Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Abstract
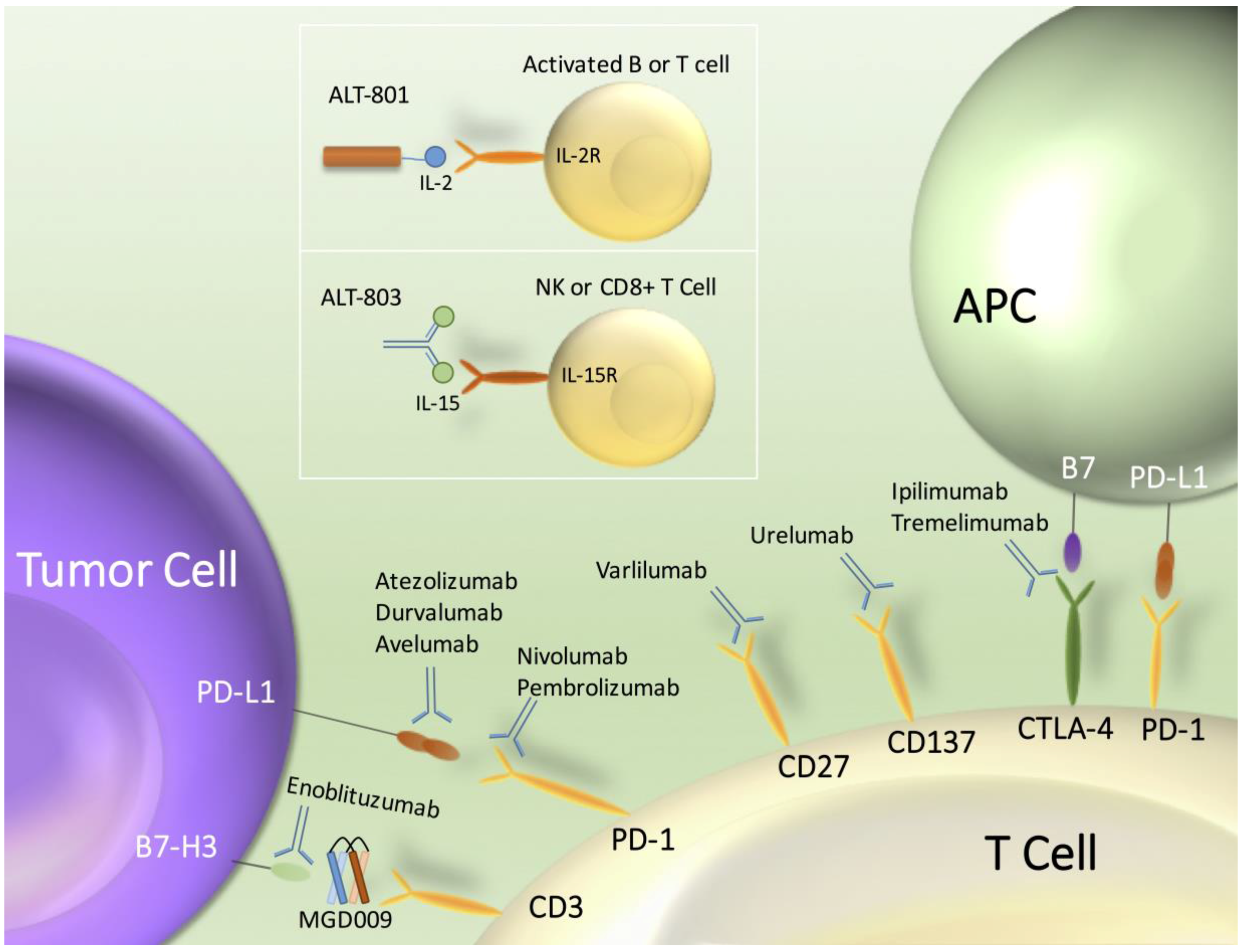
:1. Introduction
2. Immunotherapy in Metastatic Refractory Urothelial Carcinoma
2.1. Checkpoint Inhibitors
2.1.1. Atezolizumab
2.1.2. Durvalumab
2.1.3. Pembrolizumab
2.1.4. Avelumab
2.1.5. Nivolumab
2.1.6. Ipilimumab
2.1.7. Ipilimumab and Gemcitabine-Cisplatin Combination
2.1.8. Nivolumab and Ipilimumab Combination
3. First-Line Immunotherapy in Cisplatin-Ineligible Metastatic Urothelial Carcinoma
3.1. Atezolizumab
3.2. Pembrolizumab
4. Novel Tumor-Targeted Immunotherapeutics
4.1. ALT-801: Tumor-Targeted Interleukin-2 Immunotherapeutic
4.2. ALT-803: Interleukin-15 Superagonist Complex
5. Immunotherapy in Non-Muscle Invasive Bladder Cancer
6. Immunotherapy in Adjuvant and Neoadjuvant Setting
7. Immunotherapy and Radiation in Bladder Cancer
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; de Mulder, P.; Schornagel, J.H.; Theodore, C.; Fossa, S.D.; van Oosterom, A.T.; Witjes, J.A.; Spina, M.; van Groeningen, C.J.; Duclos, B.; et al. Seven year update of an eortc phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic m-vac in advanced urothelial tract tumours. Eur. J. Cancer 2006, 42, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Sternberg, C.N.; Rosenberg, J.E.; Hahn, N.M.; Galsky, M.D.; Vogelzang, N.J. Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol. 2010, 11, 861–870. [Google Scholar] [CrossRef]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.L.; et al. Mpdl3280a (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Mazieres, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Pembrolizumab [package insert]. Merck Sharp & Dohme Corp: County Cork, Ireland, 2014.
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Montgomery, R.B.; Heath, K. Pembrolizumab (MK-3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYNOTE-012. J. Clin. Oncol. 2015, 33 (Suppl. S15), 4502. [Google Scholar]
