Solvent-Free Microwave-Induced Oxidation of Alcohols Catalyzed by Ferrite Magnetic Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
2.1.1. FT-IR Spectra (Infrared Spectroscopy)
2.1.2. XRD (X-ray Diffraction Pattern)
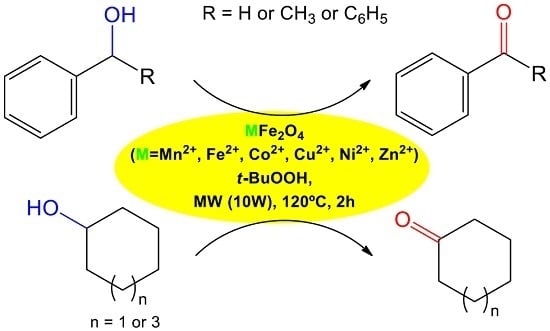
2.1.3. TEM (Transmission Electron Microscopy)
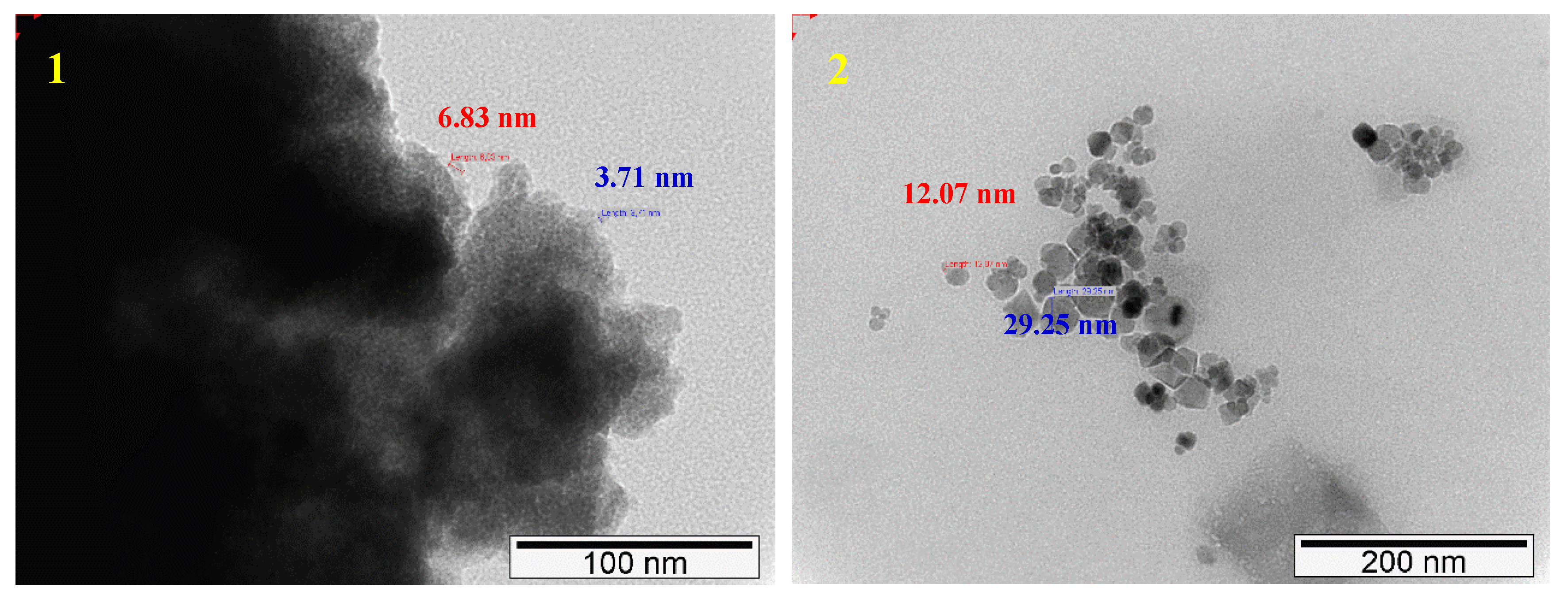
2.1.4. SEM–EDS (Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy)
2.1.5. VSM (Vibrating Sample Magnetometry)
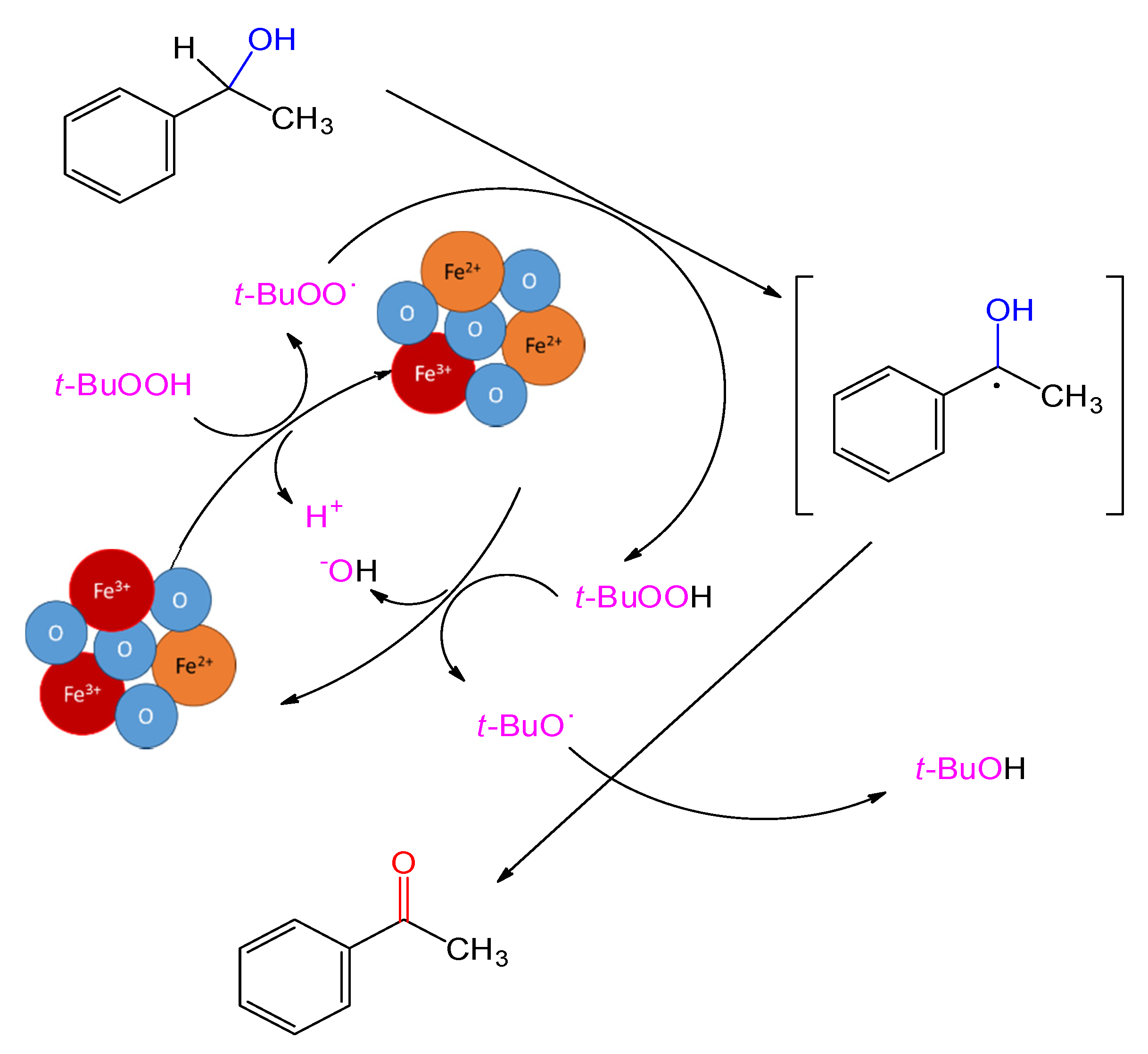
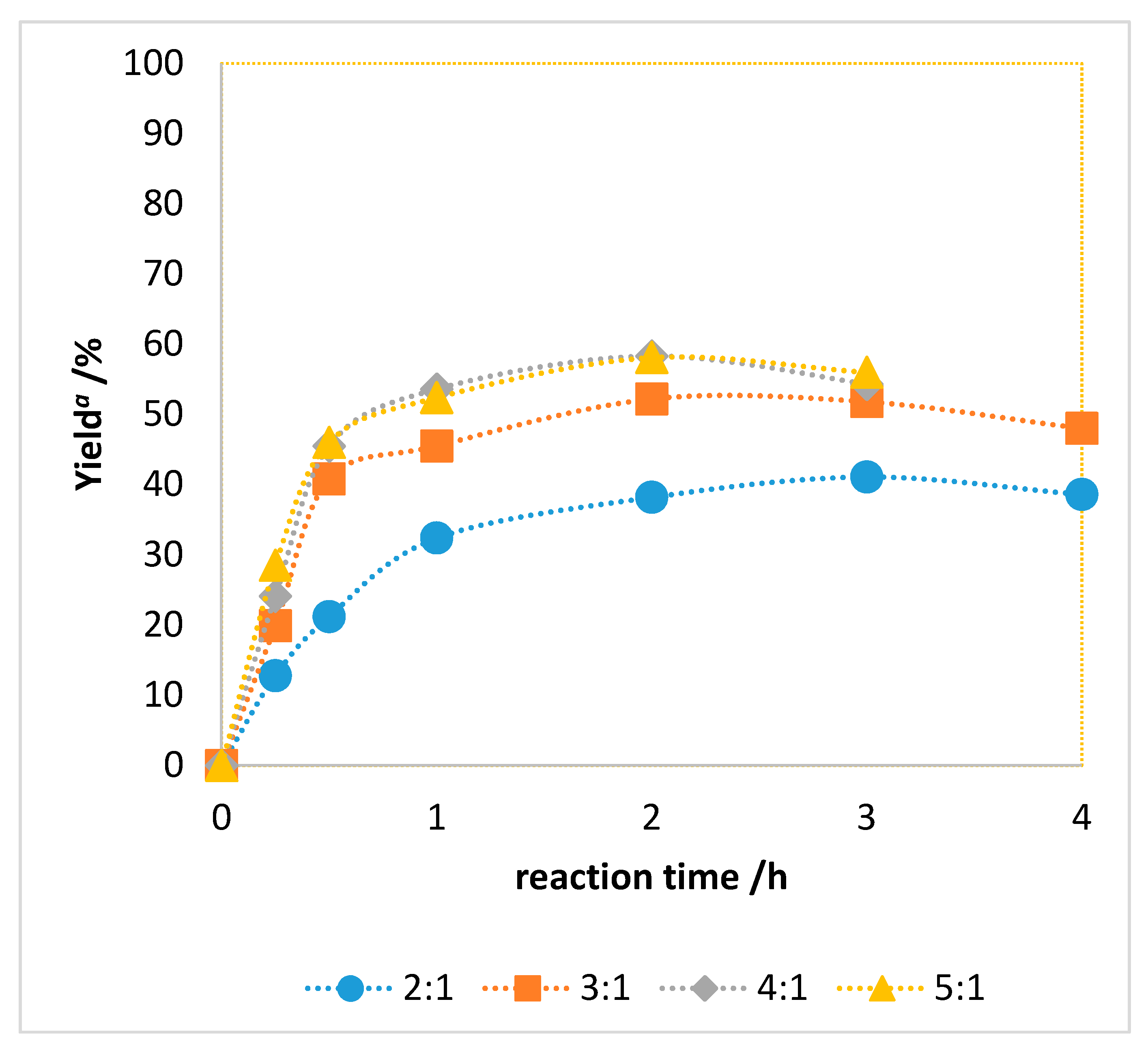
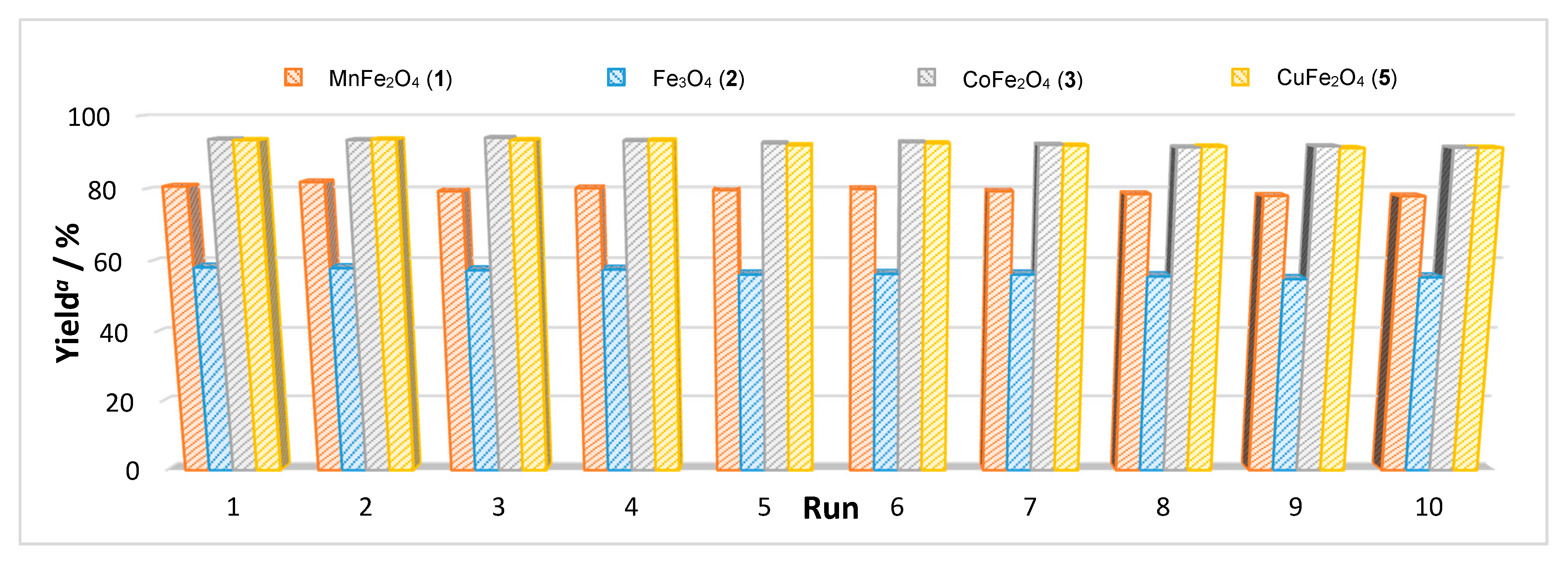
2.2. Catalytic Performance
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Catalyst Preparation
3.3. General Procedure for the Peroxidative Oxidation of Alcohols
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caruso, F.; Spasova, M.; Susha, A.; Giersig, M.; Caruso, R.A. Magnetic nanocomposite particles and hollow spheres constructed by a sequential layering approach. Chem. Mater. 2001, 13, 109–116. [Google Scholar]
- Hyeon, T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 8, 927–934. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wen, G.H.; Huang, S.; Dai, L.; Gao, R.; Wang, Z.L. Magnetic properties of fe nanoparticles trapped at the tips of the aligned carbon nanotubes. J. Magn. Magn. Mater. 2001, 231, 9–12. [Google Scholar]
- Chiba, D.; Yamanouchi, M.; Matsukura, F.; Ohno, H. Electrical manipulation of magnetization reversal in a ferromagnetic semiconductor. Science 2003, 301, 943–945. [Google Scholar] [PubMed]
- Lu, A.-H.; Schmidt, W.; Matoussevitch, N.; Bönnemann, H.; Spliethoff, B.; Tesche, B.; Bill, E.; Kiefer, W.; Schüth, F. Nanoengineering of a magnetically separable hydrogenation catalyst. Angew. Chem. Int. Ed. 2004, 43, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Jiang, Y.; Kuang, C.; Wang, S.; Liu, H.; Zhang, Y.; Wang, J. Nano-Fe2O3-catalyzed direct borylation of arenes. Chem. Commun. 2010, 46, 3170–3172. [Google Scholar] [CrossRef] [PubMed]
- Barbero, B.P.; Gamboa, J.A.; Cadús, L.E. Synthesis and characterisation of La1−xCaxFeO3 perovskite-type oxide catalysts for total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2006, 65, 21–30. [Google Scholar] [CrossRef]
- Xiao, S.H.; Jiang, W.F.; Li, L.Y.; Li, X.J. Low-temperature auto-combustion synthesis and magnetic properties of cobalt ferrite nanopowder. Mater. Chem. Phys. 2007, 106, 82–87. [Google Scholar] [CrossRef]
- Menini, L.; Pereira, M.C.; Ferreira, A.C.; Fabris, J.D.; Gusevskaya, E.V. Cobalt–iron magnetic composites as heterogeneous catalysts for the aerobic oxidation of thiols under alkali free conditions. Appl. Catal. A Gen. 2011, 392, 151–157. [Google Scholar]
