Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration
Abstract
:1. Introduction
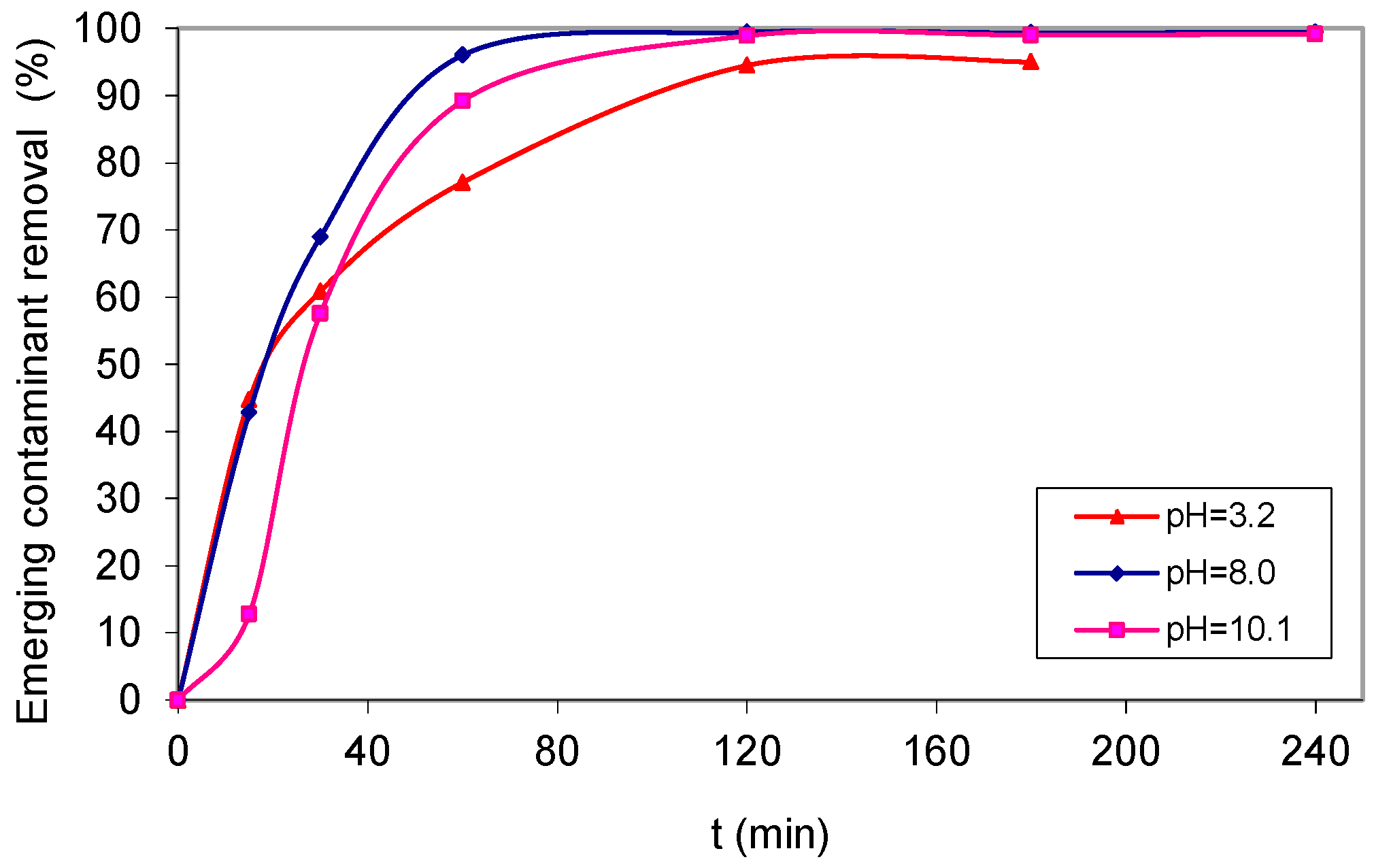
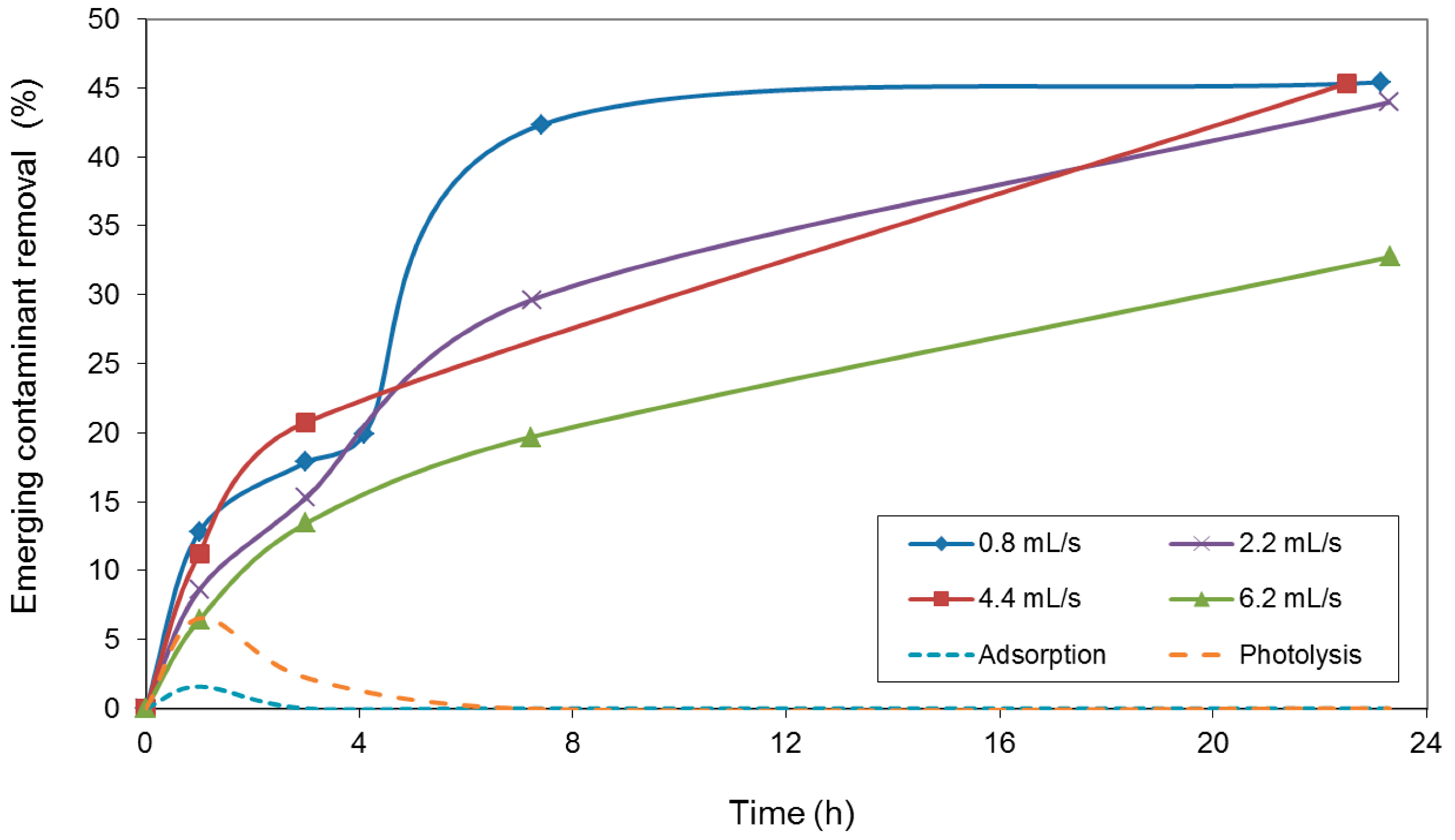
2. Results and Discussion
| XRD | N2 adsorption | Hg porosimetry | ||||
|---|---|---|---|---|---|---|
| % Anatase | % Rutile | ABET (m2/g)) | A (m2/g) | ε (%) | ρbulk (g/mL) | ρapparent (g/mL) |
| 81 | 19 | 51.1 | 63.7 | 92.46 | 0.19 | 2.58 |
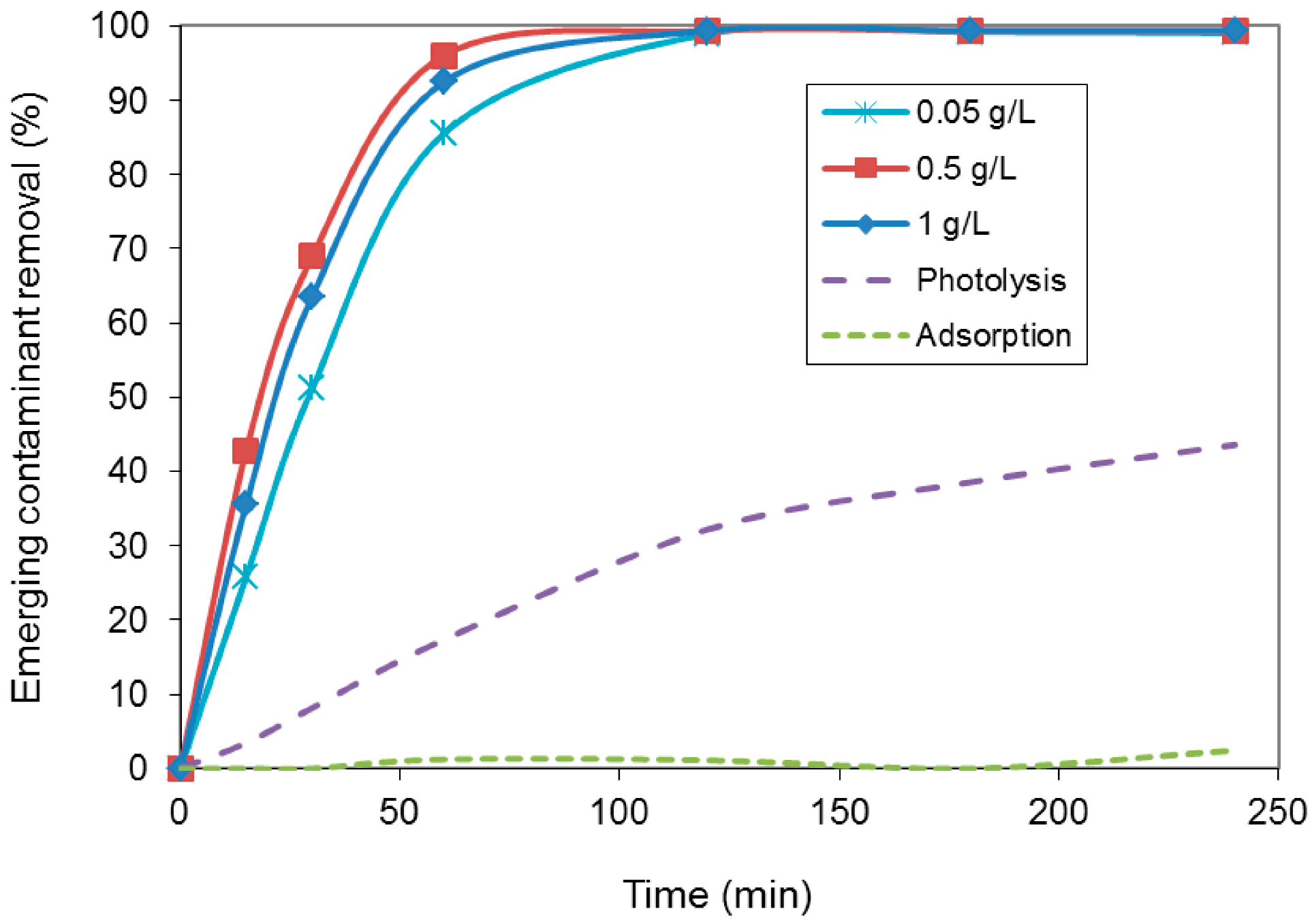
| Photocatalyst amount (g/L) | kapp (min−1) |
|---|---|
| 0.05 | 0.036 |
| 0.10 | 0.040 |
| 0.25 | 0.038 |
| 0.50 | 0.045 |
| 1.00 | 0.042 |
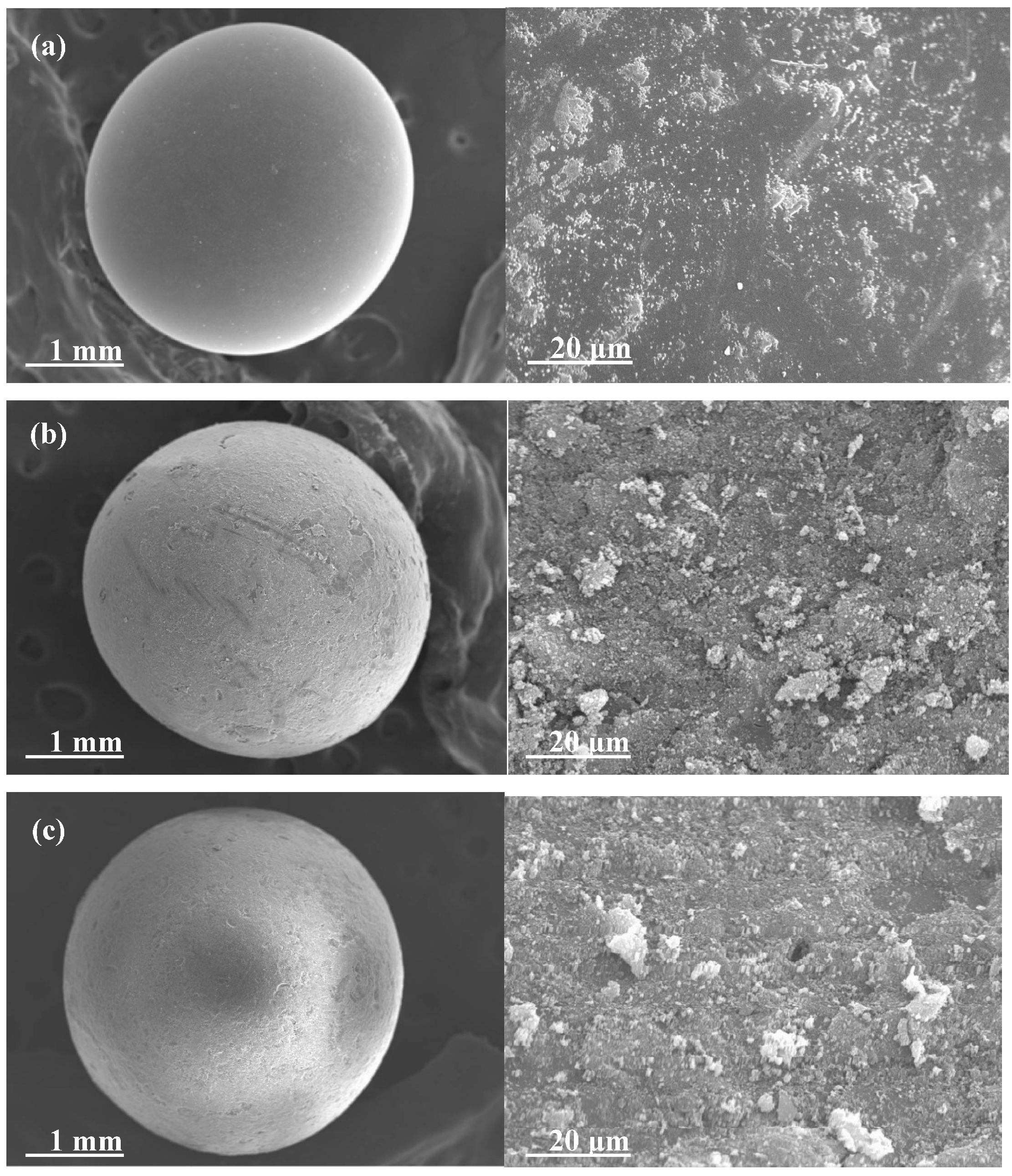
3. Experimental Section
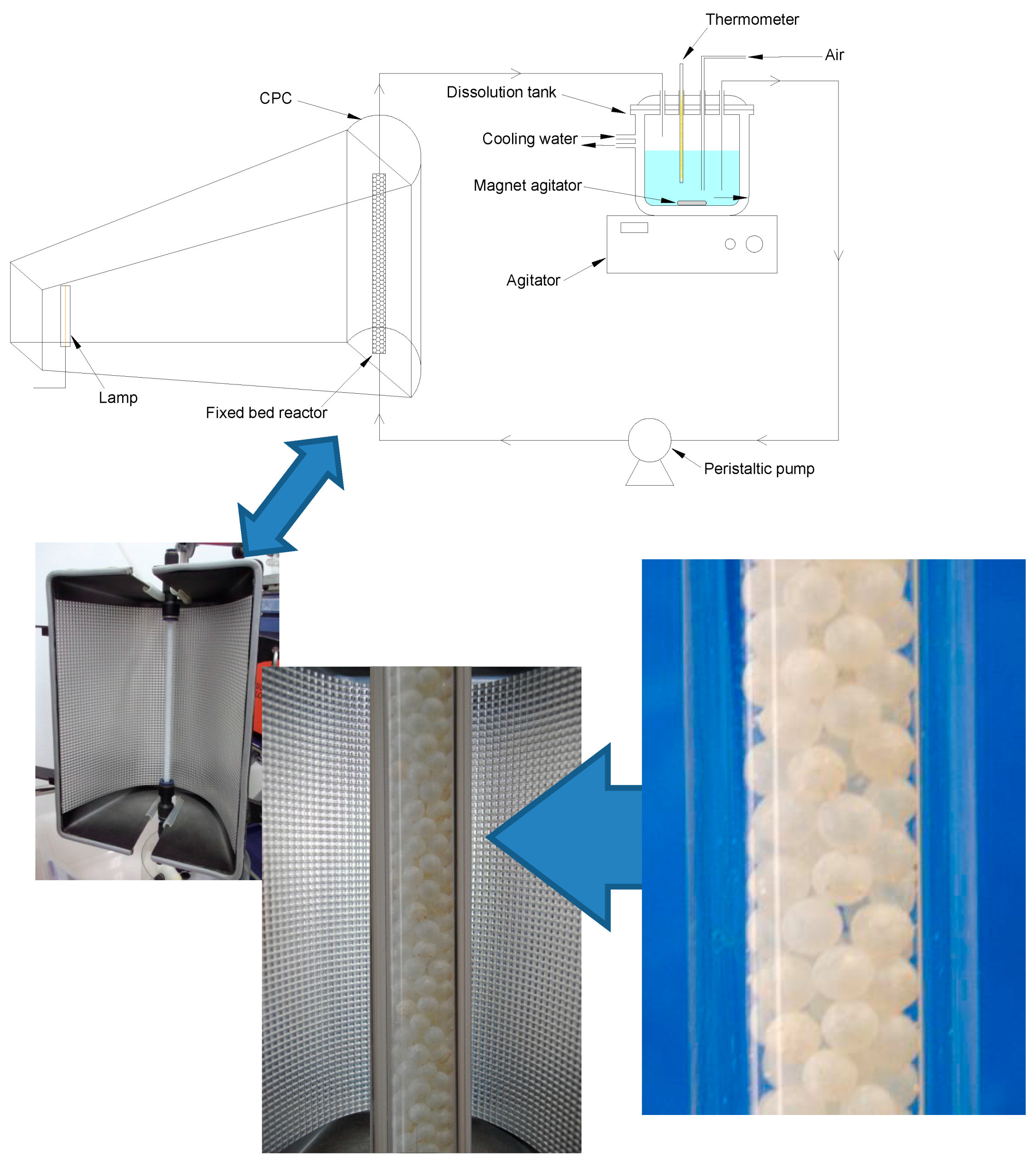
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Zhang, Z.; Hibberd, A.; Zhou, J.L. Analysis of emerging contaminants in sewage effluent and river water: Comparison between spot and passive sampling. Anal. Chimi. Acta 2008, 607, 37–44. [Google Scholar] [CrossRef]
- Miranda-García, N.; Maldonado, M.I.; Coronado, J.M.; Malato, S. Degradation study of 15 emerging contaminants at low concentration by immobilized TiO2 in a pilot plant. Catal. Today 2010, 151, 107–113. [Google Scholar] [CrossRef]
