRR (W/g) | 183 | 112 | 122 | 114 | 85 | 106 |
| PHRR (% reduction) | 0 | 39 | 33 | 38 | 54 | 42 |
| THR (kJ/g) | 14.9 | 17.5 | 16.8 | 14.3 | 8.9 | 15.9 |
| HRC (J/g·K) | 201 | 122 | 132 | 124 | 86 | 115 |
| PCFC char yield (wt%) | 45.6 | 58.4 | 55.8 | 58.2 | 53.6 | 55.3 |
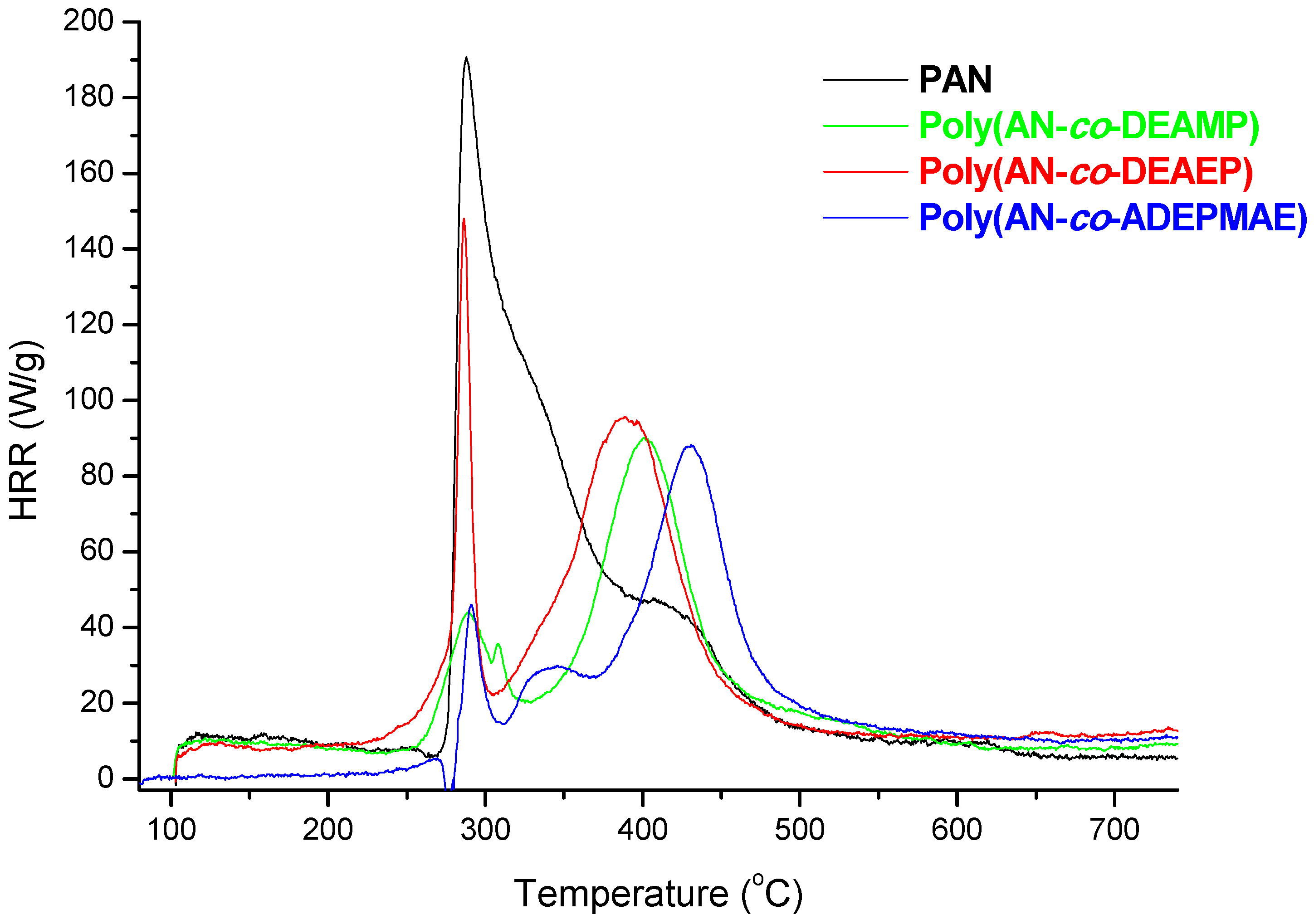
Figure 2 represents plots of HRR
versus temperature for PAN and for selected copolymers containing from 1.0 to 1.2 wt% of P as pendant groups (DEAMP, or DEAEP, or ADEPMAE). A single prominent heat release rate peak (with a maximum at 289 °C) was observed for the unmodified PAN. As it shown on
Figure 2, the incorporation even at a very nominal level of P into PAN chains as pendant groups drastically changed the general profiles of HRR curves. Indeed, two peaks were observed for copolymers: first (either small or very narrow) peak at 280–290 °C and the second one (higher and very broad peak) shifted to higher temperatures with the maximum at 390–430 °C. At the same time for the copolymers the heights of peaks as well as the areas under the curves were significantly reduced compared to PAN.