- Su, M.; He, C.; Sharma, V.K.; Abou Asi, M.; Xia, D.; Li, X.-Z.; Deng, H.; Xiong, Y. Mesoporous zinc ferrite: Synthesis, characterization, and photocatalytic activity with H2O2/visible light. J. Hazard. Mater. 2012, 211–212, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Karami, B.; Hoseini, S.J.; Nikoseresht, S.; Khodabakhshi, S. Fe3O4 nanoparticles: A powerful and magnetically recoverable catalyst for the synthesis of novel calix[4]resorcinarenes. Chin. Chem. Lett. 2012, 23, 173–176. [Google Scholar] [CrossRef]
- Conway, B.; Tilak, B. Advanced Catalysis; Academic Press: New York, NY, USA, 1992. [Google Scholar]
- Cao, H.; Suib, S.L. Highly efficient heterogeneous photooxidation of 2-propanol to acetone with amorphous manganese oxide catalysts. J. Am. Chem. Soc. 1994, 116, 5334–5342. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Cardona, F. Transition metal based catalysts in the aerobic oxidation of alcohols. Green Chem. 2012, 14, 547–564. [Google Scholar] [CrossRef]
- Trost, B.M.; Fleming, I. (Eds.) Comprehensive Organic Synthesis (Oxidation); Pergamon Press: New York, NY, USA, 1991. [Google Scholar]
- Sheldon, R.A.; Kochi, J.K. Metal Catalyzed Oxidations of Organic Compounds; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- González-Arellano, C.; Campelo, J.M.; Macquarrie, D.J.; Marinas, J.M.; Romero, A.A.; Luque, R. Efficient microwave oxidation of alcohols using low-loaded supported metallic iron nanoparticles. ChemSusChem 2008, 1, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Sadri, F.; Ramazani, A.; Massoudi, A.; Khoobi, M.; Tarasi, R.; Shafiee, A.; Azizkhani, V.; Dolatyari, L.; Joo, S.W. Green oxidation of alcohols by using hydrogen peroxide in water in the presence of magnetic Fe3O4 nanoparticles as recoverable catalyst. Green Chem. Lett. Rev. 2014, 7, 257–264. [Google Scholar] [CrossRef]
- Mori, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 2004, 126, 10657–10666. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Du, Z.; Liu, J.; Ma, H.; Xu, J. Aerobic oxidation of primary aliphatic alcohols over bismuth oxide supported platinum catalysts in water. Green Chem. 2013, 15, 2215–2221. [Google Scholar] [CrossRef]
- Mahyari, M.; Shaabani, A. Graphene oxide-iron phthalocyanine catalyzed aerobic oxidation of alcohols. Appl. Catal. A Gen. 2014, 469, 524–531. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Catalytic oxidations. In Green Chemistry and Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 133–221. [Google Scholar]