- Schriks, M.; Heringa, M.B.; van der Kooi, M.M.E.; de Voogt, P.; van Wezel, A.P. Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 2010, 44, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, L.E.; Madhumita, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Litter, M.I.; Quici, N. Photochemical advanced oxidation processes for water and wastewater treatment. Recent Pat. Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- Lydakis-Simantiris, A.; Riga, D.; Katsivela, E.; Mantzavinos, D.; Xekoukoulotakis, N.P. Disinfection of spring water and secondary treated municipal wastewater by TiO2 photocatalysis. Desalination 2010, 250, 351–355. [Google Scholar] [CrossRef]
- Robert, D.; Malato, S. Solar photocatalysis: A clean process for water detoxification. Sci. Total Environ. 2001, 291, 85–97. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.J.; Chen, C.C.; Lu, C.S.; Hsu, P.Y.; Chen, M.H. Phorate degradation by TiO2 photocatalysis: Parameter and reaction pathway investigations. Desalination 2010, 250, 869–875. [Google Scholar] [CrossRef]
- Wei, Y.-L.; Chen, K.-W.; Wang, H.P. Study of Chromium Modified TiO2 Nano Catalyst under Visible Light Irradiation. J. Nanosci. Nanotechnol. 2010, 10, 1–5. [Google Scholar] [CrossRef]
- Bahadur, N.; Jain, K.; Srivastava, A.K.; Govind; Gakhar, R.; Haranath, D.; Dulat, M.S. Effect of nominal doping of Ag and Ni on the crystalline structure and photo-catalytic properties of mesoporous titania. Mater. Chem. Phys. 2010, 124, 600–608. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Huang, G.-F.; Huang, W.-Q. Visible-light absorption and photocatalytic activity of Cr-doped TiO2 nanocrystal films. Adv. Powder Technol. 2012, 23, 8–12. [Google Scholar] [CrossRef]
- Soutsas, K.; Karayannis, V.; Poulios, I.; Riga, A.; Ntampegliotis, K.; Spiliotis, X.; Papapolymerou, G. Decolorization and degradation of reactive azo dyes via heterogeneous photocatalytic processes. Desalination 2010, 250, 345–350. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Liu, J.; Ma, M.; Chen, W.; Li, L. Activated carbon supported TiO2-photocatalysis doped with Fe ions for continuous treatment of dye wastewater in a dynamic reactor. J. Environ. Sci. 2010, 22, 1290–1296. [Google Scholar] [CrossRef]
