Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
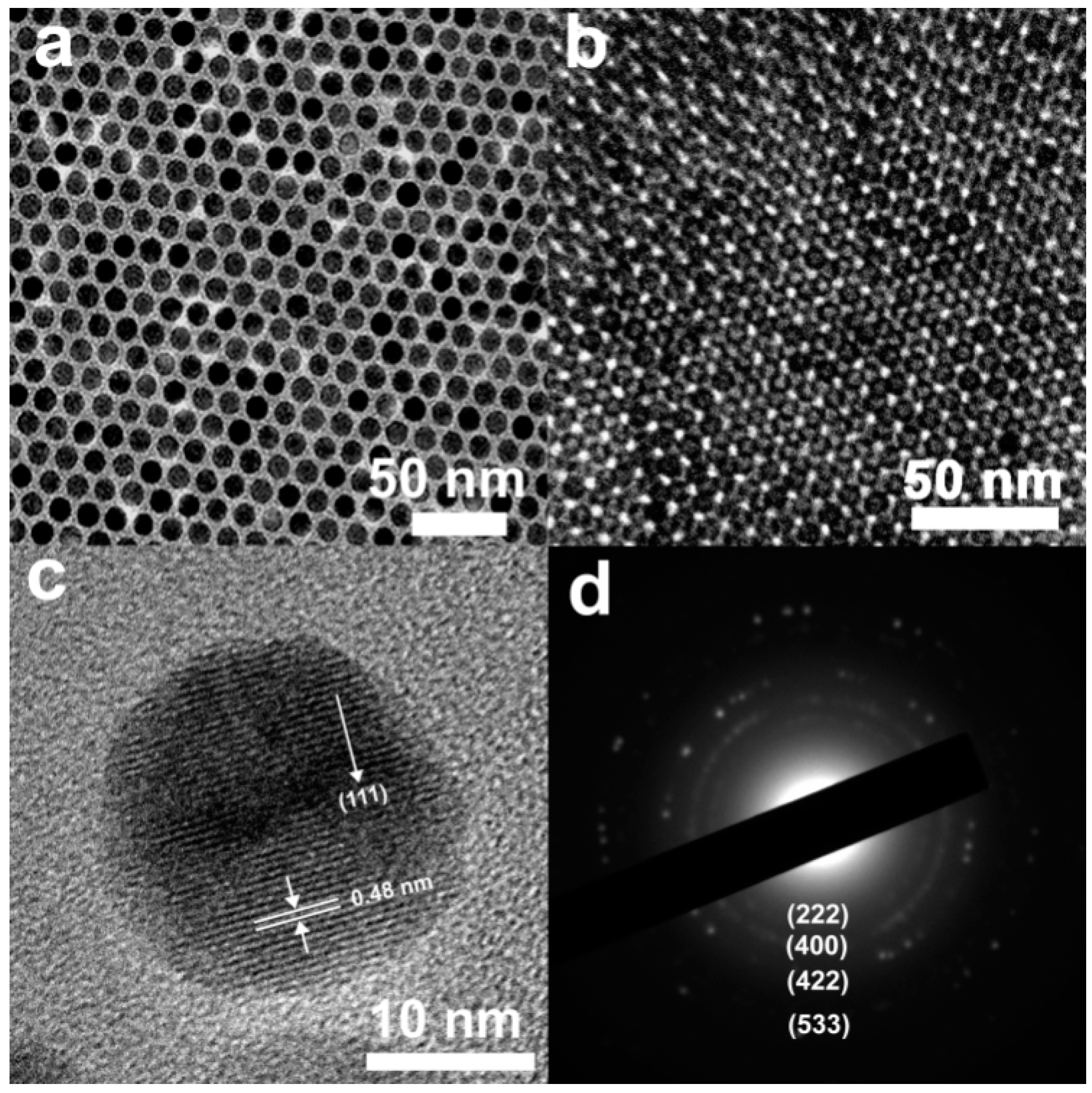
3.1. Synthesis of Magnetic Fe3O4nanoparticles
3.2. Characterization
3.3. Representative Procedure for Catalytic Reactions
- 3-morpholinopropanamide (Table 2, entry 1). 1H NMR (600 MHz, CDCl3) (ppm): δ 7.92 (s, 1H), 5.64 (s, 1H), 3.90–3.80 (m, 2H), 3.72 (s, 2H), 2.41–2.63 (m, 8H).
