Membrane Drug Transporters and Chemoresistance in Human Pancreatic Carcinoma
Abstract
: Pancreatic cancer ranks among the tumors most resistant to chemotherapy. Such chemoresistance of tumors can be mediated by various cellular mechanisms including dysregulated apoptosis or ineffective drug concentration at the intracellular target sites. In this review, we highlight recent advances in experimental chemotherapy underlining the role of cellular transporters in drug resistance. Such contribution to the chemoresistant phenotype of tumor cells or tissues can be conferred both by uptake and export transporters, as demonstrated by in vivo and in vitro data. Our studies used human pancreatic carcinoma cells, cells stably transfected with human transporter cDNAs, or cells in which a specific transporter was knocked down by RNA interference. We have previously shown that 5-fluorouracil treatment affects the expression profile of relevant cellular transporters including multidrug resistance proteins (MRPs), and that MRP5 (ABCC5) influences chemoresistance of these tumor cells. Similarly, cell treatment with the nucleoside drug gemcitabine or a combination of chemotherapeutic drugs can variably influence the expression pattern and relative amount of uptake and export transporters in pancreatic carcinoma cells or select for pre-existing subpopulations. In addition, cytotoxicity studies with MRP5-overexpressing or MRP5-silenced cells demonstrate a contribution of MRP5 also to gemcitabine resistance. These data may lead to improved strategies of future chemotherapy regimens using gemcitabine and/or 5-fluorouracil.1. Introduction
Pancreatic cancer is the fourth most common cause of cancer-related death in the Western world, with an estimated 36,800 deaths in 2010 in the United States [1]. Because early diagnostic markers are missing and due to a lack of early symptoms, less than 20% of the diagnosed pancreatic cancer patients are considered for a potentially curative resection, and many patients who undergo tumor resection suffer from recurrences and die within the first three years [2]. Despite chemotherapy, the median survival time of patients with advanced disease is only about six months and the five-year-survival rate is below 4% [3], mostly because of an almost complete resistance against established radio/chemotherapies.
Such resistance of human cells to the cytotoxic action of chemotherapeutic drugs can be the result of various mechanisms, of which three major mechanisms have been identified: a reduced uptake of the drugs into the target cells; alterations within the cells, like altered metabolism of the drugs, increased capacity to repair DNA or reduced apoptosis; and an increased efflux of the drugs from the cells [4]. Another mechanism related to chemresistance is the so-called cell-adhesion mediated drug resistance (CAM-DR) [5]. Earlier, pancreatic cancer was usually treated with 5-fluorouracil (5-FU). Today, gemcitabine chemotherapy is the standard of care for advanced and metastatic pancreatic cancer, however, 5-FU and its orally available analogue capecitabine are both acknowledged for first line therapy after surgery [6] and second-line therapy in advanced pancreatic cancer [7]. All drugs are nucleoside analogues; therefore, some of the above mentioned resistance mechanisms will be exemplified with the drugs 5-FU or gemcitabine. Water soluble drugs such as nucleoside analogues or cisplatin require specialized membrane transporters to efficiently enter the cell. The cytidine analog gemcitabine (2′-,2′-difluorodeoxycytidine) is taken up into cells primarily through the specialized concentrative or equilibrative nucleoside transporters CNT1 (gene symbol: SLC28A1), CNT3 (SLC28A3), ENT1 (SLC29A1) and ENT2 (SLC29A2) [8-11]. In pancreatic tumor cells, expression of ENT1 or CNT1 has both previously been linked to gemcitabine resistance or sensitivity of pancreatic cancer cells [12-14]. Most importantly, recent studies showed that expression levels of ENT1 and CNT3 are predictive for patient survival times after gemcitabine treatment [15,16].
Within the cells, gemcitabine has to be activated by phosphorylation to the active triphosphate metabolite dFdCTP, which then is incorporated into DNA. The enzyme deoxycytidine kinase (DCK) catalyzes the first rate-limiting step in these reactions [17]. Consequently, DCK is associated with prolonged survival of patients after adjuvant gemcitabine therapy for resected pancreatic ductal adenocarcinoma (PDAC) [18], and downregulation of DCK in vitro enhances resistance against gemcitabine in pancreatic tumor cells [19]. Moreover, the RNA-binding protein HuR modulates the translation of target mRNAs including DCK mRNA, in which case HuR overexpression elevates, and HuR silencing reduces DCK protein expression; thus, HuR expression was also found to be a outcome predictor for patients with resected PDAC during adjuvant gemcitabine therapy [20,21].
