Synthesis and Evaluation of Ni Catalysts Supported on BaCe0.5Zr0.3−xY0.2NixO3−δ with Fused-Aggregate Network Structure for the Hydrogen Electrode of Solid Oxide Electrolysis Cell
Abstract
:1. Introduction
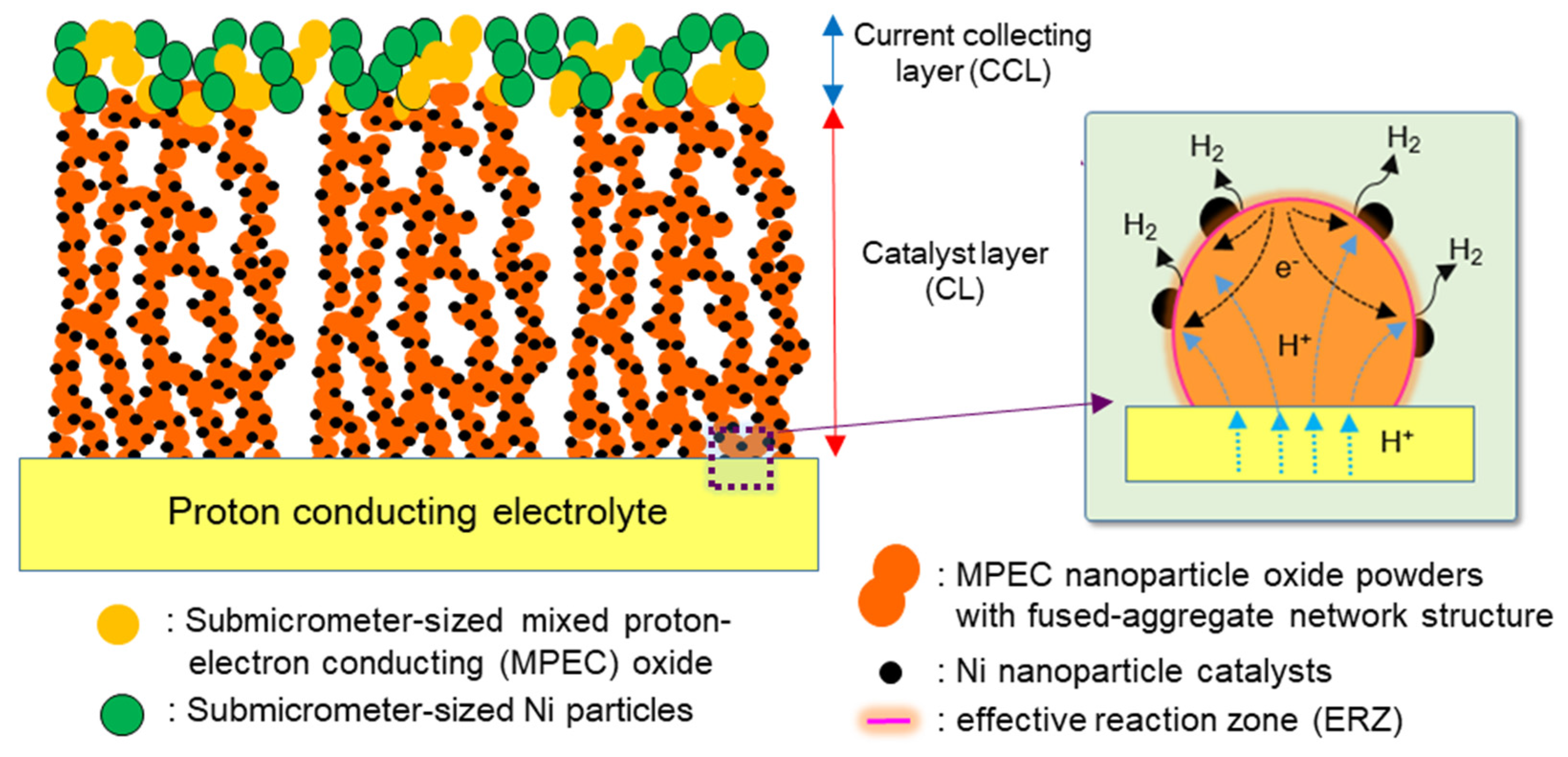
2. Results and Discussion
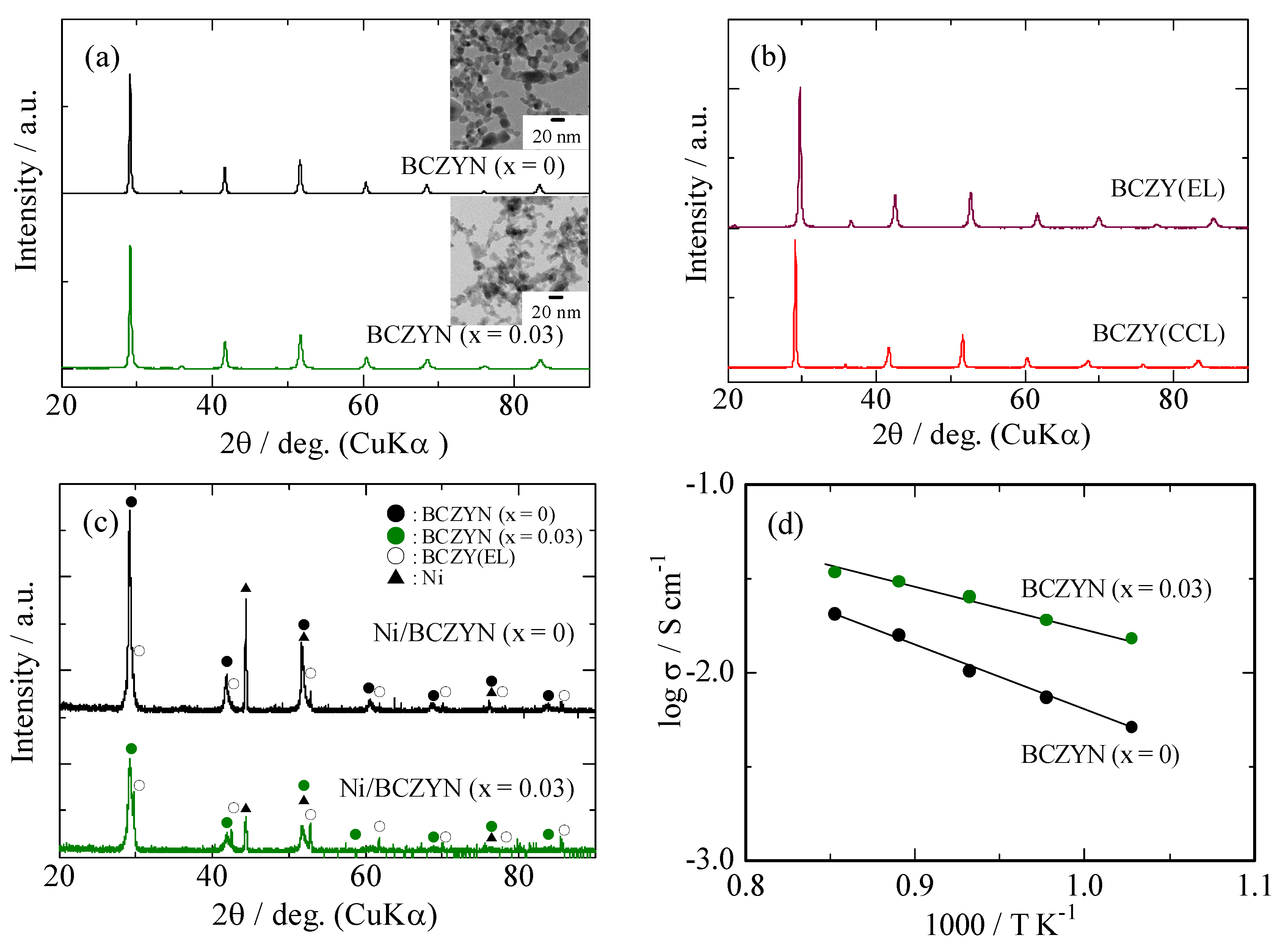
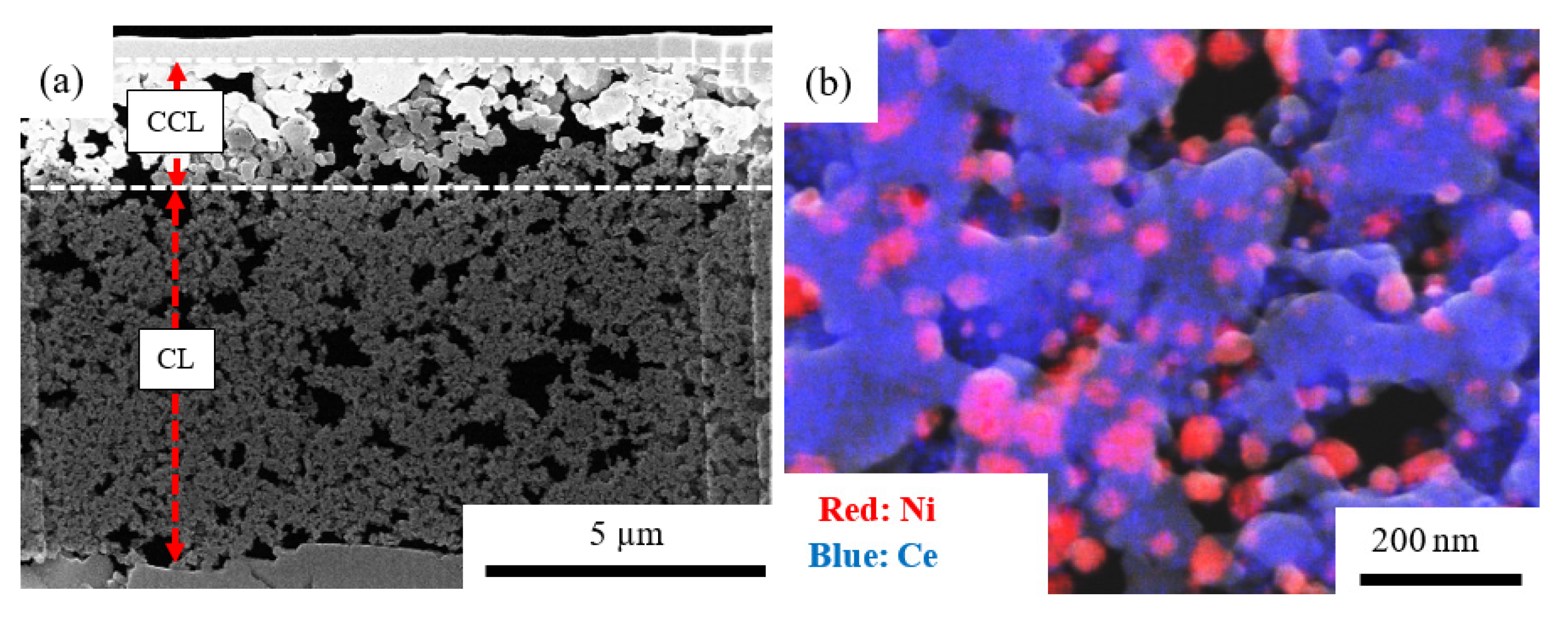
2.1. Characterization of Samples
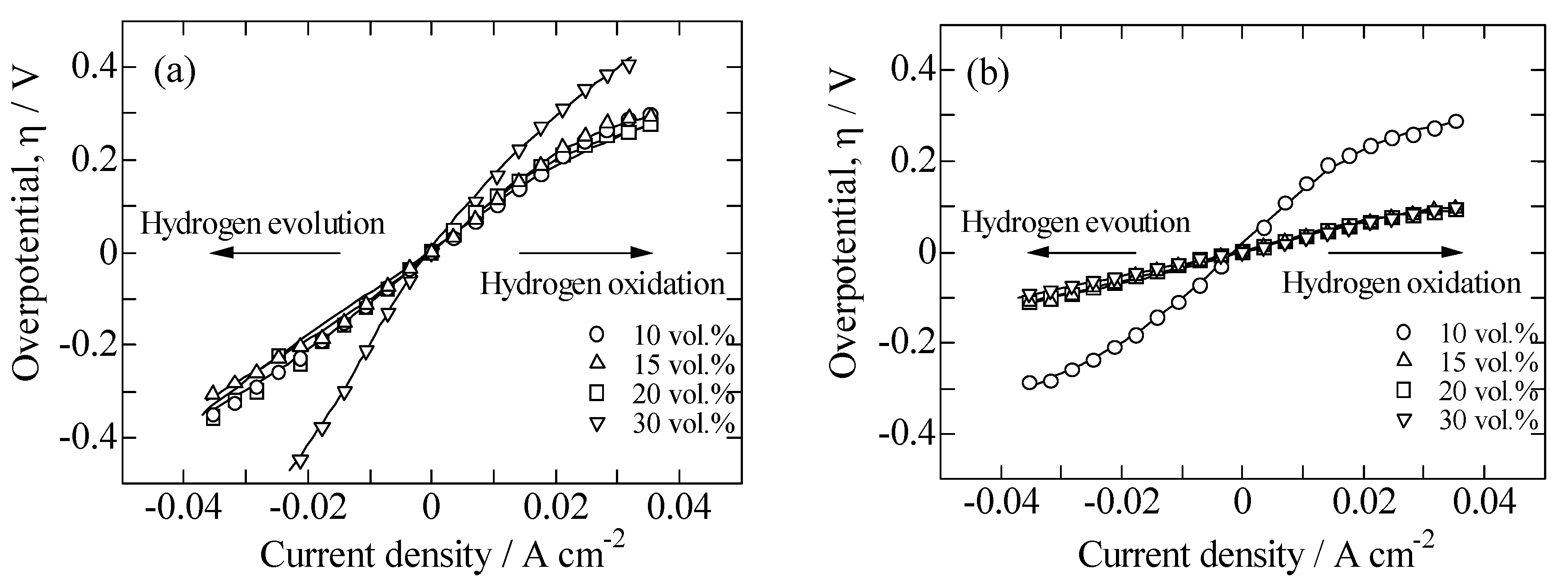
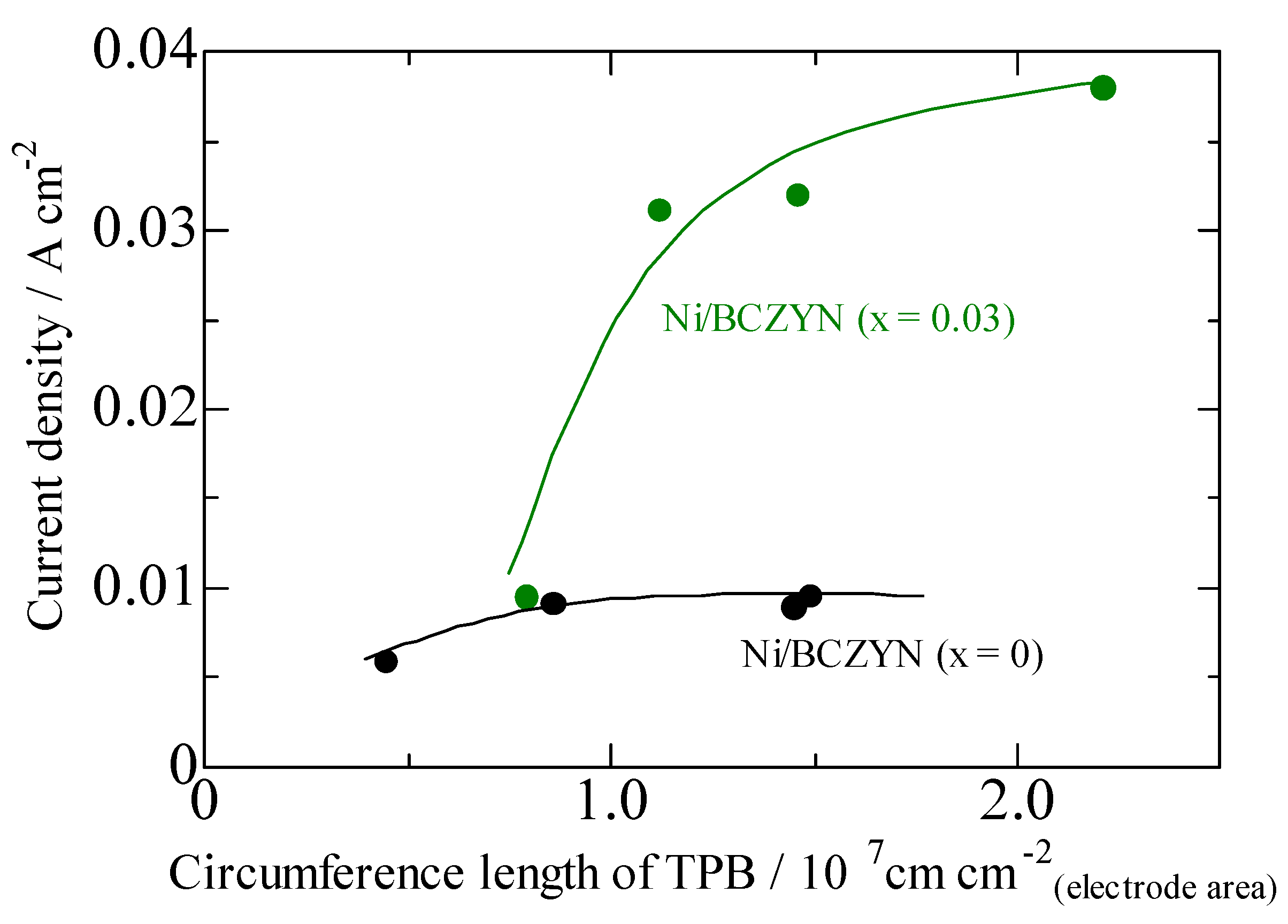
2.2. Performances of Ni/BCZYN CLs
3. Materials and Methods
3.1. Sample Preparation
3.2. Single Cell Fabrication
3.3. Characterization of Sample, CL and CCL
3.4. Electrochemical Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ni, M.; Leung, M.K.H.; Leung, D.Y.G. Electrochemical modeling of hydrogen production by proton-conducting solid oxide steam electrolyzer. Int. J. Hydrogen Energy 2008, 33, 4040–4047. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Maeda, N. High temperature fuel and steam electrolysis cells using proton conductive solid electrolytes. J. Power Sources 1982, 7, 293–301. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sakai, T.; Okuyama, Y. Proton-conducting oxide and applications to hydrogen energy devices. Pure Appl. Chem. 2013, 85, 427–435. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrad, S.; Traversa, E. Reversible Solid oxide fuel cells (R-SOFCs) with chemically stable proton-conducting oxides. Solid State Ion. 2015, 275, 101–105. [Google Scholar] [CrossRef]