- 3-(pyrrolidin-1-yl)propanamide (Table 2, entry 2). 1H NMR (600 MHz, CDCl3) (ppm): δ 8.00 (s, 1H), 5.38 (s, 1H), 2.86 (t, J = 6.2 Hz, 2H), 2.70 (s, 4H), 2.52 (t, J = 6.2 Hz, 2H), 1.91–1.80 (m, 4H).
- 3-(2-methylpiperidin-1-yl)propanamide (Table 2, entry 3). 1H NMR (600 MHz, CDCl3) (ppm): δ 8.38 (s, 1H), 5.56 (s, 1H), 3.72 (q, J = 7.0 Hz, 2H), 3.40 (d, J = 11.9 Hz, 2H), 2.81 (t, J = 11.0 Hz, 2H), 1.89 (s, 1H), 1.67 (d, J = 12.7 Hz, 2H), 1.49 (d, J = 6.5 Hz, 2H), 1.3 (t, J = 7.0 Hz, 2H), 1.12 (d, J = 6.3 Hz, 3H).
- 3-(4-methylpiperidin-1-yl)propanamide (Table 2, entry 4). 1H NMR (600 MHz, CDCl3) (ppm): δ 8.47 (s, 1H), 5.48 (s, 1H), 3.73 (q, J = 7.0 Hz, 2H), 2.94 (d, J = 11.4 Hz, 2H), 2.62–2.56 (m, 2H), 2.40 (t, J = 6.0 Hz, 2H), 1.98 (t, J = 12.3 Hz, 2H), 1.68 (d, J = 13.4 Hz, 1H), 1.25 (t, J = 8.3 Hz, 2H), 0.94 (d, J = 6.5 Hz, 3H).
- 3-(4-ethylpiperazin-1-yl)propanamide (Table 2, entry 5). 1H NMR (600 MHz, CDCl3) (ppm): δ 8.09 (s, 1H), 5.47 (s, 1H), 2.68–2.60 (m, 3H), 2.47–2.37 (m, 11H), 1.10 (t, J = 7.2 Hz, 3H).
- 3-(benzylamino)propanamide (Table 2, entry 6). 1H NMR (600 MHz, CDCl3) (ppm): δ 7.49(s, 1H), 7.23–7.25 (m, 4H), 6.26 (s, 1H), 3.71 (s, 4H), 1.33 (d, J = 6.9 Hz, 2H), 1.23 (t, J = 6.7 Hz, 2H).
- 3-morpholino-1-morpholinopropan-1-one (Table 2, entry 7). 1H NMR (600 MHz, CDCl3) (ppm): δ 3.71 (t, J = 4.5 Hz, 4H), 3.68–3.60 (m, 2H), 3.60 (s, 4H), 3.45 (d, J = 4.7 Hz, 2H), 2.74 (t, J = 7.4 Hz, 2H), 2.52 (d, J = 19.6 Hz, 4H), 2.36 (s, 4H).
- 1-morpholino-3-(pyrrolidin-1-yl)propan-1-one (Table 2, entry 8). 1H NMR (600 MHz, CDCl3) (ppm): δ 3.71–3.60 (m, 6H), 3.57 (d, J = 4.7 Hz, 2H), 2.93 (t, J = 7.3 Hz, 6H), 2.04 (s, 4H).