- Van Grieken, R.; Marugán, J.; Sordo, C.; Martínez, P.; Pablos, C. Photocatalytic inactivation of bacteria in water using suspended and immobilized silver-TiO2. Appl. Catal. B 2009, 93, 112–118. [Google Scholar] [CrossRef]
- Borges, M.E.; Alvarez-Galván, M.C.; Esparza, P.; Medina, E.; Martín-Zarza, P.; Fierro, J.L.G. Ti-containing volcanic ash as photocatalyst for degradation of phenol. Energy Environ. Sci. 2008, 1, 364–369. [Google Scholar] [CrossRef]
- Palau, J.; Colomer, M.; Penya-Roja, J.M.; Martínez-Soria, V. Photodegradation of Toluene, m-Xylene, and n-Butyl Acetate and Their Mixtures over TiO2 Catalyst on Glass Fibers. Ind. Eng. Chem. Res. 2012, 51, 5986–5994. [Google Scholar] [CrossRef]
- Song, M.Y.; Park, Y.-K.; Jurng, J. Direct coating of V2O5/TiO2 nanoparticles onto glass beads by chemical vapor deposition. Powder Technol. 2012, 231, 135–140. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Madhumita, M.B. Photocatalytic Oxidation of Paracetamol: Dominant Reactants, Intermediates, and Reaction Mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.; Morawski, A.W. New method of improving photocatalytic activity of commercial Degussa P25 for azo dyes decomposition. Appl. Catal. B 2007, 75, 118–123. [Google Scholar] [CrossRef]