- Kobayashi, T.; Abe, K.; Ukyo, Y.; Matsumoto, H. Study on current efficiency of steam electrolysis using a partial protonic conductor SrZr0.9Yb0.1O3−α. Solid State Ion. 2001, 138, 243–251. [Google Scholar] [CrossRef]
- Mullera, J.; Kreuer, K.D.; Maier, J.; Matsuo, S.; Ishigame, M. A conductivity and thermal gravimetric analysis of a Y-doped SrZrO3 single crystal. Solid State Ion. 1997, 97, 421–427. [Google Scholar] [CrossRef]
- Babilo, P.; Uda, T.; Haile, S.M. Processing of yttrium-doped barium zirconate for high proton conductivity. J. Mater. Res. 2007, 22, 1322–1330. [Google Scholar] [CrossRef]
- Ahmed, I.; Eriksson, S.G.; Ahlberg, E.; Knee, C.S.; Götlind, H.; Johansson, L.G.; Karlsson, M.; Matic, A.; Börjesson, L. Structural study and proton conductivity in Yb-doped BaZrO3. Solid State Ion. 2007, 178, 515–520. [Google Scholar] [CrossRef]
- Okuyama, Y.; Isa, K.; Lee, Y.S.; Sakai, T.; Matsumoto, H. Incorporation and conduction of proton in SrCe0.9−xZrxY0.1O3−δ. Solid State Ion. 2015, 275, 35–38. [Google Scholar] [CrossRef]
- Katahira, K.; Kohchi, Y.; Shimura, T.; Iwahara, H. Protonic conduction in Zr-substituted BaCeO3. Solid State Ion. 2000, 138, 91–98. [Google Scholar] [CrossRef]
- Gopalan, S.; Virkar, A.V. Thermodynamic stabilities of SrCeO3 and BaCeO3 using a molten salt method and galvanic cells. J. Electrochem. Soc. 1993, 140, 1060–1065. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Ogaki, K.; Nagoto, H. Nernstian hydrogen sensor using BaCeO3 - based, proton-conducting ceramics operative at 200°–900 °C. J. Electrochem. Soc. 1991, 138, 295–299. [Google Scholar] [CrossRef]