Figure 2 also shows that thermal degradation of copolymers began 20–50 °C earlier compared to PAN. The same trends were observed during TGA runs on the specimens tested in nitrogen atmosphere (data not reported here). In the case of the copolymers, values of T
PHRR for heat releases associated with earlier induction of thermal degradation (first peaks) were not reported in
Table 5.
The data summarised in
Table 5 indicates that the reactive modification of PAN with P- or P-/N-containing groups leads to the reduction of PHRR values by a factor of 1.5 to 2.2. The lowest value of PHRR was recorded for the copolymer bearing ADEPMAE groups (85 W/g). The maximum value of heat release rate divided by the heating rate of PCFC test gives a value of heat release capacity (HRC), which can be used as a reliable indicator of a polymer’s flammability [
2]. It is shown here that the presence P- or P/N-containing comonomeric units in polymeric chains lead to a decrease of HRC values, thus possibly increasing the flame retardance. The copolymer containing 1.2 wt% of P and with a synergistic element, such as nitrogen (
i.e., ADEPMAE monomeric unit), was characterised by the lowest value of HRC among all AN-based copolymers obtained in the present study. Overall, the modification of PAN with nominal amounts of P- or P-/N- containing comonomers reduced its HRC by almost 50%. The THR is the amount of heat released throughout the decomposition, in a PCFC run, and can be indicative of the total amount of fuel generated. Poly(AN-
co-ADEMPAE) had the lowest value of THR 8.9 kJ/g, which is 1.7 times lower than that of PAN (
Table 5).
Figure 2.
Plots of heat release rates versus temperature for PAN, poly(AN-co-DEAMP), poly(AN-co-DEAEP), and poly(AN-co-ADEPMAE).
Figure 2.
Plots of heat release rates versus temperature for PAN, poly(AN-co-DEAMP), poly(AN-co-DEAEP), and poly(AN-co-ADEPMAE).
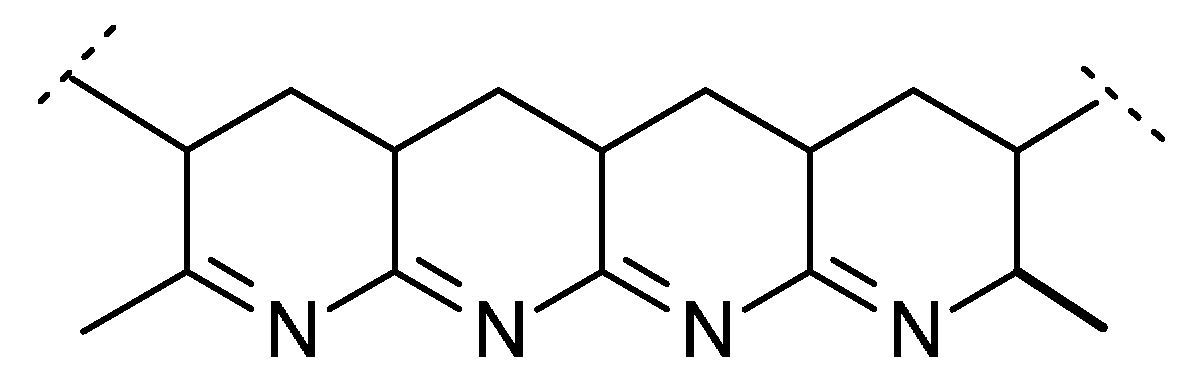
Table 5 also shows the char yields obtained in PCFC tests for PAN and for its copolymers. It should be also noted here that the percentages of residues left in TGA experiments, under nitrogen, also correlated well with the corresponding values recorded in PCFC. Even without the incorporation of phosphorus moieties, PAN produces appreciable amount of char residue (45.6 wt% in PCFC). The enhanced char production tendency of PAN can be explained by the processes of intramolecular cyclization of –C≡N side groups occurring, especially predominant, at lower heating rates [
11]. As a result, polycyclic structures are formed and nitrogen atoms become ‘locked’ onto conjugated polyene sequences leading to char precursors, as is shown in
Figure 3 below.