Finally, drug efflux from the cell is efficiently mediated by proteins belonging to the ATP-binding cassette (ABC) family of transporters [22-25]. Several members of the multidrug resistance protein family (MRPs), MDR1 P-glycoprotein and the breast cancer resistance protein (BCRP) have been demonstrated to confer chemoresistance or multidrug resistance [22-28]. These ABC transporters belong to the ABCC (MRP–MRP9; gene symbol: ABCC1–ABCC6 and ABCC10–ABCC12), ABCB (MDR1 P-glycoprotein; symbol ABCB1), and ABCG (BCRP; symbol ABCG2) families of ABC transporters, respectively. In addition to their natural endogenous substrates, the MRP proteins, MDR1, and BCRP also transport a vast array of cytotoxic substances (for review, see [4,29], and MRP3 (ABCC3), MRP4 (ABCC4), and MRP5 have been demonstrated to confer resistance against chemotherapeutic drugs such as etoposide, 5-fluorouracil (5-FU), and gemcitabine [26,27,30-32]. These transporters, among other ABC transporters, are expressed in normal or diseased human pancreas [33-38]. Conversely, the apparent cellular expression profile of transporters can be altered by chemotherapeutic drugs [26,27,38-40], leading to a cellular phenotype which can also be the result of a drug-induced selection mechanism for pre-existing subpopulations of cells.
Given the importance of ABC transporter expression for chemoresistance, the analysis of regulatory mechanisms resulting in overexpression of these transporters is mandatory to identify new targets, which may help to overcome the resistance phenotype of PDAC. For example, several ABC transporter genes are transcriptionally regulated by nuclear factor (erythroid-derived 2)-like 2 (Nrf2) protein. Overexpression of Nrf2 protein levels in pancreatic cancer cells resulted in increased drug resistance, whereas a reduction in Nrf2 protein decreased drug resistance. These changes in drug resistance or sensitivity were also positively correlated to the expression levels of Nrf2 downstream genes ABCG2, MRP1, MRP2 and MRP4 [41]. Moreover, constitutive activation of the hedgehog (HH) pathway induces chemoresistance in a variety of cancers including PDAC by regulation of ABC transporter expression, like ABCB1 and ABCG2. Interfering with HH activation sensitizes the tumor cells towards the cytotoxic effects of chemotherapy [42-46]. Moreover, a number of studies have demonstrated inhibition of ABC transporters by flavonoids, thus potentially affecting the distribution and cytotoxicity of chemotherapeutic drugs [47,48]. miRNAs, small non-coding RNAs regulating gene expression, have been shown to influence chemoresistance in several cancers. In PDAC, patients with high miR-21 expression had a significantly shorter overall survival, and low miR-21 expression was associated with benefit from adjuvant treatment [49]. In vitro, anti-miR-21 increased anticancer drug activity, while pre-miR-21 transfection significantly decreased antiproliferative effects and apoptosis induction by gemcitabine [50,51]. Moreover, hsa-miR-520h was shown to downregulate ABCG2 and to inhibit migration, invasion and side populations (SP) in pancreatic cancer cells [52].
Further, enhanced expression of ABC transporters seems to be characteristic for SP cells with cancer stem cell features [53,54]. These stem cells are more resistant against chemotherapies and may play a key role in tumor progression, recurrence and metastasis [55-58]. A further possible link between transporters and chemoresistance is represented by mucin MUC4. Interestingly, MUC4 is overexpressed in PDAC tissue with no significant expression in normal pancreatic tissue (for review: see [59]). Overexpression of MUC4 in PDAC cell lines was associated with a higher resistance against gemcitabine and treatment of MUC4-overexpressing PDAC cells with gemcitabine resulted in an enrichment of SP cells. In contrast, in MUC4 knockdown PDAC cells, all cells including the SP fraction were responsive to the cytotoxic effects induced by gemcitabine treatment [60]. Our own studies thus aim to link data on transporter expression, chemoresistance, and chemosensitivity, especially regarding gemcitabine treatment, to this complex field of pancreatic cancer cell biology in order to provide new aspects for improved future treatment of this aggressive cancer type.
2. Results and Discussion
Endogenous compounds or xenobiotics including chemotherapeutic drugs enter or leave human cells most efficiently via specialized uptake or export transporters localized at the appropriate cellular membrane domain. Thus, the detailed knowledge of the specific transporter expression profile of a certain cell, tissue, or organ is a prerequisite to understand and improve drug delivery and therapeutic effectiveness at the body site in question.