- 3-(2-methylpiperidin-1-yl)-1-morpholinopropan-1-one (Table 2, entry 9). 1H NMR (600 MHz, CDCl3) (ppm): δ 3.70 (q, J = 7.0 Hz, 4H), 3.41 (d, J = 12.7 Hz, 4H), 2.79 (t, J = 11.1 Hz, 2H), 2.52 (d, J = 7.8 Hz, 2H), 2.41 (s, 2H), 1.85 (s, 1H), 1.68 (s, 1H), 1.46 (d, J = 9.8 Hz, 2H), 1.42 (t, J = 7.0 Hz, 2H), 1.06 (s, 3H).
- 3-(4-methylpiperidin-1-yl)-1-morpholinopropan-1-one (Table 2, entry 10). 1H NMR (600 MHz, CDCl3) (ppm): δ 3.76–3.67 (m, 1H), 3.67–3.58 (m, 4H), 3.53–3.45 (m, 5H), 2.93 (d, J = 11.5 Hz, 2H), 2.79–2.71 (m, 2H), 2.63–2.55 (m, 2H), 2.06 (t, J = 11.0 Hz, 2H), 1.96 (s, 1H), 1.65 (d, J = 13.5 Hz, 2H), 0.93 (d, J = 6.5 Hz, 3H).
- 3-(4-ethylpiperazin-1-yl)-1-morpholinopropan-1-one (Table 2, entry 11). 1H NMR (600 MHz, CDCl3) (ppm): δ 3.69–3.68 (m, 6H), 3.49 (d, J = 5.0 Hz, 2H), 2.53 (d, J = 8.0 Hz, 2H), 2.45–2.29 (m, 12H), 1.11 (t, J = 7.2 Hz, 3H).
- 3-(benzylamino)-1-morpholinopropan-1-one (Table 2, entry 12). 1H NMR (600 MHz, CDCl3) (ppm): δ 7.49 (d, J = 6.2 Hz, 5H), 4.12 (s, 2H), 3.64 (d, J = 3.6 Hz, 4H), 3.54 (s, 4H), 2.97 (s, 2H), 2.03 (s, 2H), 1.24 (s, 1H).
- 3-morpholin-4-yl-N-phenyl-propionamide (Table 2, entry 13). 1H NMR (400 MHz, CDCl3) (ppm): δ 11.31 (s, 1H), 7.53 (d, J = 8.6 Hz, 2H), 7.30 (d, J = 9.9 Hz, 2H), 7.07 (t, J = 7.4 Hz, 1H), 3.04 (d, J = 11.7 Hz, 2H), 2.72–2.64 (m, 4H), 2.55–2.47 (m, 2H), 2.07 (t, J = 10.8 Hz, 4H).
- N-phenyl-3-pyrrolidin-1-yl-propionamide (Table 2, entry 14).1H NMR (600 MHz, CDCl3) (ppm): δ 9.97 (s, 1H), 7.66 (d, J = 7.9 Hz, 2H), 7.29 (d, J = 7.0 Hz, 2H), 7.09 (t, J = 7.3 Hz, 1H), 3.73 (q, J = 7.0 Hz, 2H), 3.11 (t, J = 6.5 Hz, 2H), 2.08 (s, 4H), 1.25 (t, J = 7.0 Hz, 4H).
- 3-(4-methyl-piperidin-1-yl)-N-phenyl-propionamide (Table 2, entry 15). 1H NMR (400 MHz, CDCl3) (ppm): δ 10.76 (s, 1H), 7.53 (d, J = 7.9 Hz, 2H), 7.32 (t, J = 7.8 Hz, 2H), 7.08 (t, J = 7.4 Hz, 1H), 3.82 (s, 2H), 2.87 (d, J = 23.3 Hz, 2H), 2.86 (d, J = 37.1 Hz, 2H), 2.71 (d, J = 29.6, 23.8 Hz, 2H), 1.74 (m, 1H), 1.25 (s, 4H), 0.86 (s, 3H).