- Shi, F.; Tse, M.K.; Pohl, M.-M.; Brückner, A.; Zhang, S.; Beller, M. Tuning catalytic activity between homogeneous and heterogeneous catalysis: Improved activity and selectivity of free nano-Fe2O3 in selective oxidations. Angew. Chem. Int. Ed. 2007, 46, 8866–8868. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Bo, L.; Li, Z.; Lei, Z.; Xia, C. Magnetic CoFe2O4 nanocrystal: A novel and efficient heterogeneous catalyst for aerobic oxidation of cyclohexane. J. Mol. Catal. A Chem. 2009, 307, 58–63. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.; Nogueira, I.D.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Branco, P.S. A Recyclable Ferrite–Co Magnetic Nanocatalyst for the Oxidation of Alcohols to Carbonyl Compounds. ChemPlusChem 2012, 77, 865–871. [Google Scholar] [CrossRef]
- Obermayer, D.; Balu, A.M.; Romero, A.A.; Goessler, W.; Luque, R.; Kappe, C.O. Nanocatalysis in continuous flow: Supported iron oxide nanoparticles for the heterogeneous aerobic oxidation of benzyl alcohol. Green Chem. 2013, 15, 1530–1537. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.; Satanami, M.; Ranganath, K.V.S. Efficient aerobic oxidation of alcohols using magnetically recoverable catalysts. Catal. Commun. 2014, 54, 91–93. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Bagherzadeh, M.; Karimi, H. Preparation, Preparation, characterization and catalytic activity of CoFe2O4 nanoparticles as a magnetically recoverable catalyst for selective oxidation of benzyl alcohol to benzaldehyde and reduction of organic dyes. J. Colloid Interface Sci. 2016, 465, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Chapter three-catalytic oxidation of alcohols: Recent advances. In Advances in Organometallic Chemistry; Pedro, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 63, pp. 91–174. [Google Scholar]
- Karabach, Y.Y.; Kopylovich, M.N.; Mahmudov, K.T.; Pombeiro, A.J.L. Microwave-assisted catalytic oxidation of alcohols to carbonyl compounds. In Advances in Organometallic Chemistry and Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 233–245. [Google Scholar]
- Spargo, P.L. Microwave assisted organic synthesis edited by j. P. Tierney and p. Lidstrom. Blackwell Publishing: Oxford. 2005. (Also published by CRC Press in U.S.A. and Canada, ISBN 0-8493-2371-1.). Org. Process Res. Dev. 2005, 9, 697. [Google Scholar]
- Dallinger, D.; Kappe, C.O. Microwave-assisted synthesis in water as solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef] [PubMed]
