Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Hf(OTf)4 Lewis Acid in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Ionic Radii of Lewis Acidic Metal Salts on the Catalytic Performance
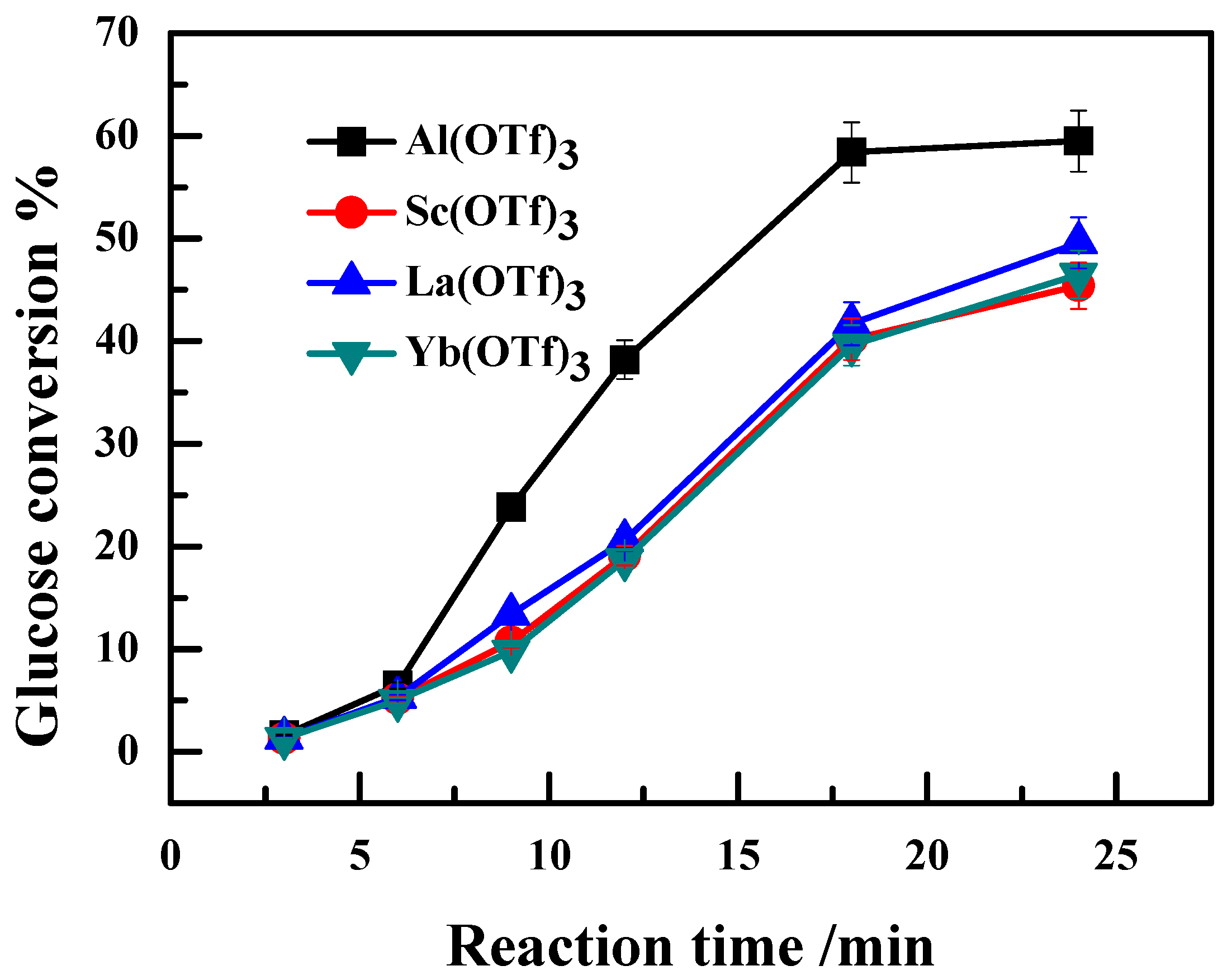
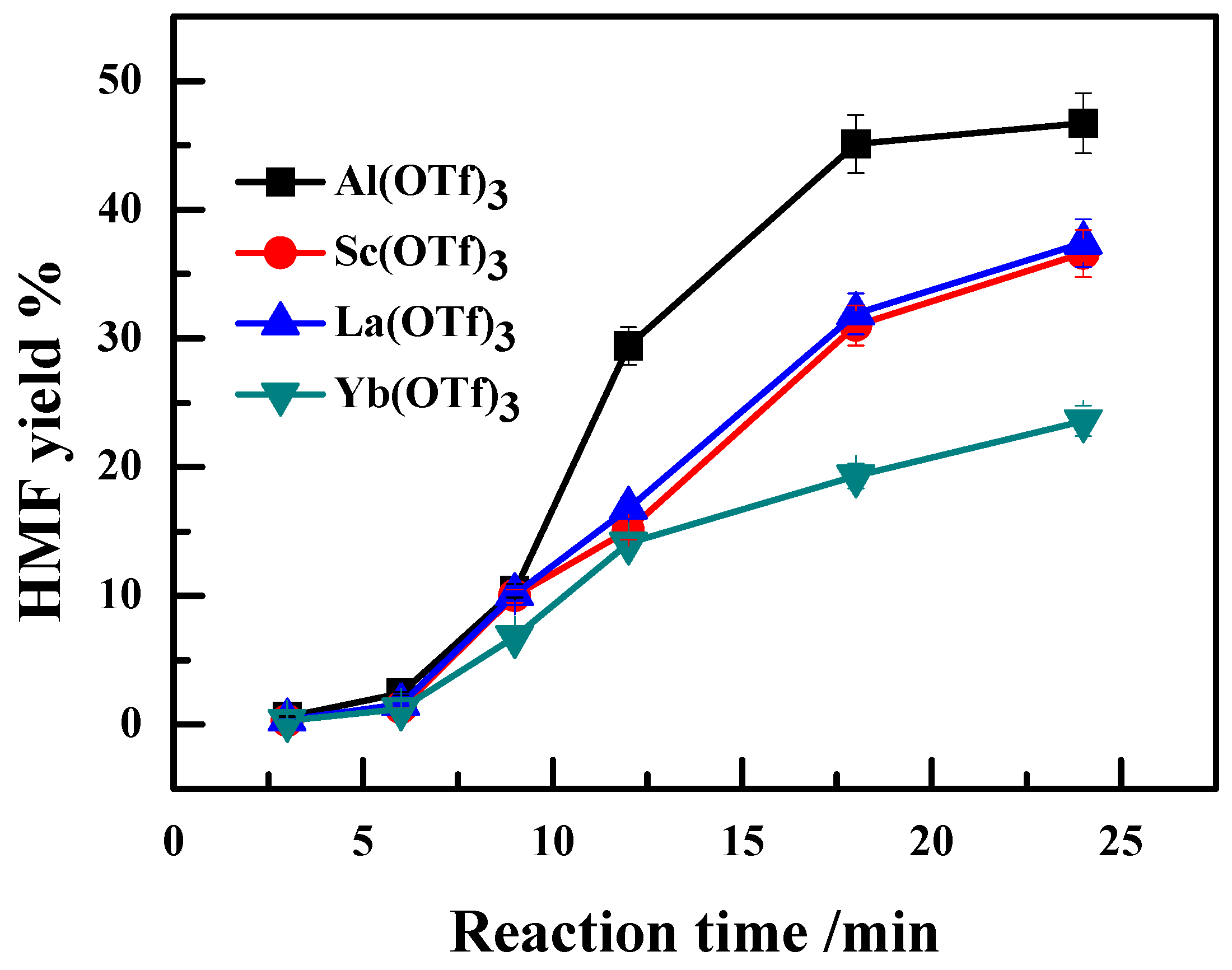
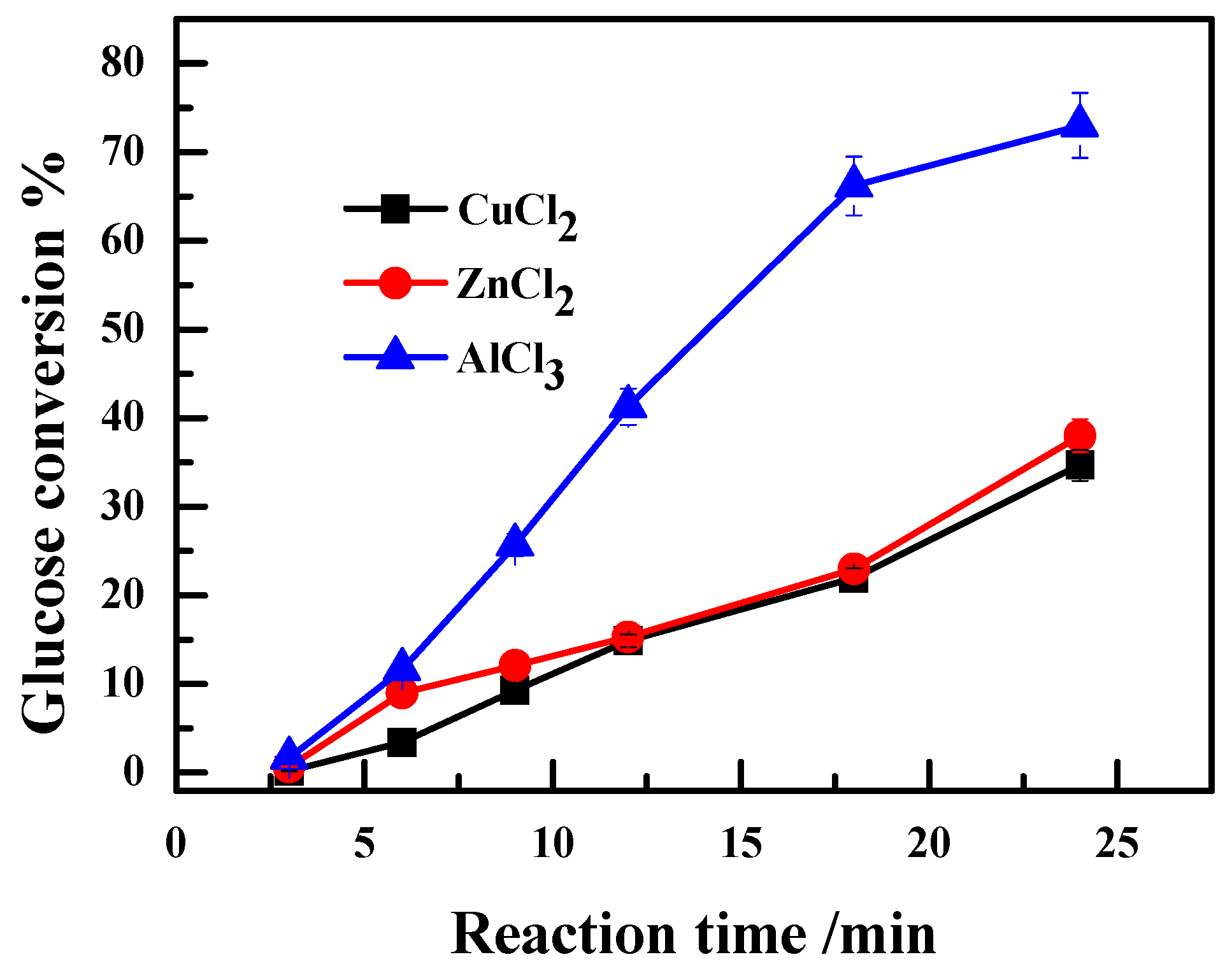
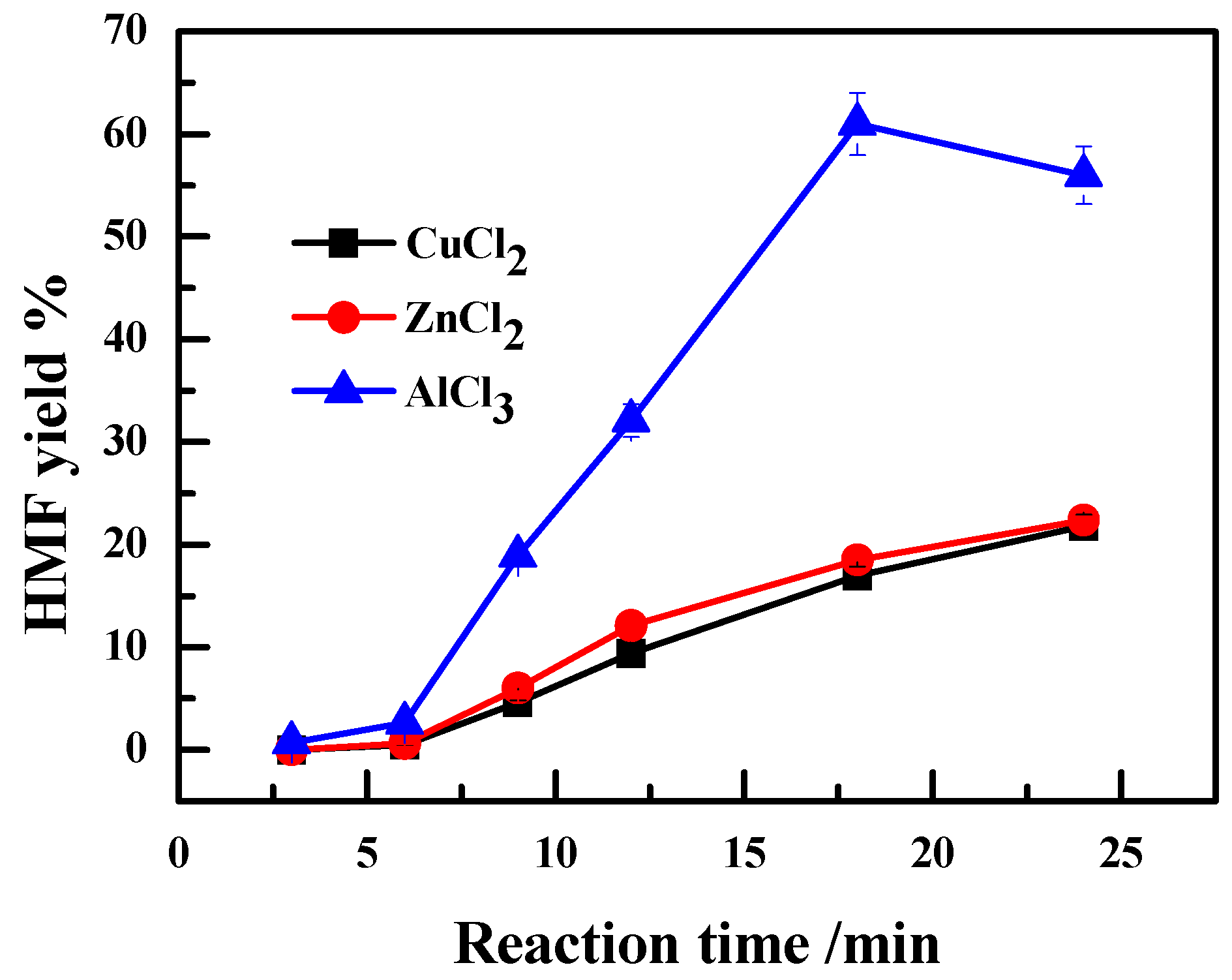
2.2. Effect of the Anion Type of Lewis Acidic Metal Salts on the Catalytic Performance
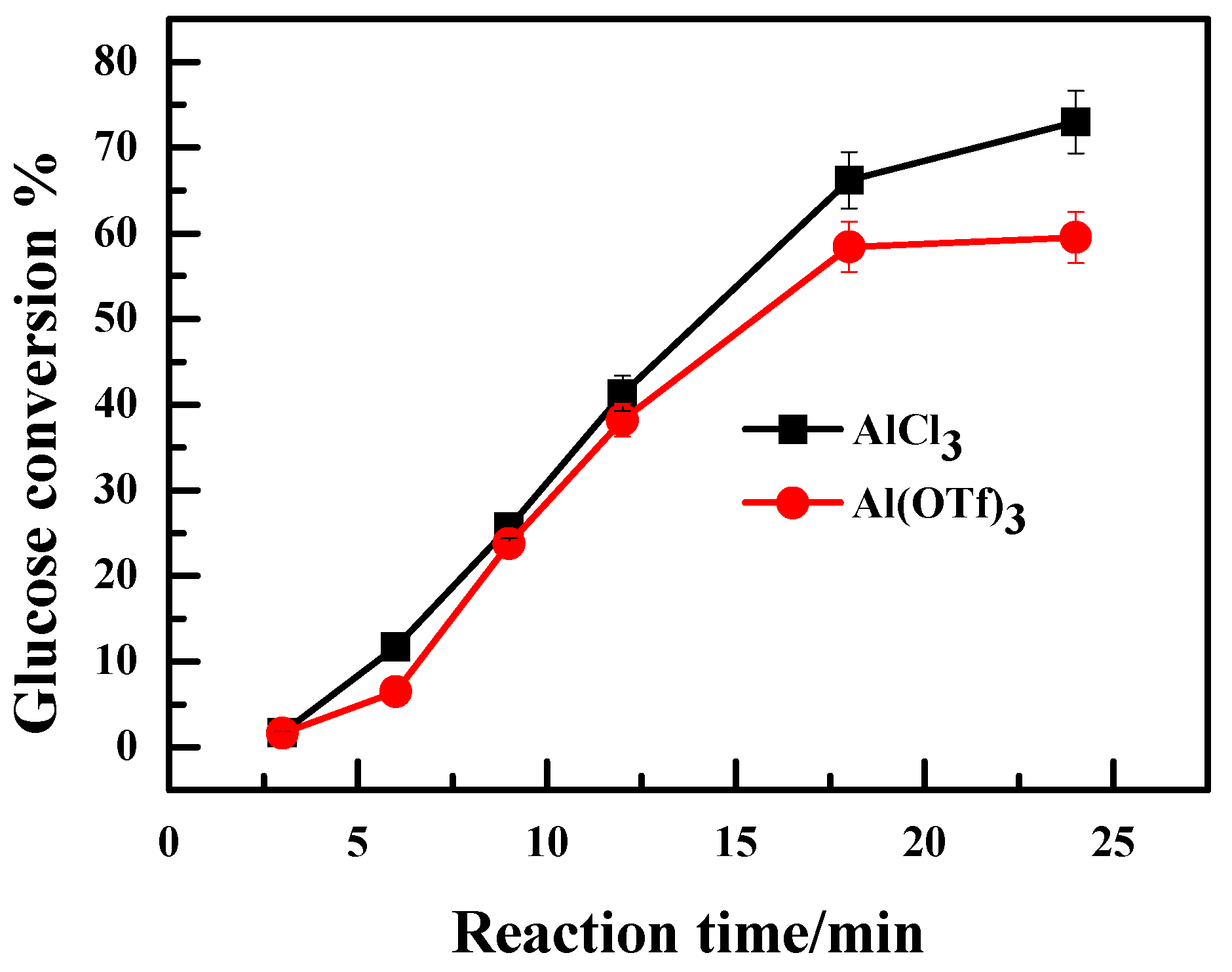
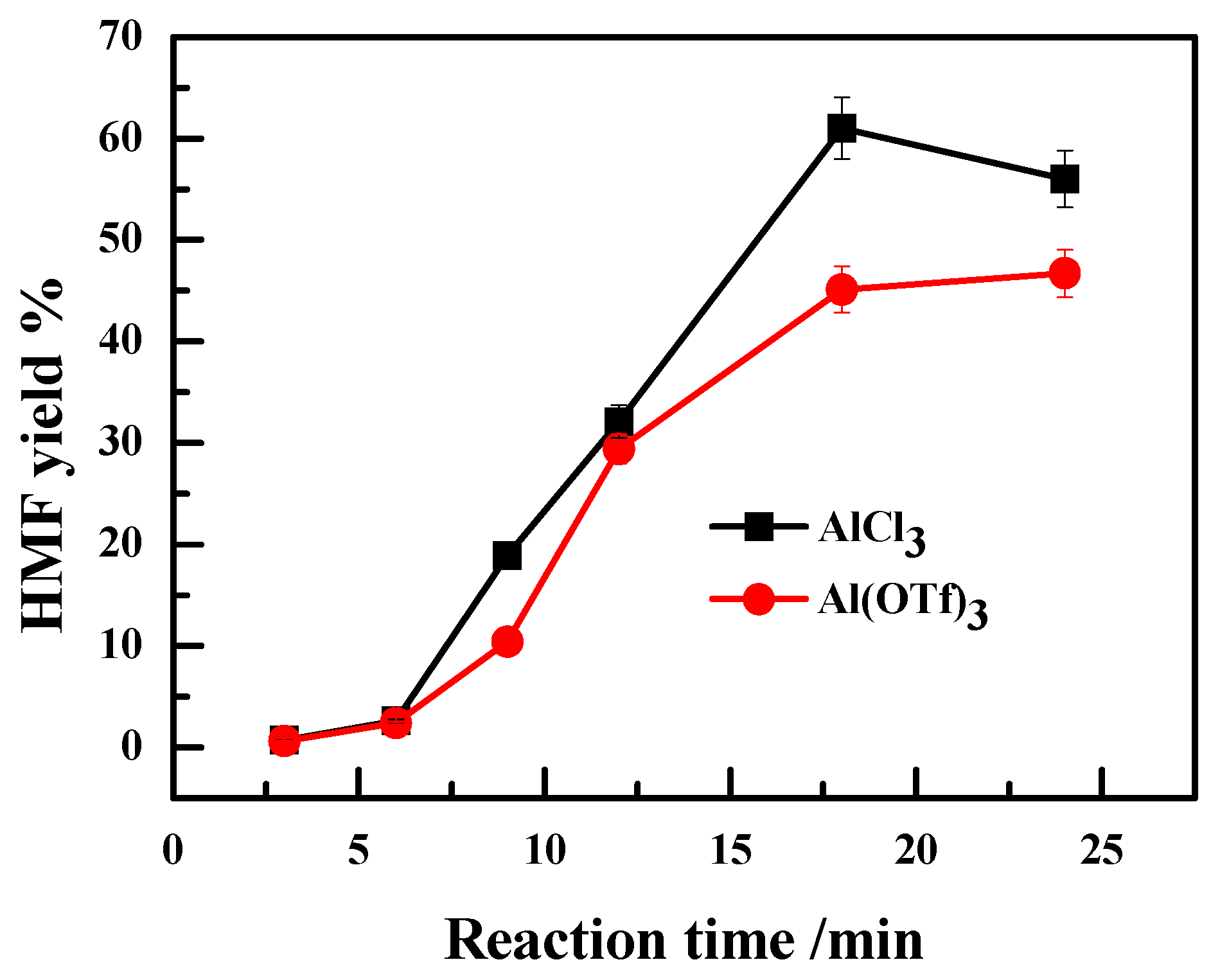
2.3. Effect of Valence State of Lewis Acidic Metal Salts on the Catalytic Performance
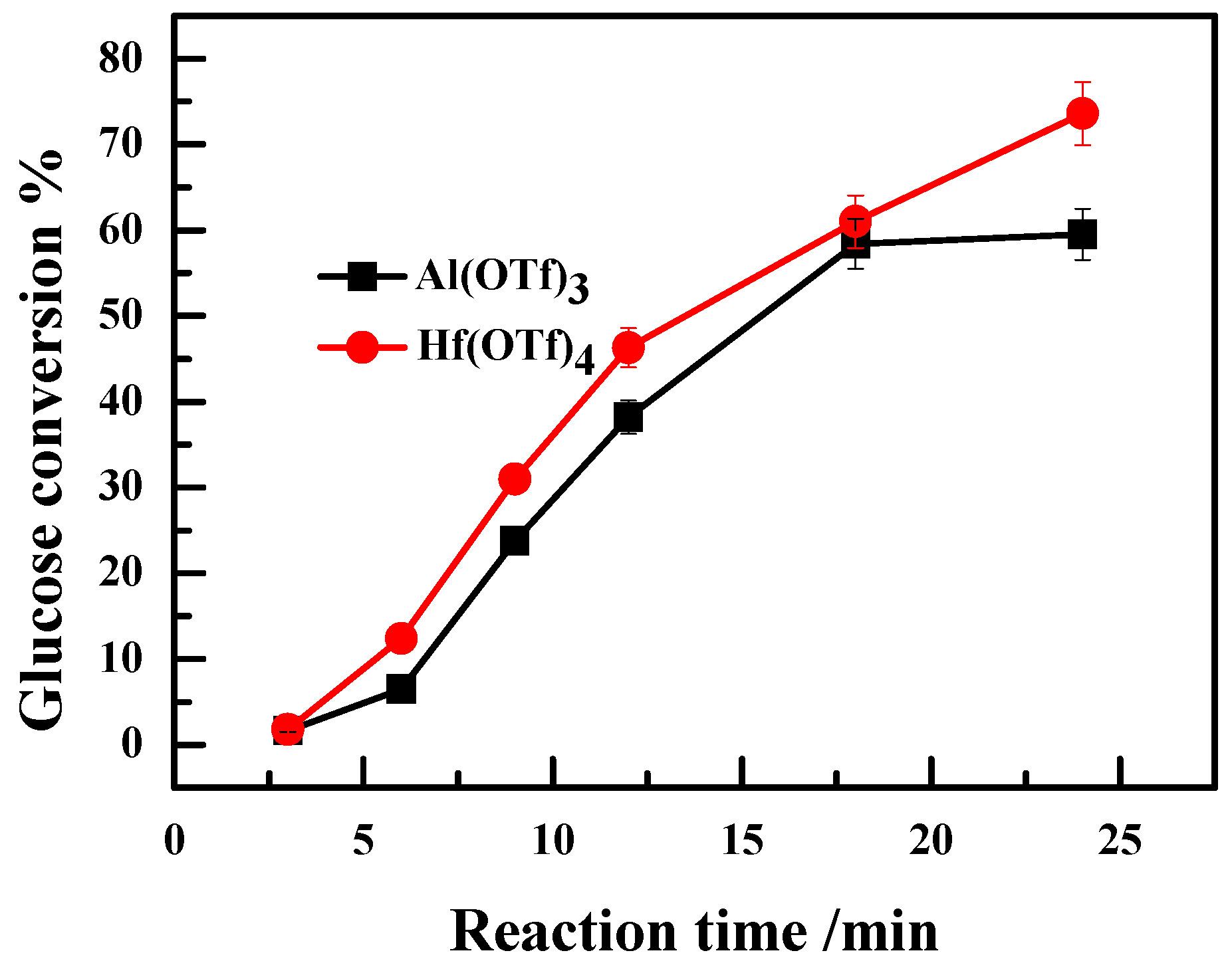
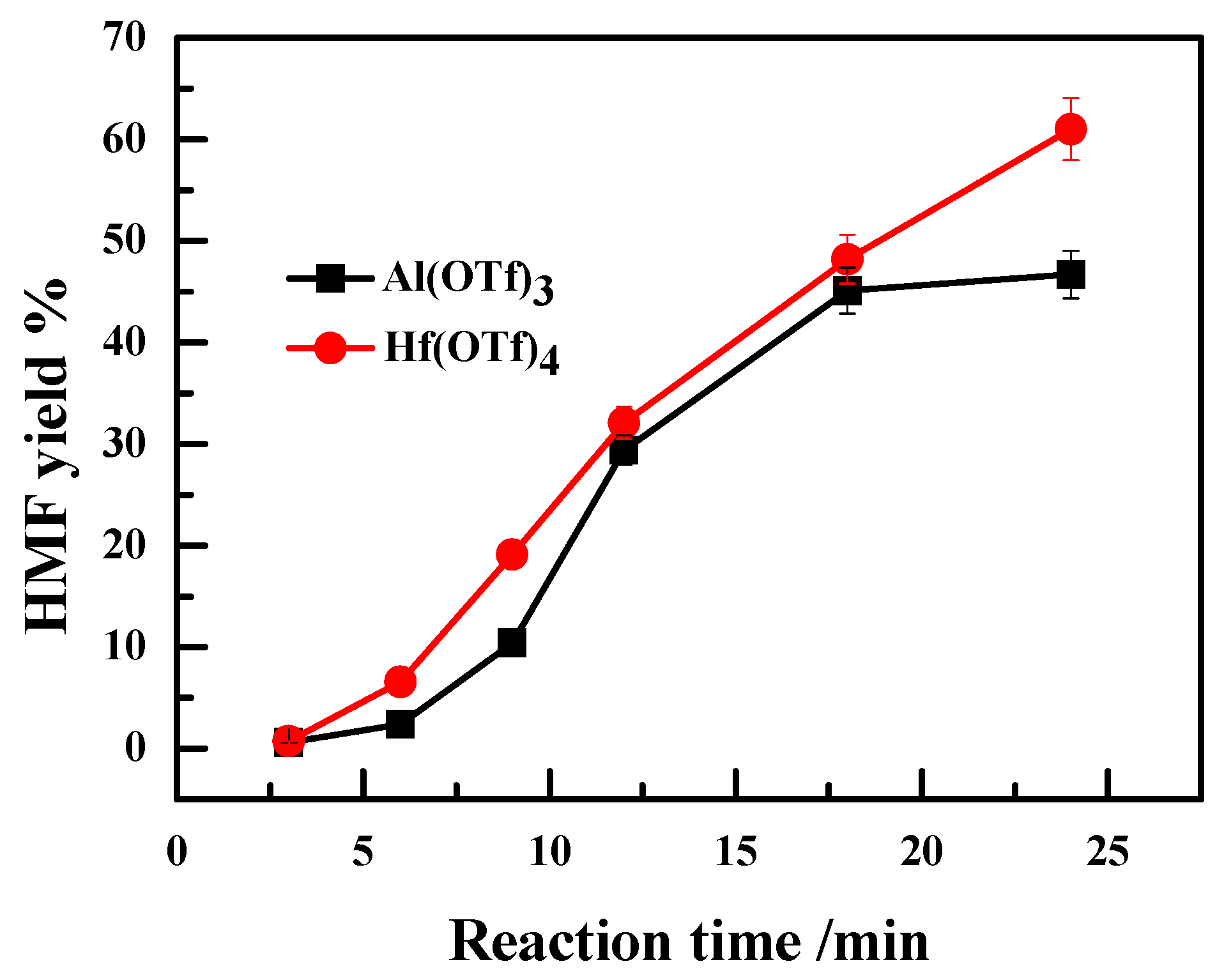
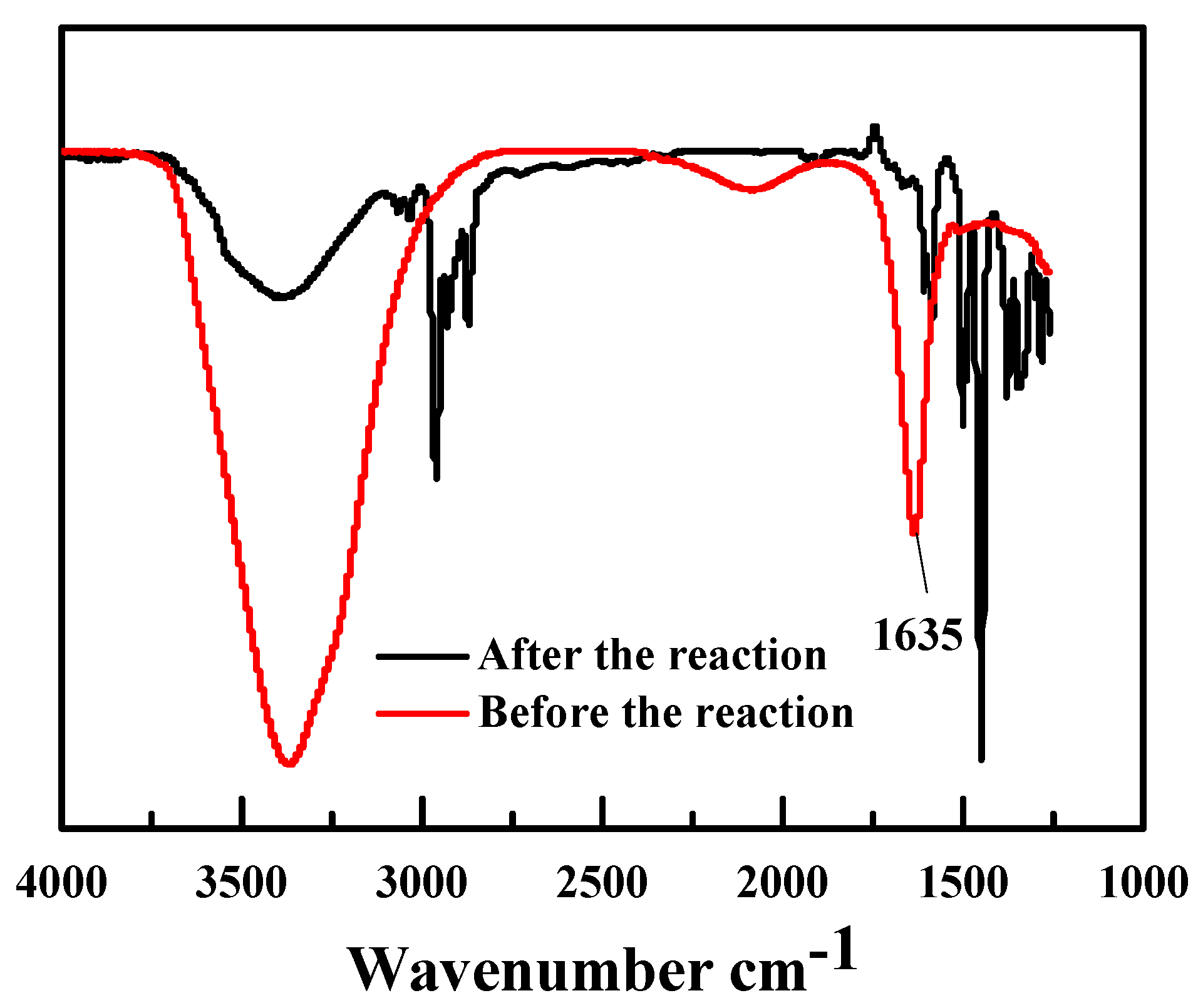
2.4. The Recyclability of Hf(OTf)4 Catalyst and Its Deactivation/Reactivation Mechanism
| Catalysts | HMF Yield % | ||||
|---|---|---|---|---|---|
| 1st run | 2nd run | 3rd run | 4th run | 5th run | |
| 25 mM Hf(OTf)4 | 60 | 26 | 16 | 9 | 4 |
| stoichiometric HCl | trace | n/a | n/a | n/a | n/a |
| 25 mM Hf(OTf)4 + stoichiometric HCl | 60 | 57 | 56 | 57 | 56 |
3. Experimental Section
3.1. Materials
3.2. Reaction Testing
3.2.1. Preparation of Reaction Solution
3.2.2. Biphasic Reaction Conditions
3.3. Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, J.A.; Kelly, J.E. Polyesters Derived from Furan and Tetrahydrofuran Nuclei. Macromolecules 1978, 11, 568–573. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic activities in the chemistry of substituted furans from carbohydrates and the ensuing polymers. Top. Catal. 2004, 27, 11–29. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Furans in Polymer Chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379. [Google Scholar] [CrossRef]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; de Vries, J.G.; Heeres, H.J. Caprolactam from Renewable Resources: Catalytic Conversion of 5-Hydroxymethylfurfural into Caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef] [PubMed]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.J., Jr.; Leesomboon, T.; Mok, W.S.; Richards, G.N. Mechanism of formation of 2-furaldehyde from D-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Ren, J.; Liu, X.; Lu, G.; Wang, Y. Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon-based solid acid. Green Chem. 2011, 13, 2678–2681. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P.; Rivalier, P.; Ros, P.; Avignon, G. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl. Catal. A 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Wang, J.; Ren, J.; Liu, X.; Xi, J.; Xia, Q.; Zu, Y.; Lu, G.; Wang, Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ren, J.; Liu, X.; Li, X.; Xia, Y.; Lu, G.; Wang, Y. Mesoporous niobium phosphate: An excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2012, 2, 2485–2491. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Williams, L.D.; Ebede, C.C. Mechanism of the dehydration of D-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: An NMR study. Carbohydr. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Moreau, C.; Finiels, A.; Vanoye, L. Dehydration of fructose and sucrose into 5-hydroxymethylfurfural in the presence of 1-H-3-methyl imidazolium chloride acting both as solvent and catalyst. J. Mol. Catal. A 2006, 253, 165–169. [Google Scholar] [CrossRef]
- Hansen, T.S.; Mielby, J.; Riisager, A. Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water. Green Chem. 2011, 13, 109–114. [Google Scholar] [CrossRef]
- Crisci, A.J.; Tucker, M.H.; Lee, M.-Y.; Jang, S.G.; Dumesic, J.A.; Scott, S.L. Acid-Functionalized SBA-15-Type Silica Catalysts for Carbohydrate Dehydration. ACS Catal. 2011, 1, 719–728. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, Z.K.; Wang, Q. Catalytic Conversion of Inulin and Fructose into 5-Hydroxymethylfurfural by Lignosulfonic Acid in Ionic Liquids. ChemSusChem 2012, 5, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, B.; Shi, J.; Ma, J.; Yang, G.; Han, B. Dehydration of Carbohydrates to 5-Hydroxymethylfurfural in Ionic Liquids Catalyzed by Hexachlorotriphosphazene. Chin. J. Chem. 2012, 30, 2079–2084. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Huang, X.; Chen, L.; Ma, L.; Li, X. Conversion of fructose into 5-hydroxymethylfurfural and alkyl levulinates catalyzed by sulfonic acid-functionalized carbon materials. Green Chem. 2013, 15, 2895–2903. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y. Efficient and Selective Dehydration of Fructose to 5-Hydroxymethylfurfural Catalyzed by Brønsted-Acidic Ionic Liquids. ChemSusChem 2010, 3, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Ma, Y.; Li, Y. An efficient catalytic dehydration of fructose and sucrose to 5-hydroxymethylfurfural with protic ionic liquids. Carbohydr. Res. 2010, 345, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, B.; Zhang, Z.; Chen, L. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by acidic ionic liquids in dimethyl sulfoxide. Ind. Crops Prod. 2013, 50, 264–269. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Deng, T.; Cui, X.; Qi, Y.; Wang, Y.; Hou, X.; Zhu, Y. Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by ZnCl2 in water. Chem. Commun. 2012, 48, 5494–5496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, B.; Zhang, L.-J.; Da, Y.-X.; Quan, Z.-J.; Yang, L.-J.; Wang, X.-C. Conversion of carbohydrates into 5-hydroxymethylfurfural using polymer bound sulfonic acids as efficient and recyclable catalysts. RSC Adv. 2013, 3, 9201–9205. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Wang, T.; Glasper, J.A.; Shanks, B.H. Kinetics of glucose dehydration catalyzed by homogeneous Lewis acidic metal salts in water. Appl. Catal. A 2015, 498, 214–221. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Saha, B. Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride catalyst in water. Green Chem. 2011, 13, 2859–2868. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Xia, Y.; Li, W.; Wang, H.; Zheng, C. Efficient process for the direct transformation of cellulose and carbohydrates to 5-(hydroxymenthyl)furfural with dual-core sulfonic acid ionic liquids and co-catalysts. RSC Adv. 2013, 3, 7782–7790. [Google Scholar] [CrossRef]
- Wu, L.; Song, J.; Zhang, B.; Zhou, B.; Zhou, H.; Fan, H.; Yang, Y.; Han, B. Very efficient conversion of glucose to 5-hydroxymethylfurfural in DBU-based ionic liquids with benzenesulfonate anion. Green Chem. 2014, 16, 3935–3941. [Google Scholar] [CrossRef]
- Liu, W.; Holladay, J. Catalytic conversion of sugar into hydroxymethylfurfural in ionic liquids. Catal. Today 2013, 200, 106–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Xie, H.; Liu, W.; Zhao, Z. Catalytic Conversion of Carbohydrates into 5-Hydroxymethylfurfural by Germanium(IV) Chloride in Ionic Liquids. ChemSusChem 2011, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Qi, W.; Su, R.; He, Z. Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem. Comm. 2010, 46, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Nikolla, E.; Roman-Leshkov, Y.; Moliner, M.; Davis, M.E. "One-Pot" Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. J. Am. Chem. Soc. 2009, 131, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, T.; Rodriguez-Rodriguez, S.; Fristrup, P.; Riisager, A. Metal-Free Dehydration of Glucose to 5-(Hydroxymethyl)furfural in Ionic Liquids with Boric Acid as a Promoter. Chem. A Eur. J. 2011, 17, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Q.; Yue, M.; Bai, X.; Du, Y. Tantalum compounds as heterogeneous catalysts for saccharide dehydration to 5-hydroxymethylfurfural. Chem. Commun. 