- 3-benzylamino-N-phenyl-propionamide (Table 2, entry 16). 1H NMR (600 MHz, CDCl3) (ppm): δ 10.44 (s, 1H), 7.52 (d, J = 7.8 Hz, 2H), 7.41–7.25 (m, 7H), 7.07 (s, 1H), 3.88 (s, 2H), 2.97 (m, 2H), 2.59–2.51 (m, 2H), 1.94 (d, J = 6.7 Hz, 1H).
- 3-butyl-N-phenyl-propionamide (Table 2, entry 17). 1H NMR (600 MHz, CDCl3) (ppm): δ 10.51 (s, 1H), 7.45 (d, J = 7.9 Hz, 2H), 7.24 (m, 2H), 6.97 (t, J = 7.4 Hz, 1H), 2.95 (m, 2H), 2.57 (t, J = 7.0 Hz, 4H), 1.37 (d, J = 8.6 Hz, 4H), 1.23 (s, 1H), 0.78 (s, 3H).
- 3-propyl-N-phenyl-propionamide (Table 2, entry 18). 1H NMR (600 MHz, CDCl3) (ppm): δ9.44 (s, 1H), 7.51 (s, 2H), 7.25 (s, 2H), 7.02 (s, 1H), 2.95 (s, 2H), 2.55 (d, J = 1.2 Hz, 4H), 1.57 (s, 1H), 1.42 (s, 2H), 0.86 (t, J = 7.3 Hz, 3H).
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Jesús, E.; Flores, J.C. Dendrimers: Solutions for catalyst separation and recycling—A review. Ind. Eng. Chem. Res. 2008, 47, 7968–7981. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, D.F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Liebscher, J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar]
- Köhler, K.; Heidenreich, R.G.; Krauter, J.G.E.; Pietsch, J. Highly active palladium/activated carbon catalysts for heck reactions: Correlation of activity, catalyst properties, and Pd leaching. Chem. Eur. J. 2002, 8, 622–631. [Google Scholar] [CrossRef]
- Yu, J.; Shen, A.; Cao, Y.; Lu, G. Preparation of Pd-Diimine@SBA-15 and its catalytic performance for the Suzuki coupling reaction. Catalysts 2016, 6, 181. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Weaver, D.W.; Ying, J.Y. Heterogeneous Heck catalysis with palladium-grafted molecular sieves. J. Am. Chem. Soc. 1998, 120, 12289–12296. [Google Scholar] [CrossRef]
- Stevens, P.D.; Li, G.F.; Fan, J.D.; Yen, M.; Gao, Y. Recycling of homogeneous Pd catalysts using superparamagnetic nanoparticles as novel soluble supports for Suzuki, Heck, and Sonogashira cross-coupling reactions. Chem. Commun. 2005, 4435–4437. [Google Scholar] [CrossRef] [PubMed]
- Papp, A.; Galbacs, G.; Molnar, R. Recyclable ligand-free mesoporous heterogeneous Pd catalysts for Heck coupling. Tetrahedron Lett. 2005, 46, 7725–7728. [Google Scholar] [CrossRef]
- Wight, A.P.; Davis, M.E. Design and preparation of organic−inorganic hybrid catalysts. Chem. Rev. 2002, 102, 3589–3613. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Chai, W.; Zhang, F.; Chen, J. Water-medium Ullmann reaction over a highly active and selective Pd/Ph-SBA-15 catalyst. Green Chem. 2007, 9, 1223–1228. [Google Scholar] [CrossRef]
- Crudden, C.M.; Sateesh, M.; Lewis, R. Mercaptopropyl-modified mesoporous silica: A remarkable support for the preparation of a reusable, heterogeneous palladium catalyst for coupling reactions. J. Am. Chem. Soc. 2005, 127, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Prockl, S.S.; Kleist, W.; Gruber, M.A.; Kohler, K. In situ generation of highly active dissolved palladium species from solid catalysts—A concept for the activation of aryl chlorides in the Heck reaction. Angew. Chem. Int. Ed. 2004, 43, 1881–1882. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Enders, D. New N-heterocyclic carbene palladium complex/ionic liquid matrix immobilized on silica: Application as recoverable catalyst for the Heck reaction. Org. Lett. 2006, 8, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; de Vries, J.G. Ligand-free Heck reactions using low Pd-loading. Chem. Commun. 2004, 35, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Q.; Wei, H.; Wang, J.; Cho, M.; Cho, H.; Terasaki, O.; Wan, Y. Aggregation-free gold nanoparticles in ordered mesoporous carbons: Toward highly active and stable heterogeneous catalysts. J. Am. Chem. Soc. 2013, 135, 11849–11860. [Google Scholar] [CrossRef] [PubMed]
- Sydnes, M.O. The Use of palladium on magnetic support as catalyst for Suzuki–Miyaura cross-coupling reactions. Catalysts 2017, 7, 35. [Google Scholar] [CrossRef]
- Carrettin, S.; Blanco, M.C.; Corma, A.; Hashmi, A.S. K. Heterogeneous gold-catalysed synthesis of phenols. Adv. Synth. Catal. 2006, 348, 1283–1288. [Google Scholar] [CrossRef]
- Witham, C.A.; Huang, W.; Tsung, C.K.; Kuhn, J.N.; Somorjai, G.A.; Toste, F.D. Converting homogeneous to heterogeneous in electrophilic catalysis using monodisperse metal nanoparticles. Nat. Chem. 2010, 2, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, H.; Zhao, Q.; Klingstedt, M.; Terasaki, O.; Zhao, D. Ordered mesoporous Pd/silica—Carbon as a highly active heterogeneous catalyst for coupling reaction of chlorobenzene in aqueous media. J. Am. Chem. Soc. 2009, 131, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.V.; Holuigue, A.; Thathagar, M.B.; ten Elshofand, E.; Rothenberg, G. Ion- and atom-leaching mechanisms from palladium nanoparticles in cross-coupling reactions. Chem. Eur. J. 2007, 13, 6908–6913. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Xue, W.; Guan, B.T.; Shi, F.B.; Shi, Z.J.; Jiang, H.; Yan, C.H. A conceptual translation of homogeneous catalysis into heterogeneous catalysis: Homogeneous-like heterogeneous gold nanoparticle catalyst induced by ceria supporter. Nanoscale 2013, 5, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis; Pergamon: Oxford, UK, 1992. [Google Scholar]
- Nising, C.F.; Bräse, S. Recent developments in the field of oxa-Michael reactions. Chem. Soc. Rev. 2012, 41, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Nising, C.F.; Bräse, S. The oxa-Michael reaction: From recent developments to applications in natural product synthesis. Chem. Soc. Rev. 2008, 37, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Vaxelaire, C.; Winter, P.; Christmann, M. One-pot reactions accelerate the synthesis of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2011, 50, 3605–3637. [Google Scholar] [CrossRef] [PubMed]
- Busacca, C.A.; Fandrick, D.R.; Song, J.J.; Senanayake, C.H. The growing impact of catalysis in the pharmaceutical industry. Adv. Synth. Catal. 2011, 353, 1825–1864. [Google Scholar] [CrossRef]
- Harrison, C.L.; Krawiec, M.; Forslund, R.E.; Nugent, W.A. Beyond the chiral pool: A general approach to β-amino-α-keto amides. Tetrahedron 2011, 67, 41–47. [Google Scholar] [CrossRef]
