Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming
Abstract
:1. Introduction
2. Results and Discussion
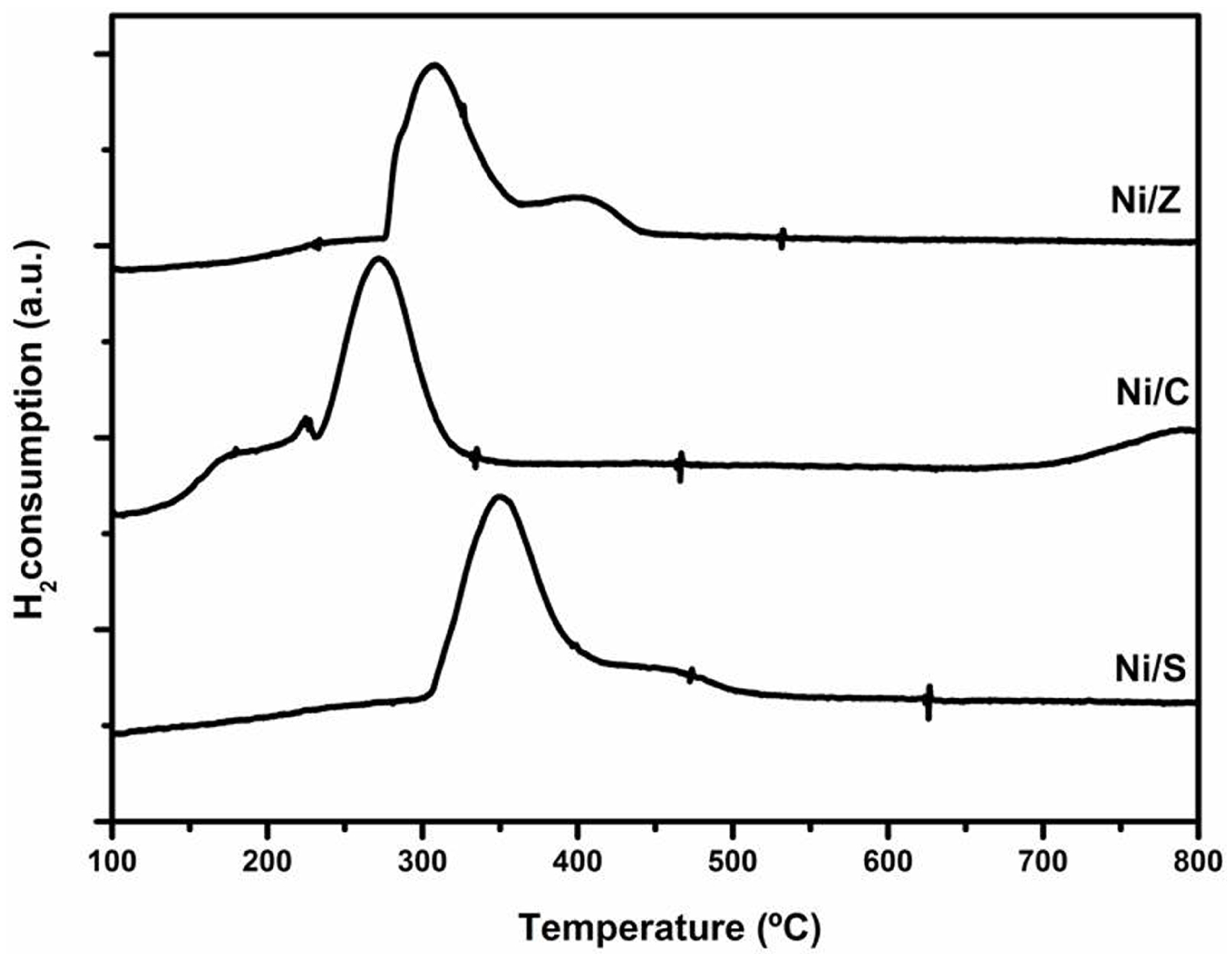
2.1. Ethanol Steam Reforming over Reference Catalysts
| Catalyst | Ni a wt% | SBET m2/g | Acidity b meq-NH3/g | nm | nm |
|---|---|---|---|---|---|
| Ni/S | 6.9 | 263 | - | 16.4 | 17.6 |
| Ni/C | 6.7 | 53 | - | - | - |
| Ni/Z | 6.8 | 1.5 | 0.275 | - | - |

| Catalyst | XEtOH mol% | Selectivity (mol%) | |||||
|---|---|---|---|---|---|---|---|
| H2 | CO2 | CO | CH4 | C2H4 | CH3CHO | ||
| Ni/S | 97.1 | 84.2 | 49.4 | 31.5 | 17.4 | 0 | 1.7 |