2011, 47, 4469–4471. [Google Scholar] [CrossRef] [PubMed]
- Heguaburu, V.; Franco, J.; Reina, L.; Tabarez, C.; Moyna, G.; Moyna, P. Dehydration of carbohydrates to 2-furaldehydes in ionic liquids by catalysis with ion exchange resins. Catal. Commun. 2012, 27, 88–91. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Lee, A.F.; Brown, D.R.; Melero, J.A.; Morales, G.; Wilson, K. Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose. Catal. Sci. Technol. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glucose dehydration to 5-hydroxymethylfurfural on zirconium containing mesoporous MCM-41 silica catalysts. Fuel 2014, 118, 265–271. [Google Scholar] [CrossRef]
- Kruger, J.S.; Nikolakis, V.; Vlachos, D.G. Aqueous-phase fructose dehydration using Brønsted acid zeolites: Catalytic activity of dissolved aluminosilicate species. Appl. Catal. A 2014, 469, 116–123. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Chen, E.Y.X. Chromium(0) Nanoparticles as Effective Catalyst for the Conversion of Glucose into 5-Hydroxymethylfurfural. ChemSusChem 2013, 6, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Huang, C.; Song, Y.; Zhang, J.; Chen, B. Efficient dehydration of glucose to 5-hydroxymethylfurfural catalyzed by the ionic liquid, 1-hydroxyethyl-3-methylimidazolium tetrafluoroborate. Bioresour. Technol. 