- Timokhin, I.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel coordination polymers with (pyrazolato)-based tectons: Catalytic activity in the peroxidative oxidation of alcohols and cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Ribera, A.; Nunes, A.V.M.; Gahramanova, S.I.; Marchetti, F.; Pombeiro, A.J.L. Mnii and cuii complexes with arylhydrazones of active methylene compounds as effective heterogeneous catalysts for solvent- and additive-free microwave-assisted peroxidative oxidation of alcohols. RSC Adv. 2015, 5, 25979–25987. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxidovanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohols. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Karmakar, A.; Martins, L.M.D.R.S.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Metal–organic frameworks with pyridyl-based isophthalic acid and their catalytic applications in microwave assisted peroxidative oxidation of alcohols and henry reaction. Cryst. Growth Des. 2016, 16, 1837–1849. [Google Scholar] [CrossRef]
- Liu, X.; An, S.; Shi, W.; Yang, Q.; Zhang, L. Microwave-induced catalytic oxidation of malachite green under magnetic cu-ferrites: New insight into the degradation mechanism and pathway. J. Mol. Catal. A Chem. 2014, 395, 243–250. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Copper(II) and iron(III) complexes with arylhydrazone of ethyl 2-cyanoacetate or formazan ligands as catalysts for oxidation of alcohols. New J. Chem. 2016, 40, 10071–10083. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Ghandoor, H.E.; Zidan, H.M.; Khalil, M.; Ismail, M.I.M. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012, 5734–5745. [Google Scholar]
- Hou, J.; Luan, Y.; Yu, J.; Qi, Y.; Wang, G.; Lu, Y. Fabrication of hierarchical composite microspheres of copper-doped Fe3O4@P4VP@ZIF-8 and their application in aerobic oxidation. New J. Chem. 2016, 40, 10127–10135. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tan, L.; Luan, Y.; Yang, M. Superparamagnetic Core–Shell Metal–Organic Framework Fe3O4/Cu3(btc)2 Microspheres and Their Catalytic Activity in the Aerobic Oxidation of Alcohols and Olefins. Eur. J. Inorg. Chem. 2016, 2016, 4906–4912. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Soinski, M.; Moses, A.J. Anisotropy in iron-based soft magnetic materials. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 1995; Chapter 4; Volume 8, pp. 325–414. [Google Scholar]
- Mattalia, J.M.; Vacher, B.; Samat, A.; Chanon, M. Mechanistic investigation of the reaction between .Alpha.-sulfonyl carbanions and polyhalogenmethanes. Electron transfer versus polar pathways. J. Am. Chem. Soc. 1992, 114, 4111–4119. [Google Scholar] [CrossRef]