| Ni/C | 99.1 | 82.5 | 48.0 | 31.6 | 19.6 | 0 | 0.7 |
| Ni/Z | 95.0 | 80.7 | 45.3 | 31.8 | 20.4 | 2.4 | 0 |
2.2. Ni/SiO2 Catalysts Modified by Ce or Zr
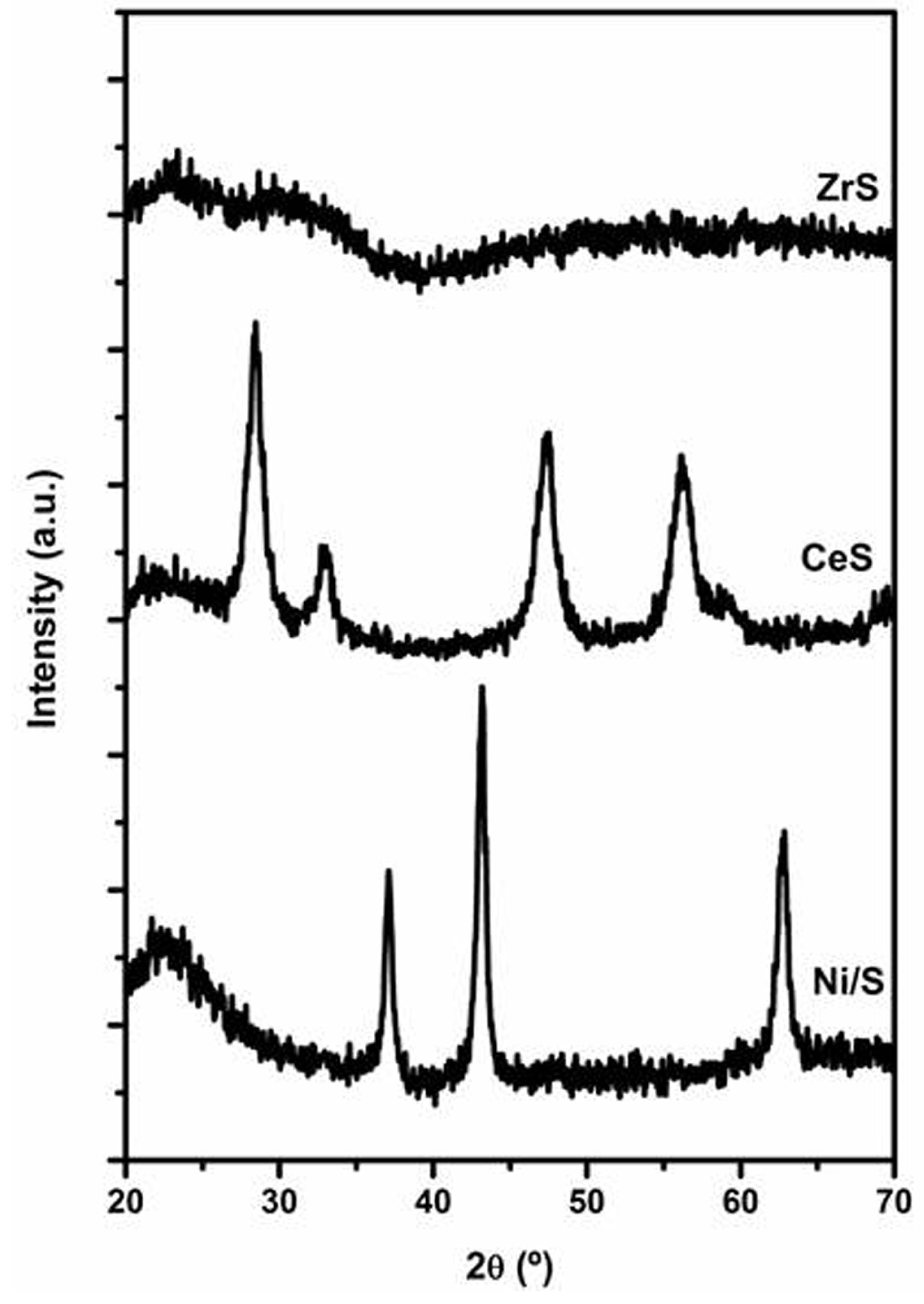
2.2.1. Characterization of Ce- or Zr-Modified Silica
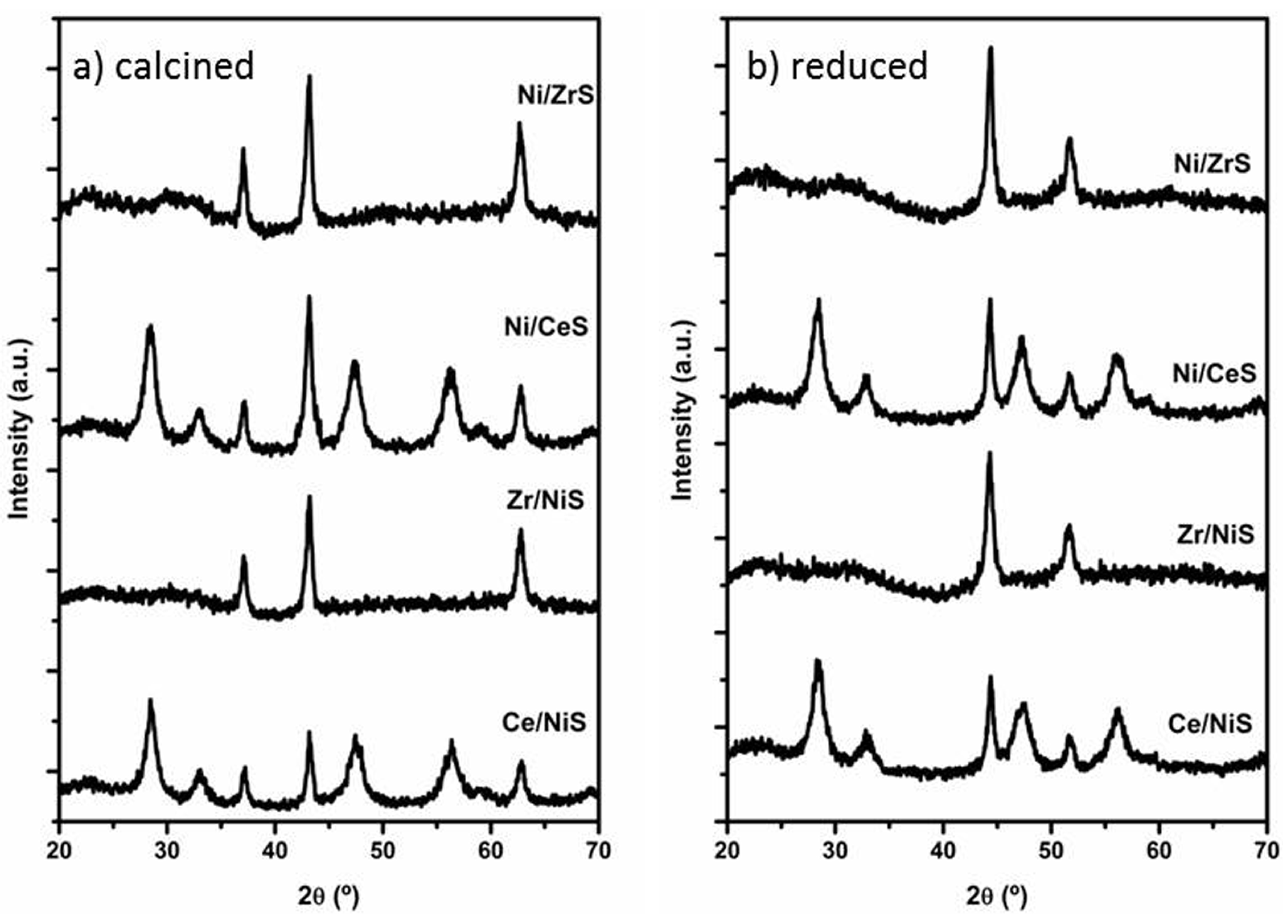
2.2.2. Characterization of Ce- or Zr-Modified Ni/SiO2 Catalysts
| Catalyst | Ni a wt% | Ce a wt% | Zr a wt% | SBET m2/g | Acidity b meq-NH3/g | nm | nm | nm |
|---|---|---|---|---|---|---|---|---|
| Ce/NiS | 6.7 | 9.7 | - | 228 | - | 8.1 | 19.8 | 19.9 |
| Zr/NiS | 6.7 | - | 9.4 | 236 | 0.144 | - | 20.0 | 19.9 |
| Ni/CeS | 6.8 | 9.2 | - | 247 | - | 7.0 | 21.8 | 23.2 |