- Hamakawa, S.; Hibino, T.; Iwahara, H. Electrochemical hydrogen permeation in a proton-hole mixed conductor and its application to a membrane reactor. J. Electrochem. Soc. 1994, 141, 1720–1725. [Google Scholar] [CrossRef]
- Iwahara, H. Proton conducting ceramics and their applications. Solid State Ion. 1996, 86–88, 9–15. [Google Scholar] [CrossRef]
- Fabbri, E.; D’Epifanio, A.; Bartolomeo, E.D.; Licoccia, S.; Traversa, E. Tailoring the chemical stability of Ba(Ce0.8−xZrx)Y0.2O3−δ protonic conductors for intermediate temperature solid oxide fuel cells (IT-SOFCs). Solid State Ion. 2008, 179, 558–564. [Google Scholar] [CrossRef]
- Bu, J.; Jönsson, P.G.; Zhao, Z. Sintering behavior of the protonic conductors BaZrxCe0.8−xLn0.2O3−δ (x = 0.8, 0.5, 0.1; Ln = Y, Sm, Gd, Dy) during the solid-state reactive-sintering process. Ceram. Int. 2015, 41, 2558–2564. [Google Scholar] [CrossRef]
- Guo, Y.; Lin, Y.; Ran, R.; Shao, Z. Zirconium doping effect on the performance of proton-conducting BaZryCe0.8−yY0.2O3−δ (0.0 ≤ y ≤ 0.8) for fuel cell applications. J. Power Sources 2009, 193, 400–407. [Google Scholar] [CrossRef]
- Sawant, P.; Varma, S.; Wani, B.N.; Bharadwaj, S.R. Synthesis, stability and conductivity of BaCe0.8−xZrxY0.2O3−δ as electrolyte for proton conducting SOFC. Int. J. Hydrogen Energy 2012, 37, 3848–3856. [Google Scholar] [CrossRef]
- Lagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Insights on thermal and transport features of BaCe0.8−xZrxY0.2O3−δ proton-conducting materials. J. Power Sources 2015, 278, 436–444. [Google Scholar] [CrossRef]