- Moiseeva, N.I.; Gekhman, A.E.; Minin, V.V.; Larin, G.M.; Bashtanov, M.E.; Krasnovskii, A.A.; Moiseev, I.I. Free radical/singlet dioxygen system under the conditions of catalytic hydrogen peroxide decomposition. Kinet. Catal. 2000, 41, 170–182. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hasheminasab, H.R. Vanadium phosphorus oxide catalyst promoted by cobalt doping for mild oxidation of benzyl alcohol to benzaldehyde in the liquid phase. Appl. Catal. A Gen. 2014, 482, 189–197. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, J.; Pan, J.; Sun, F.A.; He, M.; Chen, Q. Effect of Mg2+ on the catalytic activities of CoMgAl hydrotalcites in the selective oxidation of benzyl alcohol to benzaldehyde. Catal. Commun. 2015, 69, 1–4. [Google Scholar] [CrossRef]
- Estrada, M.; Costa, V.V.; Beloshapkin, S.; Fuentes, S.; Stoyanov, E.; Gusevskaya, E.V.; Simakov, A. Aerobic oxidation of benzyl alcohol in methanol solutions over au nanoparticles: Mg(OH)2 vs. MgO as the support. Appl. Catal. A Gen. 2014, 473, 96–103. [Google Scholar] [CrossRef]
- Cang, R.; Lu, B.; Li, X.; Niu, R.; Zhao, J.; Cai, Q. Iron-chloride ionic liquid immobilized on SBA-15 for solvent-free oxidation of benzyl alcohol to benzaldehyde with H2O2. Chem. Eng. Sci. 2015, 137, 268–275. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Yan, H.; Yi, H.; Lu, J. Precisely-controlled synthesis of Au@Pd core–shell bimetallic catalyst via atomic layer deposition for selective oxidation of benzyl alcohol. J. Catal. 2015, 324, 59–68. [Google Scholar] [CrossRef]
- Ragupathi, C.; Judith Vijaya, J.; Thinesh Kumar, R.; John Kennedy, L. Selective liquid phase oxidation of benzyl alcohol catalyzed by copper aluminate nanostructures. J. Mol. Struct. 2015, 1079, 182–188. [Google Scholar] [CrossRef]
- Zahed, B.; Hosseini-Monfared, H. A comparative study of silver-graphene oxide nanocomposites as a recyclable catalyst for the aerobic oxidation of benzyl alcohol: Support effect. Appl. Surf. Sci. 2015, 328, 536–547. [Google Scholar] [CrossRef]
- Ragupathi, C.; Judith Vijaya, J.; Narayanan, S.; Jesudoss, S.K.; John Kennedy, L. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by cobalt aluminate catalysis: A comparison of conventional and microwave methods. Ceram. Int. 2015, 41, 2069–2080. [Google Scholar] [CrossRef]