| Ni/ZrS | 6.9 | - | 9.9 | 213 | 0.133 | - | 21.6 | 19.7 |
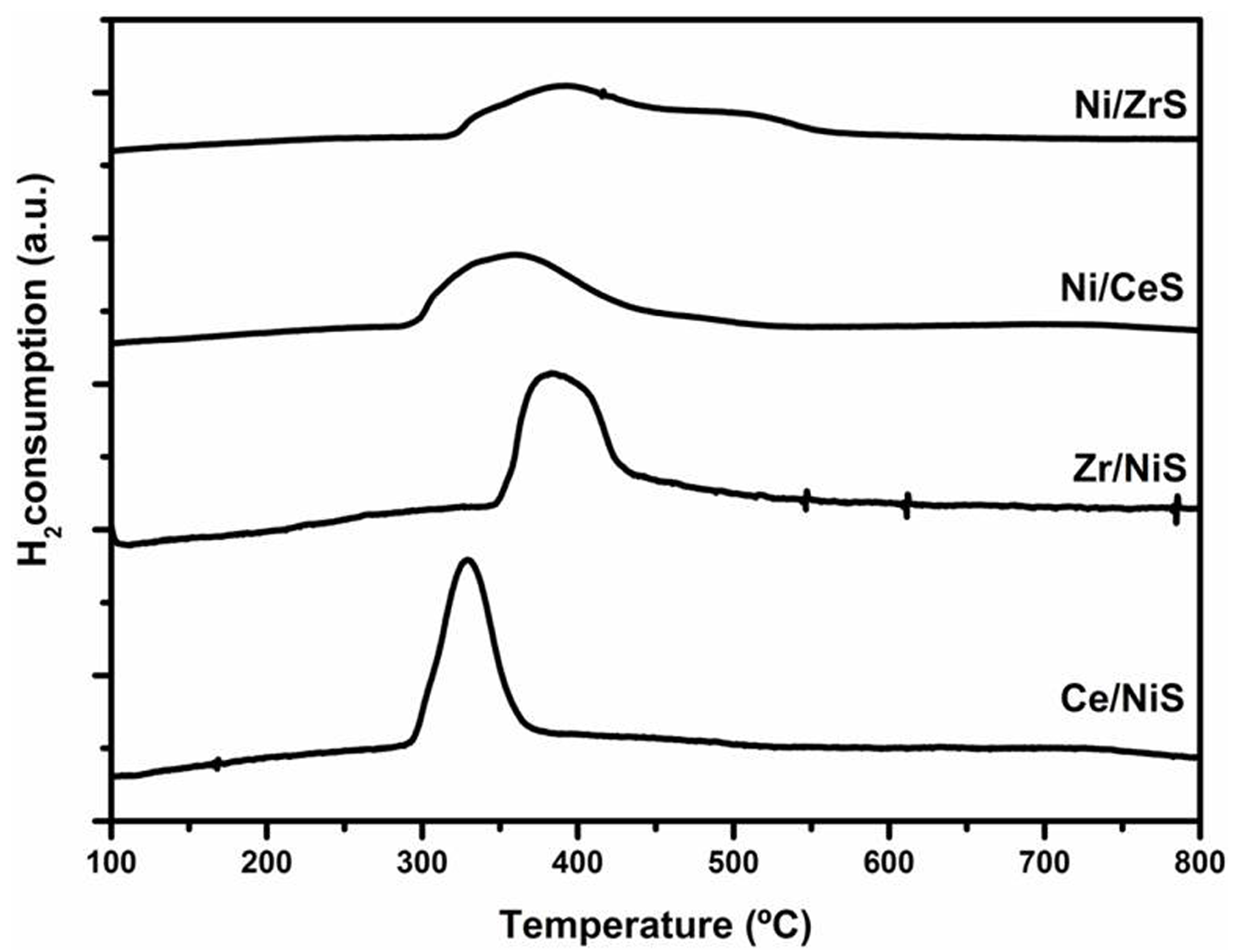
2.2.3. Catalytic Results Using Ni/SiO2 Catalysts Modified by Ce or Zr
| Catalyst | XEtOH mol% | Selectivity (mol%) | Coke | |||||
|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | CO | CH4 | C2H4 | CH3CHO | g/gcat h | ||
| NiS | 97.1 | 84.2 | 49.4 | 31.5 | 17.4 | 0 | 1.7 | 0.369 |
| Ce/NiS | 98.5 | 82.0 | 49.6 | 29.9 | 19.5 | 0 | 1.0 | 0.230 |
| Zr/NiS | 100 | 79.2 | 43.5 | 29.1 | 21.2 | 6.2 | 0 | 0.475 |
| Ni/CeS | 100 | 84.3 | 56.2 | 25.1 | 18.7 | 0.0 | 0 | 0.370 |