- Gupta, S.; O’Donnell, P.H.; Plimack, E.R.; Berger, R.; Montgomery, B.; Heath, K.; Dolled-Filhart, M.; Pathiraja, K.; Gause, C.K.; Cheng, J.; et al. A Phase 1b Study of Pembrolizumab (Pembro; MK-3475) for Advanced Urothelial Cancer. In Proceedings of the 2015 AUA Annual Meeting, New Orleans, LA, USA, 15–19 May 2015. Abstract MP68-11.
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Keynote-045: Open-label, phase III study of pembrolizumab versus investigator’s choice of paclitaxel, docetaxel, or vinflunine for previously treated advanced urothelial cancer. In Proceedings of the 2016 SITC Annual Meeting, National Harbor, MD, USA, 9–13 November 2016.
- Apolo, A.B.; Infante, J.R.; Hamid, O.; Patel, M.R.; Wang, D.; Kelly, K. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: Analysis of safety, clinical activity, and PD-L1 expression. J. Clin. Oncol. 2016, 34, 4514. [Google Scholar]
- Kazandjian, D.; Suzman, D.L.; Blumenthal, G.; Mushti, S.; He, K.; Libeg, M.; Keegan, P.; Pazdur, R. Fda approval summary: Nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016, 21, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bono, P.; Kim, J.W.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N. Efficacy and safety of nivolumab monotherapy in metastatic urothelial cancer (mUC): Results from the phase I/II CheckMate 032 study. J. Clin. Oncol. 2016, 34, 4501. [Google Scholar]
- Sharma, P.; Callahan, M.K.; Bono, P.; Kim, J.; Spiliopoulou, P.; Calvo, E.; Pillai, R.N.; Ott, P.A.; de Braud, F.; Morse, M.; et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (checkmate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016, 17, 1590–1598. [Google Scholar] [CrossRef]
- Galsky, M.D.; Retz, M.; Siefker-Radtke, A.O.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Efficacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: Results from the phase II CheckMate-275 study. In Proceedings of the 2016 ESMO Congress, Copenhagen, Denmark, 7–11 October 2016. Abstract LBA31.