- Wong, C.L.; Tan, Y.N.; Mohamed, A.R. A review on the formation of titania nanotube photocatalysts by hydrothermal treatment. J. Environ. Manag. 2011, 92, 1669–1680. [Google Scholar] [CrossRef]
- Mangalampalli, V.; Sharma, P.; Sadanandam, G.; Ratnamala, A.; Kumari, V.D.; Subrahmanyam, M. An efficient and novel porous nanosilica supported TiO2 photocatalyst for pesticide degradation using solar light. J. Hazard. Mater. 2009, 171, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, R.; Umbarkar, S.B.; Sonawane, R.S.; Takle, S.; Dongare, M.K. Vanadia-titania thin films for photocatalytic degradation of formaldehyde in sunlight. Appl. Catal. A 2010, 374, 103–109. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Yeh, D.H.; Trotz, M.A. Removing pharmaceuticals and endocrine-disrupting compounds from wastewater by photocatalysis. J. Chem. Technol. Biotechnol. 2007, 82, 121–134. [Google Scholar] [CrossRef]
- Dimitroula, H.; Daskalaki, V.M.; Frontistis, Z.; Kondarides, D.I.; Panagiotopoulou, P.; Xekoukoulotakis, N.P.; Mantzavinos, D. Solar photocatalysis for the abatement of emerging micro-contaminants in wastewater: Synthesis, characterization and testing of various TiO2 samples. Appl. Catal. B 2012, 117–118, 283–291. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Q.; Zhou, J.; Wang, B. A study on dye photoremoval on TiO2 suspension solution. J. Photochem. Photobiol. A 1997, 108, 235–238. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photodegradation of deaminobenzene acid orange 52 by three advanced oxidation process: UV-H202, Uv-TiO2 and Vis- TiO2. Comparative mechanistic and kinetics investigation. J. Photochem. Photobiol. A 2000, 130, 35–47. [Google Scholar] [CrossRef]
- Brunner, M.; Schmiedberger, A.; Schmid, R.; Jager, D.; Piegler, E.; Eichler, H.; Muller, M. Direct assesment of peripheral pharmacokinetics in humans: Comparison between cantharides blister fluid sampling, in vivo microdialisys and saliva sampling. Br. Clin. Pharmacol. 1998, 46, 425–431. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Guida, M.; Kassinos, D.; Belgiorno, V. Heterogenous photocatalytic degradation kinetics and detoxification of an urban wastewater treatment plant effluent contaminated with pharmaceuticals. Water Res. 2009, 43, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Hänel, A.; Morén, P.; Zaleska, A.; Hupka, J. Photocatalytic activity of TiO2 immobilized on glass beads. Physicochem. Probl. Miner. Process. 2010, 45, 49–56. [Google Scholar]
- Kobayakawa, K.; Sato, C.; Sato, Y.; Fujishima, A. Continuous-flow photoreactor packed with titanium dioxide immobilized on large silica gel beads to decompose oxalic acid in excess water. J. Photochem. Photobiol. A 1998, 118, 65–69. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, M.E.; García, D.M.; Hernández, T.; Ruiz-Morales, J.C.; Esparza, P. Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration. Catalysts 2015, 5, 77-87. https://doi.org/10.3390/catal5010077
Borges ME, García DM, Hernández T, Ruiz-Morales JC, Esparza P. Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration. Catalysts. 2015; 5(1):77-87. https://doi.org/10.3390/catal5010077
Chicago/Turabian StyleBorges, Mª Emma, Dulce María García, Tania Hernández, Juan Carlos Ruiz-Morales, and Pedro Esparza. 2015. "Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration" Catalysts 5, no. 1: 77-87. https://doi.org/10.3390/catal5010077