| Ni/ZrS | 100 | 88.3 | 56.5 | 28.0 | 12.9 | 2.6 | 0 | 0.518 |
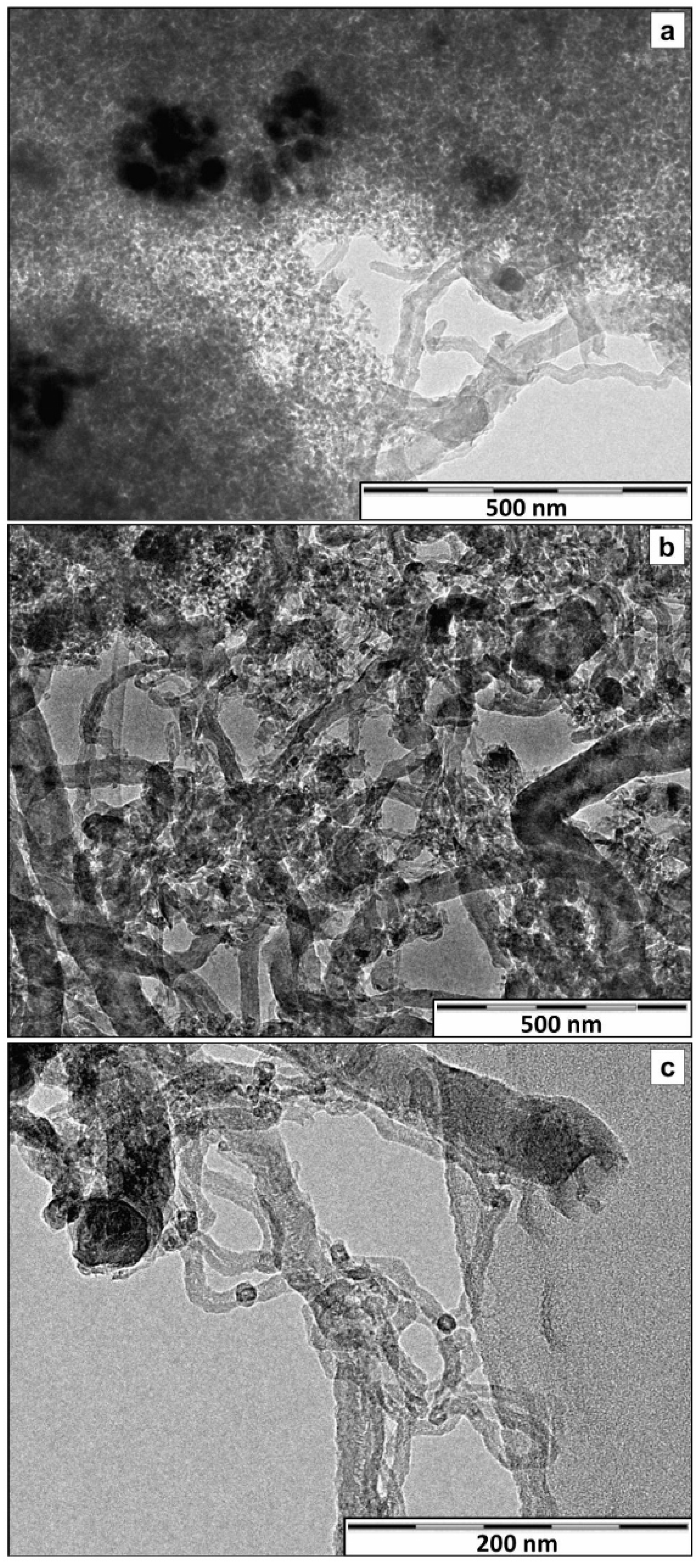
2.3. Ni/CexZr1−xO2/SiO2 Catalysts: The Effect of the Zr/Ce Ratio
2.3.1. Characterization of the Ni/CexZr1−xO2/SiO2 Catalysts
| Catalyst | Ni a awt% | Ce b awt% | Zr a awt% | SBET m2/g | Acidity b meq-NH3/g | nm | nm | nm |
|---|---|---|---|---|---|---|---|---|
| Ni/CeZrS-1/3 | 6.8 | 6.0 | 2.3 | 24.3 | 0.062 | 6.3 | 19.1 | 19.7 |
| Ni/CeZrS-1 | 7.0 | 4.2 | 4.5 | 242 | 0.077 | 5.0 | 18.1 | 16.6 |
| Ni/CeZrS-3 | 6.6 | 2.4 | 6.6 | 238 | 0.088 | 3.4 | 17.6 | 15.7 |

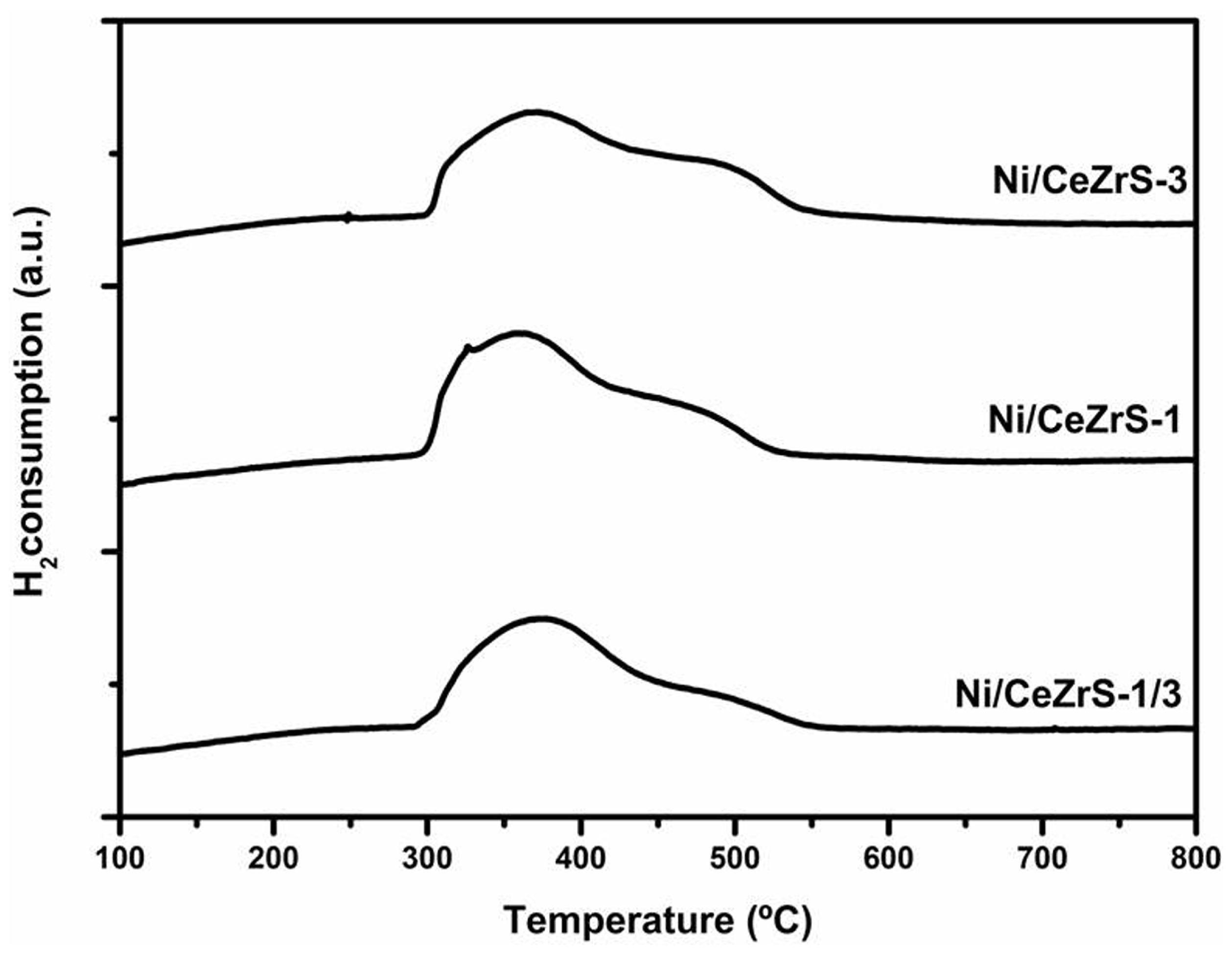