Figure 3.
Cyclic structures formed due to intramolecular cyclization in PAN.
Figure 3.
Cyclic structures formed due to intramolecular cyclization in PAN.
In case of AN-based copolymers the amounts of char produced were higher than for PAN by about 8–13 wt%. This is due to the fact that phosphorus acid species produced during early stages of the thermal degradation of copolymers had acted as nucleophilic centres, thus promoting intramolecular cyclization reactions [
7,
11]. It is very likely that the mechanism of flame retardation in the modified AN-based polymers involves a significant condensed phase activity initiated by the precursors formed during early thermal cracking (as evident also from the TGA thermograms) of the P- or P-/N-containing side groups [
10,
11]. The condensed phase activity of the P- or P/N-containing modifying groups in PAN chains, in enhancing the extent of intramolecular cyclization, thus leads to increased production of char. In addition, vapour-phase inhibitory effects of P-containing moieties (mainly oxides of phosphorus such as PO, PO
2, P
2O
4,
etc.) also need to be considered [
11].
Figure 4 shows a scatter plot of HRC values normalized to the content of P
versus the amount of char produced in PCFC tests, which might be a useful way of ranking the comonomers in their efficiency to improve flame retardance of PAN. Although the incorporation of phosphorylamino ester groups (ADEMPAE and ADEPAE) is quite effective in reducing HRC values, the amounts of corresponding char produced from these copolymers are lower than those for the copolymers containing acrylic phosphate (DEAEP) or acrylic phosphonate (DE1AEP or DEAMP) monomeric units. On the contrary, modification of PAN with DEAEP, DE1AEP and DEAMP enhances the char formation processes but seems to do not concomitantly reduce the HRC values. These inconsistencies might arise from the varying relative degrees of efficacies, shown by the modifying groups with different chemical environments, acting in the condensed and in vapour phases.
Figure 4.