- Ying, A.; Li, Z.; Yang, J.; Liu, S.; Xu, S.; Yan, H.; Wu, C. DABCO-based ionic liquids: Recyclable catalysts for aza-Michael addition of α,β-unsaturated amides under solvent-free conditions. J. Org. Chem. 2014, 79, 6510–6516. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Reichard, G.A. Enantioselective and practical syntheses of R- and S-fluoxetines. Tertrahedron Lett. 1989, 30, 5207–5210. [Google Scholar] [CrossRef]
- Duan, J.; Li, P. Asymmetric or ganocatalysis mediated by primary amines derived from cinchona alkaloids: Recent advances. Catal. Sci. Technol. 2014, 4, 311–320. [Google Scholar] [CrossRef]
- Schmidt, R.R.; Vankar, Y.D. 2-Nitroglycals as powerful glycosyl donors: Application in the synthesis of biologically important molecules. Acc. Chem. Res. 2008, 41, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; Lucchini, V.; Noe, M.; Perosa, A. Ionic liquids made with dimethyl carbonate: Solvents as well as boosted basic catalysts for the Michael reaction. Chem. Eur. J. 2009, 15, 12273–12282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, I.C.; Bergman, R.G.; Toste, F.D. Phosphine-catalyzed hydration and hydroalkoxylation of activated olefins: Use of a strong nucleophile to generate a strong base. J. Am. Chem. Soc. 2003, 125, 8696–8697. [Google Scholar] [CrossRef] [PubMed]
- Kisanga, P.B.; Illankumaran, P.; Fetterley, B.M.; Verkade, J.G. P(RNCH2CH2)3N: Efficient 1,4-addition catalysts. J. Org. Chem. 2002, 67, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Rice, P.M.; Wang, S.X.; Sun, S. Shape-controlled synthesis and shape-induced texture of MnFe2O4 nanoparticles. J. Am. Chem. Soc. 2004, 126, 11458–11459. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, E.; Shemer, G.; Markovich, G.; Markovich, G. Optimizing cobalt ferrite nanocrystal synthesis using a magneto-optical probe. Chem. Mater. 2006, 18, 465–470. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Wang, Y.; Padhan, P.; Gupta, A. A facile thermolysis route to monodisperse ferrite nanocrystals. J. Am. Chem. Soc. 2007, 129, 12374–12375. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, Z.J. Shape control and associated magnetic properties of spinel cobalt ferrite nanocrystals. J. Am. Chem. Soc. 2004, 126, 6164–6168. [Google Scholar] [CrossRef] [PubMed]
- Vestal, C.R.; Song, Q.; Zhang, Z.J. Effects of interparticle interactions upon the magnetic properties of CoFe2O4 and MnFe2O4 nanocrystals. J. Phys. Chem. B 2004, 108, 18222–18227. [Google Scholar] [CrossRef]
- Xu, D.Q.; Luo, S.P.; Wang, Y.F.; Xia, A.B.; Yue, H.D.; Wang, L.P.; Xu, Z.Y. Organocatalysts wrapped around by Poly(ethylene glycol)s (PEGs): A unique host–guest system for asymmetric Michael addition reactions. Chem. Commun. 2007, 4393–4395. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.R.; Dumbre, D.K.; Patil, S.K. FeCl3/montmorillonite K10 as an efficient catalyst for solvent-free aza-Michael reaction between amine and α,β-unsaturated compounds. RSC Adv. 2012, 2, 7061–7065. [Google Scholar] [CrossRef]
- Yang, L.; Xu, L.W.; Xia, C.G. Highly efficient KF/Al2O3-catalyzed versatile hetero-Michael addition of nitrogen, oxygen, and sulfur nucleophiles to α,β-ethylenic compounds. Tetrahedron Lett. 2005, 46, 3279–3282. [Google Scholar] [CrossRef]
- Surendra, K.; Krishnaveni, N.S.; Sridhar, R.; Rao, K.R. β-Cyclodextrin promoted aza-Michael addition of amines to conjugated alkenes in water. Tetrahedron Lett. 2006, 47, 2125–2127. [Google Scholar] [CrossRef]