2012, 121, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, I.; Teckchandani-Ortiz, A.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Selective dehydration of glucose to 5-hydroxymethylfurfural on acidic mesoporous tantalum phosphate. Appl. Catal. B 2014, 144, 22–28. [Google Scholar] [CrossRef]
- Pagan-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Bronsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Wang, T.; Pagan-Torres, Y.J.; Combs, E.J.; Dumesic, J.A.; Shanks, B.H. Water-Compatible Lewis Acid-Catalyzed Conversion of Carbohydrates to 5-Hydroxymethylfurfural in a Biphasic Solvent System. Top. Catal. 2012, 55, 657–662. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural Using Lewis and Bronsted Acid Catalysts in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Choudhary, V.; Caratzoulas, S.; Vlachos, D.G. Insights into the isomerization of xylose to xylulose and lyxose by a Lewis acid catalyst. Carbohydr. Res. 2013, 368, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; De, S.; Alam, M.I.; Abu-Omar, M.M.; Saha, B. Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J. Catal. 2012, 288, 8–15. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-W.; Abu-Omar, M.M. Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3·6H2O catalyst in a biphasic solvent system. Green Chem. 2012, 14, 509–513. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-W.; Abu-Omar, M.M. Synthesis of Furfural from Xylose, Xylan, and Biomass Using AlCl3·6H2O in Biphasic Media via Xylose Isomerization to Xylulose. Chemsuschem 2012, 5, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Rasrendra, C.; Makertihartha, I.; Adisasmito, S.; Heeres, H. Green Chemicals from D-glucose: Systematic Studies on Catalytic Effects of Inorganic Salts on the Chemo-Selectivity and Yield in Aqueous Solutions. Top. Catal. 2010, 53, 1241–1247. [Google Scholar] [CrossRef]
- Ma, Y.; Qing, S.; Wang, L.; Islam, N.; Guan, S.; Gao, Z.; Mamat, X.; Li, H.; Eli, W.; Wang, T. Production of 5-hydroxymethylfurfural from fructose by a thermo-regulated and recyclable Bronsted acidic ionic liquid catalyst. RSC Adv. 2015, 5, 47377–47383. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, Y.; Wang, L.; Song, Z.; Li, H.; Wang, T.; Li, H.; Eli, W. Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Hf(OTf)4 Lewis Acid in Water. Catalysts 2016, 6, 1. https://doi.org/10.3390/catal6010001
Li J, Ma Y, Wang L, Song Z, Li H, Wang T, Li H, Eli W. Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Hf(OTf)4 Lewis Acid in Water. Catalysts. 2016; 6(1):1. https://doi.org/10.3390/catal6010001
Chicago/Turabian StyleLi, Junjie, Yubo Ma, Lei Wang, Zean Song, Huiping Li, Tianfu Wang, Hongyi Li, and Wumanjiang Eli. 2016. "Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Hf(OTf)4 Lewis Acid in Water" Catalysts 6, no. 1: 1. https://doi.org/10.3390/catal6010001