Ratio of HRC to content of P versus PCFC char residue for copolymers of AN.
Figure 4.
Ratio of HRC to content of P versus PCFC char residue for copolymers of AN.
In the case of MMA- and S-based systems, generally, relatively lower yields of final recovered products were obtained. Furthermore, as with AN-based polymers, it was difficult to achieve the similar mole fractions of modifying groups for all copolymers, and thus the level of P incorporation varied in the polymeric products obtained (
Table 6 and
Table 7).
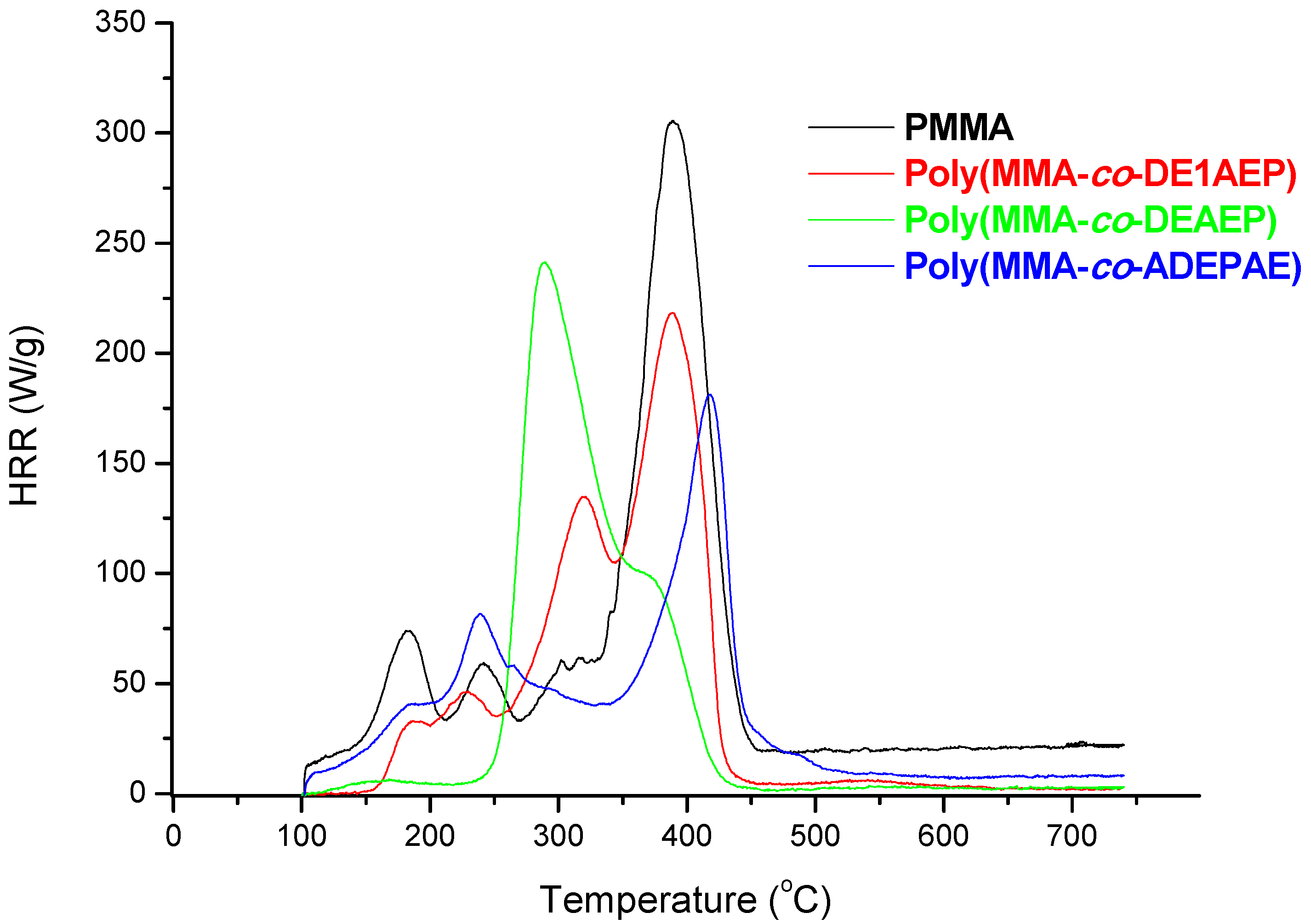
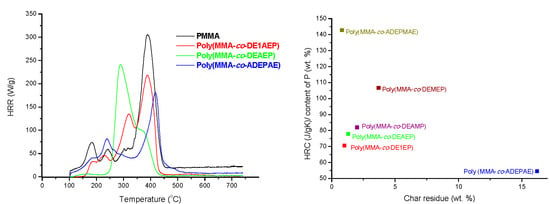
In
Figure 5, a plot of heat release rates
versus temperature for PMMA and for the copolymers with closer levels of P loadings, from 3.2 to 3.4 wt% are shown. As it is evident from this figure, the incorporation of P- or P-/N-containing groups substantially lowers the PHRR of copolymers as compared to homopolymer. This effect was most pronounced for poly(MMA-
co-ADEPAE) that contained both P and N atoms in the pendent groups. The temperatures to PHRR associated with the main degradation steps of this copolymer were shifted to higher temperatures compared to PMMA. The PCFC parameters for MMA-based polymeric systems are collated in
Table 6. These data also confirm that the highest level of flame retardation for MMA-based copolymers was achieved by incorporating ADEPAE units (0.126 mole fractions, 3.3 wt% P) into MMA chains. Indeed, the presence of P and N-containing moieties had led to the decrease, compared to the values recorded for PMMA, of: PHRR values by 141 W/g; THR values by 9.2 kJ/g; HRC values by 158 J/g·K. At the same time, the amount of residue of poly(MMA-
co-ADEPAE) was significantly greater (16.2 wt% of the initial sample weight) than for unmodified PMMA (0.7 wt%).
Figure 5.
Plots of HRR versus temperature for PMMA, poly(MMA-co-DE1AEP), poly(MMA-co-DEAEP), and poly(MMA-co-ADEPAE).
Figure 5.
Plots of HRR versus temperature for PMMA, poly(MMA-co-DE1AEP), poly(MMA-co-DEAEP), and poly(MMA-co-ADEPAE).
Table 6.
PCFC parameters for homo- and co-polymers of MMA.
Table 6.