2.3.2. Catalytic Results Using the Ni/CexZr1−xO2/SiO2 Catalysts
| Catalyst | XEtOH mol% | Selectivity (mol%) | Coke | |||||
|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | CO | CH4 | C2H4 | CH3CHO | g/gcat h | ||
| Ni/CeZrS-1/3 | 100 | 84.6 | 52.4 | 29.6 | 18.0 | 0 | 0 | 0.500 |
| Ni/CeZrS-1 | 100 | 85.6 | 54.0 | 29.0 | 17.0 | 0 | 0 | 0.443 |
| Ni/CeZrS-3 | 100 | 85.8 | 54.3 | 28.9 | 16.8 | 0 | 0 | 0.308 |
3. Experimental Section
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: the future energy carrier. Phil. Trans. R. Soc. A 2012, 368, 3329–3342. [Google Scholar] [CrossRef]
- Guo, L.J.; Zhao, L.; Jing, D.W.; Lu, Y.J.; Yang, Y.J.; Bai, B.F.; Zhang, X.M.; Ma, L.J.; Wu, X.M. Solar hydrogen production and its development in China. Energy 2009, 34, 1073–1090. [Google Scholar] [CrossRef]
- Olateju, B.; Kumar, A. Hydrogen production from wind energy in Western Canada for upgrading bitumen from oil sands. Energy 2011, 36, 6326–6339. [Google Scholar] [CrossRef]
- Zahedi, A. Hydrogen as storage option for intermittent renewable technologies such as solar and wind. In Proceedings of the 2006 Australasian Universities Power Engineering Conference (AUPEC’06), Melbourne, Australia, 10–13 December 2006.