- Tamiolakis, I.; Lykakis, I.N.; Armatas, G.S. Mesoporous CdS-sensitized TiO2 nanoparticle assemblies with enhanced photocatalytic properties: Selective aerobic oxidation of benzyl alcohols. Catal. Today 2015, 250, 180–186. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Wei, H.; Zheng, J.; Su, H.; Wang, X. Hydrotalcite-supported gold catalysts for a selective aerobic oxidation of benzyl alcohol driven by visible light. J. Mol. Catal. A Chem. 2014, 395, 128–136. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, S.; Sun, Q.; Shi, L.; Lu, A.-H. Uniformly dispersed Pd nanoparticles on nitrogen-doped carbon nanospheres for aerobic benzyl alcohol oxidation. Chin. J. Catal. 2015, 36, 612–619. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.-C.; Hao, G.-P.; Hao, Y.; Sun, Q.; Zhang, X.-Q.; Lu, A.-H. Temperature-programmed precise control over the sizes of carbon nanospheres based on benzoxazine chemistry. J. Am. Chem. Soc. 2011, 133, 15304–15307. [Google Scholar] [CrossRef] [PubMed]
- Dimitratos, N.; Villa, A.; Wang, D.; Porta, F.; Su, D.; Prati, L. Pd and pt catalysts modified by alloying with au in the selective oxidation of alcohols. J. Catal. 2006, 244, 113–121. [Google Scholar] [CrossRef]
- Harada, T.; Ikeda, S.; Miyazaki, M.; Sakata, T.; Mori, H.; Matsumura, M. A simple method for preparing highly active palladium catalysts loaded on various carbon supports for liquid-phase oxidation and hydrogenation reactions. J. Mol. Catal. A Chem. 2007, 268, 59–64. [Google Scholar] [CrossRef]
- Harada, T.; Ikeda, S.; Hashimoto, F.; Sakata, T.; Ikeue, K.; Torimoto, T.; Matsumura, M. Catalytic activity and regeneration property of a Pd nanoparticle encapsulated in a hollow porous carbon sphere for aerobic alcohol oxidation. Langmuir 2010, 26, 17720–17725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Zhang, X. Selective oxidation of benzyl alcohol catalyzed by palladium nanoparticles supported on carbon-coated iron nanocrystals. Chin. J. Catal. 2011, 32, 1693–1701. [Google Scholar] [CrossRef]




| Entry | Catalyst | M (mg) | Cat/Alcohol Molar Ratio | Yield b (%) |
|---|---|---|---|---|
| 1 | none | - | - | 1.8 |
| 2 | FeCl2·6H2O | 19.9 | 0.04 | 12.9 |
| 3 | FeCl3 | 16.2 | 0.04 | 22.7 |
| 4 | FeCl2·6H2O + 2FeCl3 | 5.3 + 13.3 | 0.01 + 0.03 | 15.2 |
| 5 | Fe3O4 (2) | 2.9 | 0.005 | 3.1 |
| 6 | Fe3O4 (2) | 29.6 | 0.05 | 17.8 |
| 7 c | Fe3O4 (2) | 29.7 | 0.05 | 12.2 |
| Entry | Oxidant | Additive | Yield b (%) |
|---|---|---|---|
| § | - | - | no reaction |
| 2 | O2 (1 atm) | - | 1.3 |
| 3 | H2O2 30% | - | 11.1 |
| 4 | t-BuOOH 70% | - | 17.8 |
| 5 c | t-BuOOH 70% | TEMPO 2.5% mol vs. subs | 7.3 |
| 6 d | t-BuOOH 70% | Ph2NH 100% mol vs. subs | 0.2 |
| 7 d | t-BuOOH 70% | CBrCl3 100% mol vs. subs | 0.7 |
| Entry | Temperature (°C) | Yield b (%) |
|---|---|---|