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Carthon, B.C.; Wolchok, J.D.; Yuan, J.; Kamat, A.; Ng Tang, D.S.; Sun, J.; Ku, G.; Troncoso, P.; Logothetis, C.J.; Allison, J.P.; et al. Preoperative ctla-4 blockade: Tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 2010, 16, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Hahn, N.M.; Albany, C.; Fleming, M.T.; Starodub, A.; Twardowski, P.; Pal, S.K.; Hauke, R.J.; Sonpavde, G.; Oh, W.K.; et al. Phase II trial of gemcitabine + cisplatin + ipilimumab in patients with metastatic urothelial cancer. J. Clin. Oncol. 2016, 34, 357. [Google Scholar]
- Balar, A.V.; Galsky, M.D.; Loriot, Y.; Dawson, N.A.; Necchi, A.; Srinivas, S. Atezolizumab (atezo) as first-line (1L) therapy in cisplatin-ineligible locally advanced/metastatic urothelial carcinoma (mUC): Primary analysis of IMvigor210 cohort 1. J. Clin. Oncol. 2016, 34, LBA4500. [Google Scholar]
- Balar, A.; Bellmunt, J.; O’Donnell, P.; Castellano, D.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.; Hahn, N.; de Wit, R. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: Preliminary results from the phase 2 keynote-052 study. Ann. Oncol. 2016, 27, LBA32_PR. [Google Scholar]
- Wen, J.; Zhu, X.; Liu, B.; You, L.; Kong, L.; Lee, H.I.; Han, K.P.; Wong, J.L.; Rhode, P.R.; Wong, H.C. Targeting activity of a TCR/IL-2 fusion protein against established tumors. Cancer Immunol. Immunother. 2008, 57, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.N.; Thompson, J.A.; Pennock, G.K.; Gonzalez, R.; Diez, L.M.; Daud, A.I.; Weber, J.S.; Huang, B.Y.; Tang, S.; Rhode, P.R.; et al. Phase I trial of ALT-801, an interleukin-2/T-cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin. Cancer Res. 2011, 17, 7765–7775. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.N.; Vaena, D.A.; Singh, P.; Picus, J.; Vaishampayan, U.N.; Slaton, J.; Mahoney, J.F.; Agarwala, S.S.; Rosser, C.J.; Landau, D.; et al. Phase Ib/II study of an IL-2/T-cell receptor fusion protein in combination with gemcitabine and cisplatin in advanced or metastatic chemo-refractory urothelial cancer (UC). J. Clin. Oncol. 2015, 33, 4515. [Google Scholar]
- Kim, P.S.; Kwilas, A.R.; Xu, W.; Alter, S.; Jeng, E.K.; Wong, H.C.; Schlom, J.; Hodge, J.W. Il-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15Sa/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of nk and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016, 7, 16130–16145. [Google Scholar] [PubMed]
- Gomes-Giacoia, E.; Miyake, M.; Goodison, S.; Sriharan, A.; Zhang, G.; You, L.; Egan, J.O.; Rhode, P.R.; Parker, A.S.; Chai, K.X.; et al. Intravesical ALT-803 and bcg treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and nk cell expansion. PLoS ONE 2014, 9, e96705. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Pettus, J.A.T.; Herr, H.W.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Russo, P.; Boyle, M.G.; Milowsky, M.I.; Bajorin, D.F. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer 2008, 113, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
| Immunotherapy Trials in Metastatic or Unresectable Bladder Cancer, 1st Line Therapy Setting | ||||
| Intervention | Indication | Arms/Phase | Primary Endpoint | Trial ID |
| Durvalumab ± Tremelimumab | Metastatic BC | chemotherapy/III | PFS | NCT02516241 |
| n = 525 | ||||
| Nivolumab | Metastatic BC or metastatic melanoma | ±Ipilimumab/II | ORR | NCT02553642 |
| n = 120 | ||||
| Cisplatin/gemcitabine + Ipilimumab | BC | Single/II | 1-year OS | NCT01524991 |