PCFC parameters for homo- and co-polymers of MMA.
| Parameter | PMMA | Poly(MMA-co-DEAMP) | Poly(MMA-co-DE1AEP) | Poly(MMA-co-DEAEP) | Poly(MMA-co-DEMEP) | Poly(MMA-co-ADEPMAE) | Poly(MMA-co-ADEPAE) |
|---|
| [M]/[co-M] (mole fraction) | 0 | 0.890/0.110 | 0.880/0.120 | 0.870/0.130 | 0.900/0.100 | 0.934/0.066 | 0.874/0.126 |
| P content (wt%) | 0 | 3.0 | 3.2 | 3.4 | 2.4 | 1.8 | 3.3 |
| TPHRR (°C) | 379 | 413 | 394 | 291 | 395 | 411 | 414 |
| PHRR (W/g) | 308 | 227 | 206 | 242 | 235 | 234 | 167 |
| PHRR (% reduction) | 0 | 26 | 33 | 21 | 24 | 24 | 46 |
| THR (kJ/g) | 31.1 | 27.4 | 22.9 | 23.1 | 31.8 | 25.7 | 21.9 |
| HRC (J/g·K) | 338 | 246 | 226 | 265 | 256 | 257 | 180 |
| PCFC char yield (wt%) | 0.7 | 2.0 | 1.0 | 1.3 | 3.7 | 0.8 | 16.2 |
Generally, the values of PHRR, THR and HRC of copolymers containing only P atoms (
i.e., as phosphonate groups, DEAMP and DE1AEP, or as phosphate groups, DEAEP and DEMEP) were lower than for unmodified PMMA, but higher compared to the copolymer containing both P and N atoms (ADEPAE). The ranking exercise demonstrated that the most efficient reacting comonomer is ADEPAE (
Figure 6). Furthermore, DE1AEP appears to be the most efficient, in terms of reducing the flammability, among the copolymers containing P atom only.
Figure 6.
Ratio of HRC to the content of P versus PCFC char residue for copolymers of MMA.
Figure 6.
Ratio of HRC to the content of P versus PCFC char residue for copolymers of MMA.
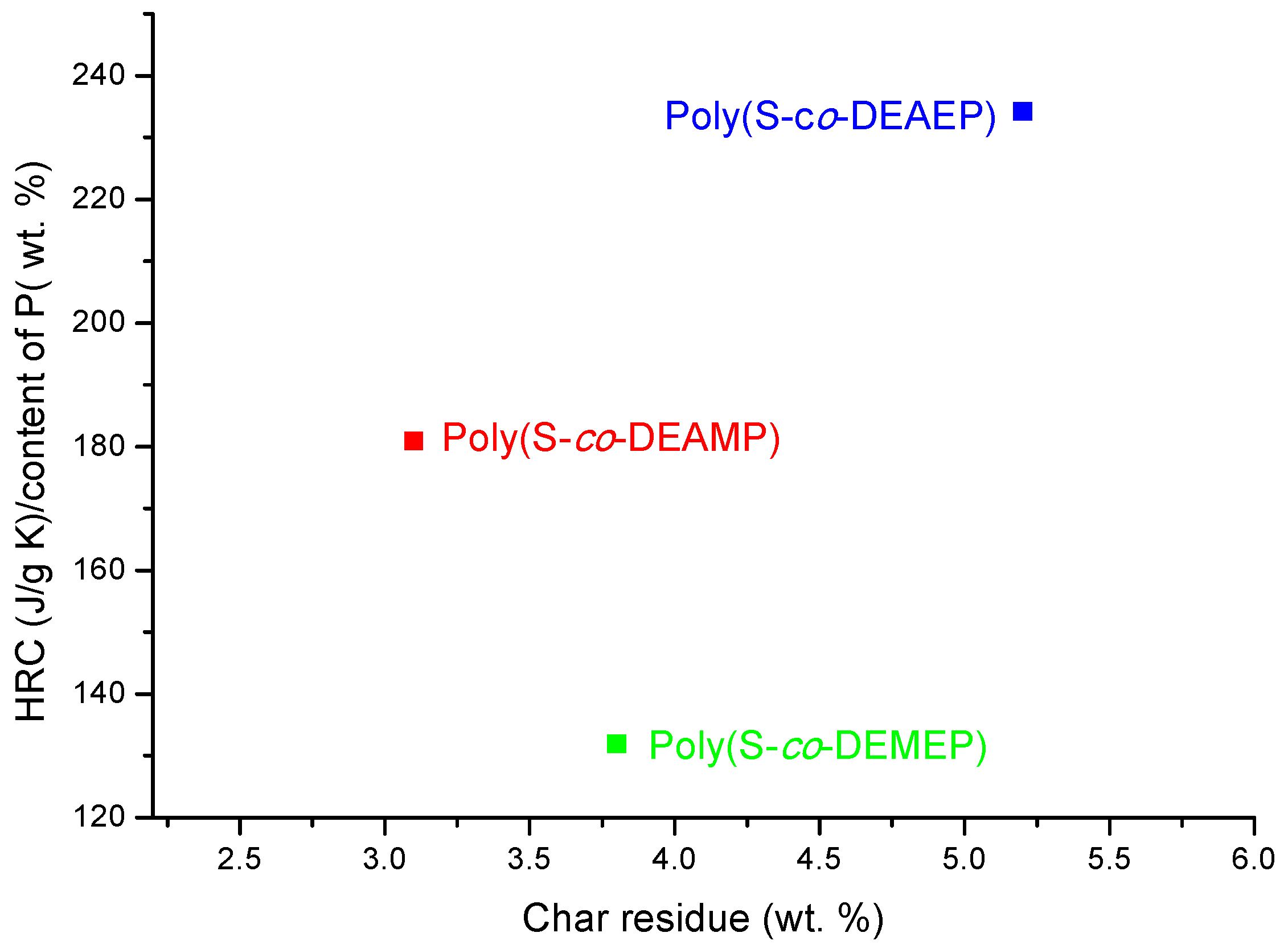
As it follows from
Table 7 and
Figure 7, for S-based polymeric systems the general trends were similar to those of the systems based on PMMA especially, in terms of HRR-temperature profiles and char production tendencies. As opposed to the unmodified counterpart, reactive modification of PS with DEMEP comonomeric units (0.127 mole fraction, 3.2 wt% of P) had led to the most drastic decrease of PHRR and HRC values, whilst the amounts of residue registered by PCFC increased by three-fold. It is interesting to note that copolymerization reaction with P- and N-containing comonomer (e.g., with ADEPAE) achieved the lowest level of P incorporation (only 0.2 wt%). Nevertheless, even the lowest loading of ADEPAE comonomeric units led to the reduction of HRC values from 729 (J/g·K) for PS to 541 (J/g·K) for poly(S-