| 1 | 50 | 9.2 |
| 2 | 80 | 17.8 |
| 3 | 100 | 18.3 |
| 4 | 120 | 21.2 |
| 5 | 150 | 18.6 |
| Entry | Substrate | Product | Yield b (%) |
|---|---|---|---|
| 1 | 1-Phenylethanol | Acetophenone | 58.3 |
| 2 | Benzyl alcohol | Benzaldehyde | 51.2 |
| 3 | Benzhydrol | Benzophenone | 53.5 |
| 4 | Cinnamyl alcohol | Cinnamaldehyde | 38.3 |
| 5 | Cyclopentanol | Cyclopentanone | 39.2 |
| 6 | Cyclohexanol | Cyclohexanone | 41.1 |
| 7 | Cycloheptanol | Cycloheptanone | 45.1 |
| 8 | Cyclooctanol | Cyclooctanone | 46.8 |
| Catalyst | Acetophenone Yield b (%) | Benzaldehyde Yield b (%) |
|---|---|---|
| MnFe2O4 (1) | 80.8 | 79.1 |
| Fe3O4 (2) | 58.3 | 51.2 |
| CoFe2O4 (3) | 93.7 | 88.9 |
| NiFe2O4 (4) | 52.4 | 46.2 |
| CuFe2O4 (5) | 93.5 | 86.7 |
| ZnFe2O4 (6) | 48.0 | 44.7 |
| Entry | Catalyst | Reaction Conditions | Conversion a (%) | Selectivity b (%) | Reference |
|---|---|---|---|---|---|
| 1 | Au/Mg-350 | Methanol, 10 atm (O2), 110 °C, 10 h | 96 | 33 | [51] |
| 2 | Au/Mg-500 | Methanol, 10 atm (O2), 110 °C, 10 h | 78 | 66 | [51] |
| 3 | VPO | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 50 | 100 | [49] |
| 4 | VPO-Co-n (0.01) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 70 | 84 | [49] |
| 5 | VPO-Co-n (0.03) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 67 | 67 | [49] |
| 6 | VPO-Co-n (0.06) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 56 | 100 | [49] |
| 7 | VPO-Co-n (0.1) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 65 | 77 | [49] |
| 8 | VPO-Co-a (0.2) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 66 | 74 | [49] |
| 9 | VPO-Co-a (0.5) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 57 | 93 | [49] |
| 10 | VPO-Co-a (1) | MeCN, t-BuOOH, 90 °C (reflux), 8 h | 66 | 82 | [49] |
| 11 | SIL-FeCl3 (loading FeCl3 = 0.71) | Solvent-free, H2O2, 90 °C, 5 h | 62.2 | 70.7 | [52] |
| 12 | Au@1Pd/SiO2 | Solvent-free, O2, 90 °C, 6 h | 8.8 | 91 | [53] |
| 13 | Au@5Pd/SiO2 | Solvent-free, O2, 90 °C, 6 h | 54 | 93 | [53] |
| 14 | Au@8Pd/SiO2 | Solvent-free, O2, 90 °C, 6 h | 91 | 87 | [53] |
| 15 | Au@10Pd/SiO2 | Solvent-free, O2, 90 °C, 6 h | 88 | 91 | [53] |
| 16 | Au@15Pd/SiO2 | Solvent-free, O2, 90 °C, 6 h | 90 | 88 | [53] |
| 17 | Au@20Pd/SiO2 | Solvent-free, O2, 90 °C, 6 h | 94 | 88 | [53] |
| 18 | CuAl2O4 | MeCN, H2O2, 80 °C, 8 h | 99 | 98 | [54] |
| 19 | AgNPs/rGO | MeCN, NHPI, O2, 80 °C, 24 h | 12 | 8 | [55] |
| 20 | Ag NPs/GO | MeCN, NHPI, O2, 80 °C, 24 h | 33 | 55 | [55] |
| 21 | Ag NPs/GOSH | MeCN, NHPI, O2, 80 °C, 24 h | 61 | 58 | [55] |
| 22 | CoAl2O4 | MeCN, O2, 80 °C, 5 h | 80.9 | 96.7 | [56] |