- He, F.; Song, D.; Peng, R.; Meng, G.; Yang, S. Electrode performance and analysis of reversible solid oxide fuel cells with proton conducting electrolyte of BaCe0.5Zr0.3Y0.2O3−δ. J. Power Sources 2010, 195, 3359–3364. [Google Scholar] [CrossRef]
- Rao, Y.; Zhong, S.; He, F.; Wang, Z.; Peng, R.; Lu, Y. Cobalt-doped BaZrO3: A single phase air electrode material for reversible solid oxide cells. Int. J. Hydrogen Energy 2012, 37, 12522–12527. [Google Scholar] [CrossRef]
- Yoo, Y.; Lim, N. Performance and stability of proton conducting solid oxide fuel cells based on yttrium-doped barium cerate-zirconate thin-film electrolyte. J. Power Sources 2013, 229, 48–57. [Google Scholar] [CrossRef]
- Watanabe, M.; Uchida, H.; Yoshida, M. Effect of ionic conductivity of zirconia electrolytes on the polarization behavior of ceria-based anodes in solid oxide fuel cells. J. Electrochem. Soc. 1997, 144, 1739–1743. [Google Scholar] [CrossRef]
- Watanabe, M.; Uchida, H.; Shibata, M.; Mochizuki, N.; Amikura, K. High performance catalyzed-reaction layer for medium temperature operating solid oxide fuel cells. J. Electrochem. Soc. 1994, 141, 342–346. [Google Scholar] [CrossRef]
- Uchida, H.; Mochizuki, N.; Watanabe, M. High-performance electrode for medium-temperature operating solid oxide fuel cells polarization property of ceria-based anode with highly dispersed ruthenium catalysts in (H2+CO2+H2O) gas. J. Electrochem. Soc. 1996, 143, 1700–1704. [Google Scholar] [CrossRef]