co-ADEPAE). While this result is quite encouraging, it cannot be easily explained (a normalized value of 2705 is not plotted in
Figure 8).
Table 7.
PCFC parameters for S-based polymers.
Table 7.
PCFC parameters for S-based polymers.
| Parameter | PS | Poly(S-co-DEAMP) | Poly(S-co-DEAEP) | Poly(S-co-DEMEP) | Poly(S-co-ADEPAE) |
|---|
| [M]/[co-M] (mole fraction) | 0 | 0.880/0.120 | 0.902/0.098 | 0.873/0.127 | 0.992/0.008 |
| P content (wt%) | 0 | 3.1 | 2.6 | 3.2 | 0.2 |
| TPHRR (°C) | 420 | 383 | 392 | 419 | 423 |
| PHRR (W/g) | 703 | 512 | 557 | 389 | 499 |
| PHRR (% reduction) | 0 | 27 | 21 | 45 | 29 |
| THR (kJ/g) | 38.5 | 33.2 | 33.1 | 33.6 | 40.1 |
| HRC (J/g·K) | 729 | 561 | 609 | 423 | 541 |
| PCFC char yield (wt. %) | 1.1 | 3.1 | 5.2 | 3.8 | 1.0 |
Figure 7.
Plots of HRR versus temperature for PS, poly(S-co-DEAMP), poly(S-co-DEMEP) and poly(S-co-ADEPAE).
Figure 7.
Plots of HRR versus temperature for PS, poly(S-co-DEAMP), poly(S-co-DEMEP) and poly(S-co-ADEPAE).
Figure 8.
Ratio of HRC to the content of P versus PCFC char residue for the copolymers of S.
Figure 8.
Ratio of HRC to the content of P versus PCFC char residue for the copolymers of S.
The mechanistic aspects of flame retardation in MMA- and S-based systems ought to be different to that of PAN as the primary modes of degradation of both PS and PMMA differ from that of PAN owing to the differences in their chemical constitution. In any case, the initial point of degradation is believed to be activated by the modifying groups containing only P atoms or both P and N atoms. As is shown previously, in the case of MMA-based polymers trans-esterification route is highly probable leading to a formation of char precursors [
8,
11,
12], whilst in the case of PS the elements of mechanism are less obvious [
11,
13].