| n = 3 | ||||
| ALT-801 + cisplatin/gemcitabine | BC | Single/Ib-II | Safety | NCT01326871 |
| n = 90 | ||||
| CVA21 + Pembrolizumab + standard chemotherapy | BC | Single/I | Safety | NCT02043665 |
| n = 90 | ||||
| Immunotherapy Trials in Metastatic or Unresectable Bladder Cancer, 2nd Line Therapy Setting | ||||
| Intervention | Indication | Arms/Phase | Primary Endpoint | Trial ID |
| Atezolizumab | Metastatic BC | Single/IV | n/a | NCT02589717 |
| n = n/a | ||||
| Atezolizumab | Metastatic BC | Taxane or Vinflunine/III | OS | NCT02302807 |
| n = 931 | ||||
| Avelumab | Maintenance after chemotherapy | BSC/III | OS | NCT02603432 |
| n = 668 | ||||
| Pembrolizumab | Metastatic BC | Taxane or Vinflunine/III | OS and PFS | NCT02256436 |
| n = 470 | ||||
| Atezolizumab | Metastatic BC | Single/II | ORR | NCT02108652 |
| n = 439 | ||||
| Pembrolizumab | Metastatic BC | Single/II | ORR | NCT02335424 |
| n = 350 | ||||
| Nivolumab | Metastatic BC | Single/II | ORR | NCT02387996 |
| n = 242 | ||||
| Pembrolizumab | Maintenance after chemotherapy | Placebo/II | PFS | NCT02500121 |
| n = 200 | ||||
| Tremelimumab, followed by Durvalumab vs. combo | Many cancer types | Two arms/II | ORR | NCT02527434 |
| n = 76 | ||||
| aCP-196+ Pembrolizumab | Metastatic BC | Single/II | ORR | NCT02351739 |
| n = 74 | ||||
| EphB4-HAS + Pembrolizumab | Metastatic BC | Single/II | OS | NCT02717156 |
| n = 64 | ||||
| Nivolumab | Multiple solid tumors | ±ipilimumab/ Ib-II | ORR | NCT01928394 |
| n = 1100 | ||||
| PLX3397 + Pembrolizumab | Multiple solid tumors | Single/Ib-II | Safety | NCT02452424 |
| n = 400 | ||||
| Urelumab + Nivolumab | Metastatic BC | Single/Ib-II | Safety | NCT02253992 |
| n = 200 | ||||
| Ulocuplumab + Nivolumab | Multiple solid tumors | Single/Ib-II | Safety | NCT02472977 |
| n = 195 | ||||
| Lenvatinib + Pembrolizumab | Metastatic BC | Single/Ib-II | Safety, ORR | NCT02501096 |
| n = 150 | ||||
| Varlilumab + Atezolizumab | Metastatic BC | Single/Ib-II | Safety, ORR | NCT02543645 |
| n = 55 | ||||
| Pembrolizumab + Vorinostat | Metastatic BC or RCC | Single/Ib-II | Safety | NCT02619253 |
| n = 42 | ||||
| CDX-1401 + Poly ICLC + Pembrolizumab | Metastatic BC | Single/Ib-II | Safety | NCT02661100 |
| n = 26 | ||||
| Durvalumab ± AZD4547, Olaparib, AZD1775 | Metastatic BC | Four arms/Ib | Safety | NCT02546661 |
| n = 40 | ||||
| Avelumab | Multiple solid tumors | Single/I | Safety | NCT01772004 |
| n = 1670 | ||||
| CPI-444 ± Atezolizumab | Multiple solid tumors | Single/I | Safety | NCT02655822 |
| n = 534 | ||||
| Lirilumab + Nivolumab | Many cancer types | Single/I | Safety | NCT01714739 |
| n = 162 | ||||
| Enoblituzumab (MGA271) | Many cancer types | Single/I | Safety | NCT01391143 |
| n = 151 | ||||
| MGD009 | Many cancer types | Single/I | Safety | NCT02628535 |
| n = 114 | ||||
| Ramucirumab + Pembrolizumab | Multiple solid tumors | Single/I | Safety | NCT02443324 |
| n = 92 | ||||
| Ipililumumab + MGA271 | Many cancer types | Single/I | Safety | NCT02381314 |
| n = 84 | ||||
| Cabozantinib + Nivolumab | Metastatic BC | ±ipilimumab/I | Safety | NCT02496208 |
| n = 66 | ||||
| Pembrolizumab + gemcitabine or Docetaxel | Metastatic BC | Docetaxel or gemcitabine/I | Safety | NCT02437370 |
| n = 38 | ||||
| Enadenotucirev + Pembrolizumab | Multiple solid tumors | Single/I | Safety | NCT02636036 |
| n = 30 | ||||
| Interferon Gamma + Nivolumab | Metastatic BC | Single/I | Safety | NCT02614456 |