- Suzuki, S.; Uchida, H.; Watanabe, M. Interaction of samaria-doped ceria anode with highly dispersed Ni catalysts in a medium-temperature solid oxide fuel cell during long-term operation. Solid State Ion. 2006, 177, 359–365. [Google Scholar] [CrossRef]
- Uchida, H.; Osuga, T.; Watanabe, M. High-performance electrode for medium-temperature solid oxide fuel cell control of microstructure of ceria-based anodes with highly dispersed ruthenium electrocatalysts. J. Electrochem. Soc. 1999, 146, 1677–1682. [Google Scholar] [CrossRef]
- Uchida, H.; Watanabe, S.; Tao, Y.; Osada, N.; Watanabe, M. Double Layer-Type Electrodes for Reversible Solid Oxide Fuel Cells Cell Design, Processing, and Performance. ECS Trans. 2007, 7, 365–371. [Google Scholar] [CrossRef]
- Uchida, H.; Puengjinda, P.; Miyano, K.; Shimura, K.; Nishino, H.; Kakinuma, K.; Brito, M.E.; Watanabe, M. Effect of Microstructure on Performances of Hydrogen and Oxygen Electrodes for Reversible SOEC/SOFC. ECS Trans. 2015, 68, 3307–3313. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Ricote, S.; Sullivan, N.P. Characterization of ionic transport through BaCe0.2Zr0.7Y0.1O3−δ membranes in galvanic and electrolytic operation. Int. J. Hydrogen Energy 2015, 40, 9278–9286. [Google Scholar] [CrossRef]
- Costa, R.; Grünbaum, N.; Berger, M.-H.; Dessemond, L.; Thorel, A. On the use of NiO as sintering additive for BaCe0.9Y0.1O3−α. Solid State Ion. 2009, 180, 891–895. [Google Scholar] [CrossRef]