- Goltsova, V.A.; Veziroglu, T.N.; Goltsova, L.F. Hydrogen civilization of the future—A new conception of the IAHE. Int. J. Hydrogen Energy 2006, 31, 153–159. [Google Scholar]
- Grzegorczyk, W.; Denis, A.; Gac, W.; Ioannides, T.; Machocki, A. Hydrogen Formation via Steam Reforming of Ethanol Over Cu/ZnO Catalyst Modified with Nickel, Cobalt and Manganese. Catal. Lett. 2009, 128, 443–448. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen Production from Bioethanol. In Hydrogen Production: Prospects and Processes; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 247–294. [Google Scholar]
- Papadopoulou, E.; Delimaris, D.; Denis, A.; Machocki, A.; Ioannides, T. Alcohol reforming on cobalt-based catalysts prepared from organic salt precursors. Int. J. Hydrogen Energy 2007, 37, 16375–16381. [Google Scholar] [CrossRef]
- Navarro, R.M.; Peña, M.A.; Fierro, J.L.G. Hydrogen production reactions from crbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Auprête, F.; Descorme, C.; Duprez, D. Bio-ethanol catalytic steam reforming over supported metal catalysts. Catal. Commun. 2002, 3, 263–267. [Google Scholar] [CrossRef]
- Comas, J.; Mariño, F.; Laborde, M.; Amadeo, N. Bio-ethanol steam reforming on Ni/Al2O3 catalyst. Chem. Eng. J. 2004, 98, 61–68. [Google Scholar] [CrossRef]
- Fatsikostas, A.N.; Verykios, X.E. Reaction network of steam reforming of ethanol over Ni–based catalysts. J. Catal. 2004, 225, 439–452. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 production for MC fuel cell by steam reforming of ethanol over MgO supported Pd, Rh, Ni and Co catalysts. Catal. Commun. 2004, 5, 611–615. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Ethanol steam reforming over Ni/M xOy-Al2O3 (M = Ce, La, Zr and Mg) catalysts: Influence of support on the hydrogen production. Int. J. Hydrogen Energy 2007, 32, 1462–1471. [Google Scholar] [CrossRef]
- Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Ce and La modification of mesoporous Cu-Ni/SBA-15 catalysts for hydrogen production through ethanol steam reforming. Micropor. Mesopor. Mater. 2009, 119, 200–207. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Ethanol steam reforming on Mg- and Ca-modified Cu-Ni/SBA-15 catalysts. Catal. Today 2009, 146, 63–70. [Google Scholar] [CrossRef]
- Lindo, M.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Ethanol steam reforming on Ni/Al-SBA-15 catalysts: Effect of the aluminium content. Int. J. Hydrogen Energy 2010, 35, 5895–5901. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Lindo, M.; Carrero, A.; Calles, J.A. Hydrogen production by steam reforming of ethanol using Ni catalysts based on ternary mixed oxides prepared by coprecipitation. Int. J. Hydrogen Energy 2012, 37, 1985–1992. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Carrero, A.; Calles, J.A. Hydrogen production by ethanol steam reforming over Cu-Ni supported catalysts. Int. J. Hydrogen Energy 2007, 32, 1450–1461. [Google Scholar] [CrossRef]
- Biswas, P.; Kunzru, D. Steam reforming of ethanol for production of hydrogen over Ni/CeO2-ZrO2 catalysts: Effect of support and metal loading. Int. J. Hydrogen Energy 2007, 32, 969–980. [Google Scholar] [CrossRef]
- Biswas, P.; Kunzru, D. Oxidative steam reforming of ethanol over Ni/CeO2-ZrO2 catalyst. Chem. Eng. J. 2008, 136, 41–49. [Google Scholar] [CrossRef]
- Youn, M.H.; Seo, J.G.; Cho, K.M.; Park, S.; Park, D.R.; Jung, J.C.; Song, I.K. Hydrogen production by auto-thermal reforming of ethanol over nickel catalysts supported on Ce-modified mesoporous zirconia: Effect of Ce/Zr molar ratio. Int. J. Hydrogen Energy 2008, 33, 5052–5059. [Google Scholar] [CrossRef]
- Wan, H.; Li, X.; Ji, S.; Huang, B.; Wang, K.; Li, C. Effect of Ni loading and CexZr1−xO2 promoter on Ni-based SBA-15 catalysts for steam reforming of methane. J. Nat. Gas Chem. 2007, 16, 139–147. [Google Scholar] [CrossRef]
- Rocchini, E.; Vicario, M.; Llorca, J.; Leitenburg, C.; Dolcetti, G.; Trovarelli, A. Reduction and oxygen storage behavior of noble metals supported on silica-doped ceria. J. Catal. 2002, 211, 407–421. [Google Scholar] [CrossRef]