| 23 | CdS-MTA | MeCN, O2, 5 °C, UV–vis, 1 h | 65 | >99 | [57] |
| 24 | Au/HT-3 | O2, in visible light, 24 h | 57.4 | 93.8 | [58] |
| 25 | Au/HT-3 | O2, in the dark, 24 h | 3.7 | 91.5 | [58] |
| 26 | Co2Al-LDH | MeCN, t-BuOOH, 60 °C, 30 min | 26.9 | 91.1 | [50] |
| 27 | Co2Mg0.5Al-LDH | MeCN, t-BuOOH, 60 °C, 30 min | 38.1 | 88.9 | [50] |
| 28 | Co2MgAl-LDH | MeCN, t-BuOOH, 60 °C, 30 min | 39.5 | 89.2 | [50] |
| 29 | Co2Mg1.5Al-LDH | MeCN, t-BuOOH, 60 °C, 30 min | 33.8 | 92.0 | [50] |
| 30 | Co2Mg2Al-LDH | MeCN, t-BuOOH, 60 °C, 30 min | 32.1 | 94.9 | [50] |
| 31 | Pd@PBFS-500 | H2O, O2, 80 °C, 0.1 MPa, 0.5 h | 87 | >99 | [59] |
| 32 | Pd/CB | H2O, O2, 80 °C, 0.1 MPa, 2 h | 10 | >99 | [60] |
| 33 | Pd/AC | H2O, O2, 80 °C, 0.15 MPa, 3 h | 18 | 91 | [61] |
| 34 | Pd/CMK-3 | 1,4-Dioxane, O2, 80 °C, 0.1 MPa, 1 h | 55 | 80 | [62] |
| 35 | Pd@hmC | H2O, K2CO3, O2, 80 °C, 0.1 MPa, 1 h | 48 | 77 | [63] |
| 36 | Pd/Fe@C | H2O, K2CO3, O2, 80 °C, 0.1 MPa, 1 h | 63 | 67 | [64] |
| 37 | Cu-FPZ | MeCN, NaHCO3, TEMPO, O2, 60 °C, 12 h | 99 | 99 | [43] |
| 38 | Fe3O4 | Toluene, O2, 80 °C, 12 h | 80 | 100 | [29] |
| 39 | Fe3O4/Cu3(BTC)2 | MeCN, Na2CO3, TEMPO, O2, 75 °C, 6 h | >99 | >99 | [44] |
| 40 | CoFe2O4 | Solvent-free, H2O2, 110 °C, 5 h | >99 | 100 | [30] |
| 41 | MnFe2O4 (1) | Solvent-free, t-BuOOH, MW 120 °C, 2 h | 79.1 | >99 | This work |
| 42 | Fe3O4 (2) | Solvent-free, t-BuOOH, MW 120 °C, 2 h | 51.2 | >99 | This work |
| 43 | CoFe2O4 (3) | Solvent-free, t-BuOOH, MW 120 °C, 2 h | 88.9 | >99 | This work |
| 44 | NiFe2O4 (4) | Solvent-free, t-BuOOH, MW 120 °C, 2 h | 46.2 | >99 | This work |
| 45 | CuFe2O4 (5) | Solvent-free, t-BuOOH, MW 120 °C, 2 h | 86.7 | >99 | This work |
| 46 | ZnFe2O4 (6) | Solvent-free, t-BuOOH, MW 120 °C, 2 h | 44.7 | >99 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, N.M.R.; Martins, L.M.D.R.S.; Amorim, C.O.; Amaral, V.S.; Pombeiro, A.J.L. Solvent-Free Microwave-Induced Oxidation of Alcohols Catalyzed by Ferrite Magnetic Nanoparticles. Catalysts 2017, 7, 222. https://doi.org/10.3390/catal7070222
Martins NMR, Martins LMDRS, Amorim CO, Amaral VS, Pombeiro AJL. Solvent-Free Microwave-Induced Oxidation of Alcohols Catalyzed by Ferrite Magnetic Nanoparticles. Catalysts. 2017; 7(7):222. https://doi.org/10.3390/catal7070222
Chicago/Turabian StyleMartins, Nuno M. R., Luísa M. D. R. S. Martins, Carlos O. Amorim, Vitor S. Amaral, and Armando J. L. Pombeiro. 2017. "Solvent-Free Microwave-Induced Oxidation of Alcohols Catalyzed by Ferrite Magnetic Nanoparticles" Catalysts 7, no. 7: 222. https://doi.org/10.3390/catal7070222