- Gorbova, E.; Maragou, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Influence of sintering additives of transition metals on the properties of gadolinium-doped barium cerate. Solid State Ion. 2008, 179, 887–890. [Google Scholar] [CrossRef]
- Tao, S.; Irvine, J.T.S. Conductivity studies of dense yttrium-doped BaZrO3 sintered at 1325 °C. J. Solid State Chem. 2007, 180, 3493–3503. [Google Scholar] [CrossRef]
- Nikodemski, S.; Tong, J.; Duan, C.; O’Hayre, R. Ionic transport modification in proton conducting BaCe0.6Zr0.3Y0.1O3−δ with transition metal oxide dopants. Solid State Ion. 2016, 294, 37–42. [Google Scholar] [CrossRef]
- Kakinuma, K.; Uchida, M.; Kamino, T.; Uchida, H.; Watanabe, M. Synthesis and electrochemical characterization of Pt catalyst supported on Sn0.96Sb0.04O2−δ with a network structure. Electrochim. Acta 2011, 56, 2881–2887. [Google Scholar] [CrossRef]
- Senoo, Y.; Taniguchi, K.; Kakinuma, K.; Uchida, M.; Uchida, H.; Deki, S.; Watanabe, M. Cathodic performance and high potential durability of Ta-SnO2−δ-supported Pt catalysts for PEFC cathodes. Electrochem. Commun. 2015, 51, 37–40. [Google Scholar] [CrossRef]
- Senoo, Y.; Kakinuma, K.; Uchida, M.; Uchida, H.; Deki, S.; Watanabe, M. Improvements in electrical and electrochemical properties of Nb-doped SnO2−δ supports for fuel cell cathodes due to aggregation and Pt loading. RSC Adv. 2014, 4, 32180–32188. [Google Scholar] [CrossRef]