| n = 15 | ||||
| p53MVA + Pembrolizumab | Metastatic BC | Single/I | Safety | NCT02432963 |
| n = 12 | ||||
| Immunotherapy Trials in Non-Muscle Invasive Bladder Cancer Setting | ||||
|---|---|---|---|---|
| Intervention | Indication | Arms/Phase | Primary Endpoint | Trial ID |
| Recombinant Adenovirus CG0070 | Superficial BC after BCG failure | Single arm/III | Durable CR | NCT02365818 |
| n = 122 | ||||
| Pembrolizumab | Superficial BC after BCG failure | Single arm/II | CR, DFS | NCT02625961 |
| n = 260 | ||||
| Recombinant Adenovirus CG0070 | Superficial BC after BCG failure | Four arms/II | Durable CR, CR | NCT01438112 |
| n = 222 | ||||
| Pembrolizumab + gemcitabine + radiation + TURBT | Superficial BC with bladder preservation | Single/II | Two-year DFS | NCT02621151 |
| n = 54 | ||||
| Atezolizumab | Superficial BC after BCG failure; neoadjuvant therapy for invasive BC ineligible for platinum | Single arm/II | CR | NCT02451423 |
| n = 42 | ||||
| Pembrolizumab + cisplatin + radiation | Superficial BC after TURBT with bladder preservation | Single/II | Safety | NCT02662062 |
| n = 30 | ||||
| ALT-803 + BCG | Superficial BC | BCG/Ib-II | Safety | NCT02138734 |
| n = 115 | ||||
| HS-410 ± BCG | Superficial BC after BCG failure | ±BCG/I-II | Safety, one-year DFS | NCT02010203 |
| n = 110 | ||||
| ALT-801 + gemcitabine | Superficial BC after BCG failure | Single arm/Ib-II | Safety | NCT01625260 |
| n = 52 | ||||
| Pembrolizumab + BCG | Superficial BC after BCG failure | Single arm/I | Safety | NCT02324582 |
| n = 15 | ||||
| Immunotherapy Trials in Neoadjuvant Therapy Setting | ||||
| Intervention | Indication | Arms/Phase | Primary Endpoint | Trial ID |
| Pembrolizumab + Cystectomy | Invasive BC | Single/II | CR | NCT02736266 |
| n = 90 | ||||
| Atezolizumab | Invasive BC | Single/II | CR | NCT02662309 |
| n = 85 | ||||
| Pembrolizumab + cisplatin + gemcitabine | Invasive BC | Single/II | Downstaging | NCT02690558 |
| n = 39 | ||||
| Pembrolizumab + cisplatin ± gemcitabine | Invasive BC, Italian locations only | Single/Ib-II | Safety, CR | NCT02365766 |
| n = 81 | ||||
| Immunotherapy Trials in Adjuvant Therapy Setting | ||||
| Intervention | Indication | Arms/Phase | Primary Endpoint | Trial ID |
| Nivolumab | Invasive BC | Placebo/III | ORR | NCT02632409 |
| n = 640 | ||||
| Atezolizumab | Invasive BC, no neoadjuvant therapy, PD-L1 positive stain | Observation/III | DFS | NCT02450331 |
| n = 440 | ||||
| Combination of Immunotherapy and Radiation Therapy in Superficial Urothelial Carcinoma | ||||
| Pembrolizumab + radiation | Superficial BC, ineligible for surgery or concurrent chemotherapy | Single/I | Safety | NCT02560636 |
| n = 34 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Gill, D.; Poole, A.; Agarwal, N. Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions. Cancers 2017, 9, 15. https://doi.org/10.3390/cancers9020015
Gupta S, Gill D, Poole A, Agarwal N. Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions. Cancers. 2017; 9(2):15. https://doi.org/10.3390/cancers9020015
Chicago/Turabian StyleGupta, Shilpa, David Gill, Austin Poole, and Neeraj Agarwal. 2017. "Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions" Cancers 9, no. 2: 15. https://doi.org/10.3390/cancers9020015
APA StyleGupta, S., Gill, D., Poole, A., & Agarwal, N. (2017). Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions. Cancers, 9(2), 15. https://doi.org/10.3390/cancers9020015