- Perin, G.; Borges, E.L.; Rosa, P.C.; Carvalho, P.N.; Lenardão, E.J. Simple cleavage of diorganyl diselenides with NaBH4/PEG-400 and direct Michael addition to electron-deficient alkenes. Tetrahedron Lett. 2013, 54, 1718–1721. [Google Scholar] [CrossRef]
- Wang, X.; Quan, Z.; Zhang, Z. Michael additions of dihydropyrimidines and 2-amino-1,3,4-thiadiazoles to α,β-ethylenic compounds: Using polyethylene glycols as a green reaction media. Tetrahedron 2007, 63, 8227–8233. [Google Scholar] [CrossRef]
- Alleti, R.; Woon, S.O.; Perambuduru, M.; Ramena, C.V.; Reddy, V.P. Imidazolium-based polymer supported gadolinium triflate as a heterogeneous recyclable Lewis acid catalyst for Michael additions. Tetrahedron Lett. 2008, 49, 3466–3470. [Google Scholar] [CrossRef]
- Jin, J.; Oskam, P.C.; Karmee, S.K.; Straathof, A.J.; Hanefeld, U. MhyADH catalysed Michael addition of water and in situ oxidation. Chem. Commun. 2010, 46, 8588–8590. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.F.; Guan, Z.; He, Y.H. The lipase-catalyzed asymmetric C–C Michael addition. J. Mol. Catal. B Enzym. 2011, 68, 240–244. [Google Scholar] [CrossRef]
- Madalińska, L.; Kwiatkowska, M.; Cierpiał, T.; Kiełbasiński, P. Investigations on enzyme catalytic promiscuity: The first attempts at a hydrolytic enzyme-promoted conjugate addition of nucleophiles to α,β-unsaturated sulfinyl acceptors. J. Mol. Catal. B Enzym. 2012, 81, 25–30. [Google Scholar] [CrossRef]
- Ying, A.; Liu, S.; Ni, Y.; Qiu, F.; Xu, S.; Tang, W. Ionic tagged DABCO grafted on magnetic nanoparticles: A water-compatible catalyst for the aqueous aza-Michael addition of amines to α,β-unsaturated amides. Catal. Sci. Technol. 2014, 4, 2115–2125. [Google Scholar] [CrossRef]




| Entry | Acryl Amide | Amine | Catalyst | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  | - | 38.1 |
| 2 |  |  | Fe3O4 nanoparticles | 89.0 |
| 3 |  |  | - | 36.2 |
| 4 |  |  | Fe3O4 nanoparticles | 85.2 |
| 5 |  |  | - | 39.6 |
| 6 |  |  | Fe3O4 nanoparticles | 93.2 |

| Entry | Acryl Amides | Amine | Product | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  |  | 89.0 |
| 2 |  |  |  | 90.6 |
| 3 |  |  |  | 90.4 |
| 4 |  |  |  | 91.7 |
| 5 |  |  |  | 90.1 |
| 6 |  |  |  | 80.2 |
| 7 |  |  |  | 85.2 |
| 8 |  |  |  | 86.9 |
| 9 |  |  |  | 84.3 |
| 10 |  |  |  | 85.6 |
| 11 |  |  |  | 80.2 |
| 12 |  |  |  | 90.1 |
| 13 |  |  |  | 93.2 |
| 14 |  |  |  | 81.2 |
| 15 |  |  |  | 84.6 |
| 16 |  |  |  | 80.1 |
| 17 |  |  |  | 78.6 |
| 18 |  |  |  | 77.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-X.; Luo, D.; Li, M.-M.; Xing, X.-F.; Ma, Z.-Z.; Xu, H. Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field. Catalysts 2017, 7, 219. https://doi.org/10.3390/catal7070219
Li Z-X, Luo D, Li M-M, Xing X-F, Ma Z-Z, Xu H. Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field. Catalysts. 2017; 7(7):219. https://doi.org/10.3390/catal7070219
Chicago/Turabian StyleLi, Zhen-Xing, Dan Luo, Ming-Ming Li, Xiao-Fei Xing, Zheng-Zheng Ma, and Hao Xu. 2017. "Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field" Catalysts 7, no. 7: 219. https://doi.org/10.3390/catal7070219