- Kakinuma, K.; Chino, Y.; Senoo, Y.; Uchida, M.; Kamino, T.; Uchida, H.; Deki, S.; Watanabe, M. Characterization of Pt catalysts on Nb-doped and Sb-doped SnO2−δ support materials with aggregated structure by rotating disk electrode and fuel cell measurements. Electrochim. Acta 2013, 110, 316–324. [Google Scholar] [CrossRef]
- Kakinuma, K.; Kim, I.-T.; Senoo, Y.; Yano, H.; Watanabe, M.; Uchida, M. Electrochemical oxidation of hydrolyzed poly oxymethylene-dimethyl ether by PtRu Catalysts on Nb-Doped SnO2−δ Supports for direct oxidation fuel cells. ACS Appl. Mater. Interfaces 2014, 6, 22138–22145. [Google Scholar] [CrossRef] [PubMed]
- Chino, Y.; Taniguchi, K.; Senoo, Y.; Kakinuma, K.; Hara, M.; Watanabe, M.; Uchida, M. Effect of added graphitized CB on both performance and durability of Pt/Nb-SnO2 cathodes for PEFCs. J. Electrochem. Soc. 2015, 162, F736–F743. [Google Scholar] [CrossRef]
- Chino, Y.; Kakinuma, K.; Tryk, D.A.; Watanabe, M.; Uchida, M. Influence of Pt loading and cell potential on the HF ohmic resistance of an Nb-Doped SnO2-supported Pt cathode for PEFCs. J. Electrochem. Soc. 2016, 163, F97–F105. [Google Scholar] [CrossRef]
- Osada, N.; Uchida, H.; Watanabe, M. Polarization behavior of SDC cathode with highly dispersed Ni catalysts for solid oxide electrolysis cells. J. Electrochem. Soc. 2006, 153, A816–A820. [Google Scholar] [CrossRef]
- Bi, L.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Nishino, H.; Kakinuma, K.; Brito, M.E.; Uchida, H. High durability of La0.6Sr0.4Co0.2Fe0.8O3−δ/samaria-doped ceria (SDC) composite oxygen electrode with SDC interlayer for reversible solid oxide fuel cell/solid oxide electrolysis cell. J. Ceram. Soc. Jpn. 2017, 125, 218–222. [Google Scholar] [CrossRef]