- Raju, V.; Jaenicke, S.; Chuah, G.K. Effect of hydrothermal treatment and silica on thermal stability and oxygen storage capacity of ceria-zirconia. Appl. Catal. B 2009, 191, 92–100. [Google Scholar] [CrossRef]
- Reddy, B.M.; Thrimurthulu, G.; Saikia, P.; Bharali, P. Silica supported ceria and ceria-zirconia nanocomposite oxides for selective dehydration of 4-methylpentan-2-ol. J. Mol. Catal. A 2007, 275, 167–173. [Google Scholar] [CrossRef]
- Reddy, B.M.; Saikia, P.; Bharali, P.; Katta, L.; Thrimurthulu, G. Highly dispersed ceria and ceria-zirconia nanocomposites over silica surface for catalytic applications. Catal. Today 2009, 141, 109–114. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, M.; Tomiyama, S. Addition of zirconia in Ni/SiO2 catalyst for improvement of steam resistance. Appl. Catal. A 2004, 273, 211–215. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Song, I.K. Effect of SiO2-ZrO2 supports prepared by a grafting method on hydrogen production by steam reforming of liquefied natural gas over Ni/SiO2-ZrO2 catalysts. J. Power Sources 2007, 168, 251–257. [Google Scholar] [CrossRef]
- Roh, H.S.; Potdar, H.S.; Jun, K.W. Carbon dioxide reforming of methane over co-precipitated Ni-CeO2, Ni-ZrO2 and Ni-Ce-ZrO2 catalysts. Catal. Today 2004, 93–95, 39–44. [Google Scholar]
- Trovarelli, A. Catalysis by Ceria and Related Materials; Imperial College Press: London, UK, 2002. [Google Scholar]
- Djinovic, P.; Batista, J.; Pintar, A. WGS reaction over nanostructures CuO-CeO2 catalysts prepared by hard template method: Characterization, activity and deactivation. Catal. Today 2009, 147, 191–197. [Google Scholar] [CrossRef]
- Andreeva, D.; Ivanov, I.; Ilieva, L.; Sobczak, J.W.; Avdeev, G.; Tabakova, T. Nanosized gold catalysts supported on ceria and ceria-alumina for WGS reaction: Influence of preparation method. Appl. Catal. A 2007, 333, 153–160. [Google Scholar] [CrossRef]
- Trim, D.L. Coke formation and minimisation during steam reforming reactions. Catal. Today 1997, 37, 233–238. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Hydrogen production by ethanol steam reforming over Cu-Ni/SBA-15 supported catalysts prepared by direct synthesis and impregnation. Appl. Catal. A 2007, 327, 82–94. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q. Reforming of methane with carbon dioxide over Ni/Al2O2 catalysts: Effect of nickel precursor. Appl. Catal. 1998, 69, 271–280. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Effect of potassium content in the activity of K-promoted Ni/Al2O2 catalysts for the dry reforming of methane. Appl. Catal. 2006, 301, 9–15. [Google Scholar] [CrossRef]
- Carrero, A.; Calles, J.A.; Vizcaíno, A.J. Effect of Mg and Ca addition on coke deposition over Cu-Ni/SiO2 catalysts for ethanol steam reforming. Chem. Eng. J. 2010, 163, 395–402. [Google Scholar] [CrossRef]
- Helveg, S.; Sehested, J.; Rostrup-Nielsen, J.R. Whisker carbon in perspective. Catal. Today 2011, 178, 42–46. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Watson, R.B.; Wang, X.; Ozkan, U.S. Deactivation characteristics of lanthanide-promoted sol-gel Ni/Al2O2 catalysts in propane steam reforming. J. Catal. 2005, 234, 496–508. [Google Scholar]
- Bellido, J.D.A.; Assaf, E.M. Nickel catalysts supported on ZrO2, Y2O3-stabilized ZrO2 and CaO-stabilized ZrO2 for the steam reforming of ethanol: Effect of the support and nickel load. J. Power Sources 2008, 177, 24–32. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calles, J.A.; Carrero, A.; Vizcaíno, A.J.; Lindo, M. Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming. Catalysts 2015, 5, 58-76. https://doi.org/10.3390/catal5010058
Calles JA, Carrero A, Vizcaíno AJ, Lindo M. Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming. Catalysts. 2015; 5(1):58-76. https://doi.org/10.3390/catal5010058
Chicago/Turabian StyleCalles, Jose Antonio, Alicia Carrero, Arturo Javier Vizcaíno, and Montaña Lindo. 2015. "Effect of Ce and Zr Addition to Ni/SiO2 Catalysts for Hydrogen Production through Ethanol Steam Reforming" Catalysts 5, no. 1: 58-76. https://doi.org/10.3390/catal5010058