| x | Ni Loading Amount/vol % | Ni Particle Size/nm | Circumference Length of TPB/107 cm cm−2 (Electrode Area) |
|---|---|---|---|
| 0 | 10 | 48.3 ± 15.4 | 0.857 |
| 15 | 45.8 ± 20.6 | 1.49 | |
| 20 | 55.3 ± 21.2 | 1.45 | |
| 30 | 84.1 ± 47.3 | 0.444 | |
| 0.03 | 10 | 49.9 ± 19.3 | 0.792 |
| 15 | 52.9 ± 18.4 | 1.12 | |
| 20 | 58.2 ± 22.5 | 1.31 | |
| 30 | 58.7 ± 21.3 | 2.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, R.; Kakinuma, K.; Nishino, H.; Brito, M.E.; Gopalan, S.; Uchida, H. Synthesis and Evaluation of Ni Catalysts Supported on BaCe0.5Zr0.3−xY0.2NixO3−δ with Fused-Aggregate Network Structure for the Hydrogen Electrode of Solid Oxide Electrolysis Cell. Catalysts 2017, 7, 223. https://doi.org/10.3390/catal7070223
Nishikawa R, Kakinuma K, Nishino H, Brito ME, Gopalan S, Uchida H. Synthesis and Evaluation of Ni Catalysts Supported on BaCe0.5Zr0.3−xY0.2NixO3−δ with Fused-Aggregate Network Structure for the Hydrogen Electrode of Solid Oxide Electrolysis Cell. Catalysts. 2017; 7(7):223. https://doi.org/10.3390/catal7070223
Chicago/Turabian StyleNishikawa, Ryosuke, Katsuyoshi Kakinuma, Hanako Nishino, Manuel E. Brito, Srikanth Gopalan, and Hiroyuki Uchida. 2017. "Synthesis and Evaluation of Ni Catalysts Supported on BaCe0.5Zr0.3−xY0.2NixO3−δ with Fused-Aggregate Network Structure for the Hydrogen Electrode of Solid Oxide Electrolysis Cell" Catalysts 7, no. 7: 223. https://doi.org/10.3390/catal7070223