2.1. Expression Profile of Uptake Transporters of the OATP Family in Pancreatic Cells
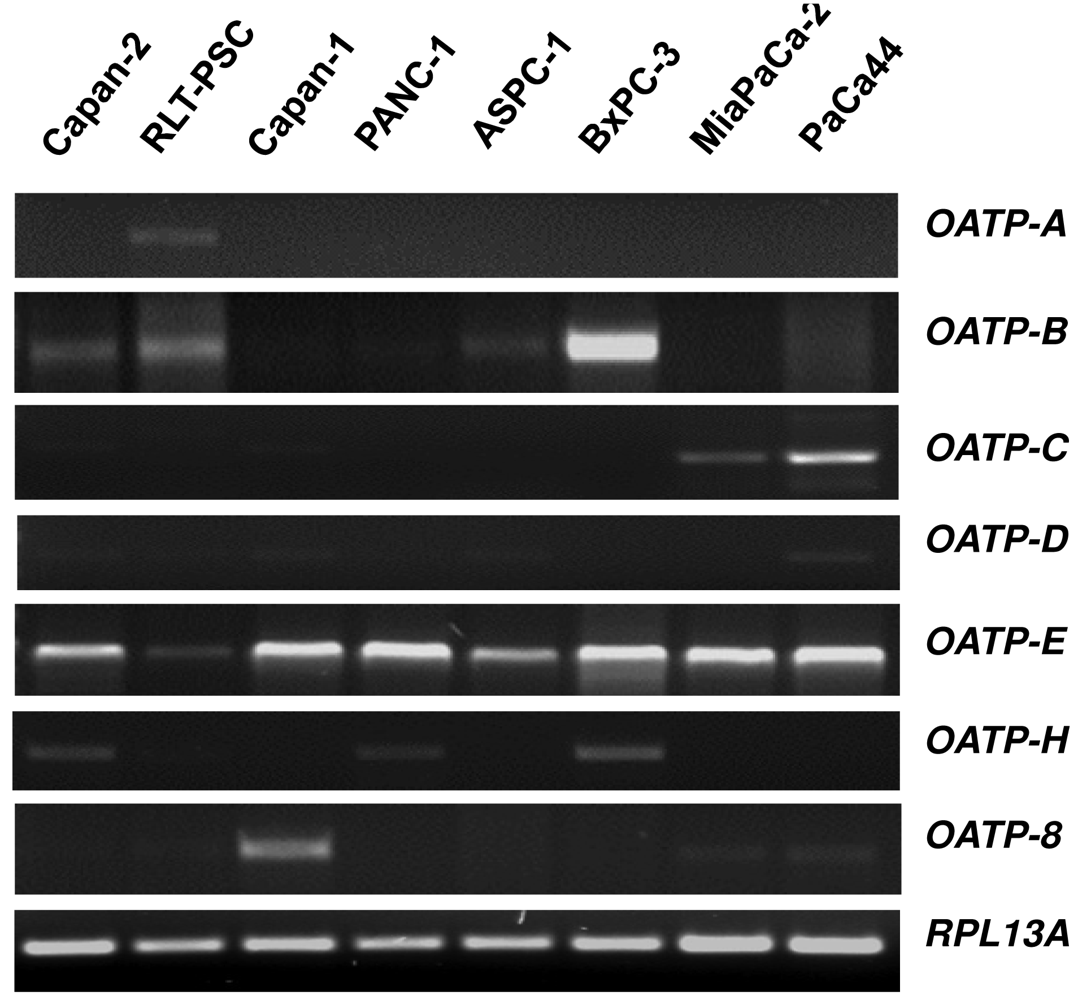
Organic anion transporting polypeptides (OATPs) represent membrane transport proteins mediating the sodium-independent transport of a wide range of amphipathic organic compounds including bile salts, organic dyes, steroid conjugates, thyroid hormones, anionic oligopeptides, drugs and other xenobiotic substances [61]. Whereras some OATPs are apparently selectively expressed in liver [62,63], most OATPs are expressed in multiple tissues [64]. Our novel data on the OATP mRNA expression spectrum using a panel of eight different human pancreatic cells showed that among the seven OATP family members studied, only OATP-E was expressed in all pancreatic cells (Figure 1), whereas OATP-F, OATP-I, and PGT were not detectable (data not shown). OATP-B, OATP-C and OATP-H were detected in some cell lines, but OATP-A, OATP-D, and OATP-8 only in one of these cells. Although our in vitro mRNA data do not necessarily reflect the respective cellular OATP protein expression, they may indicate that OATP-E is the main family member of these uptake transporters present in pancreatic cells. OATP-E has been reported to mediate uptake of taurocholate, thyroid hormones, and PGE2 [65]. So far, OATP-E has not been demonstrated to transport chemotherapeutic drugs. Further studies will thus clarify whether OATP-E is also involved in the uptake of chemotherapeutic drugs in pancreatic cells.
2.2. mRNA Expression Profile of ABC Transporters of the MRP Family in Pancreatic Cells
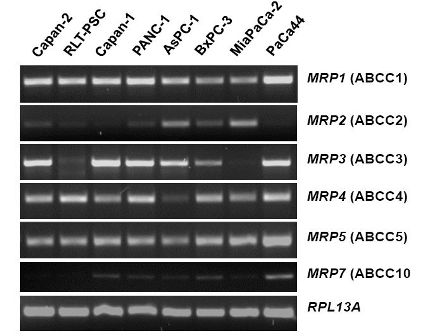
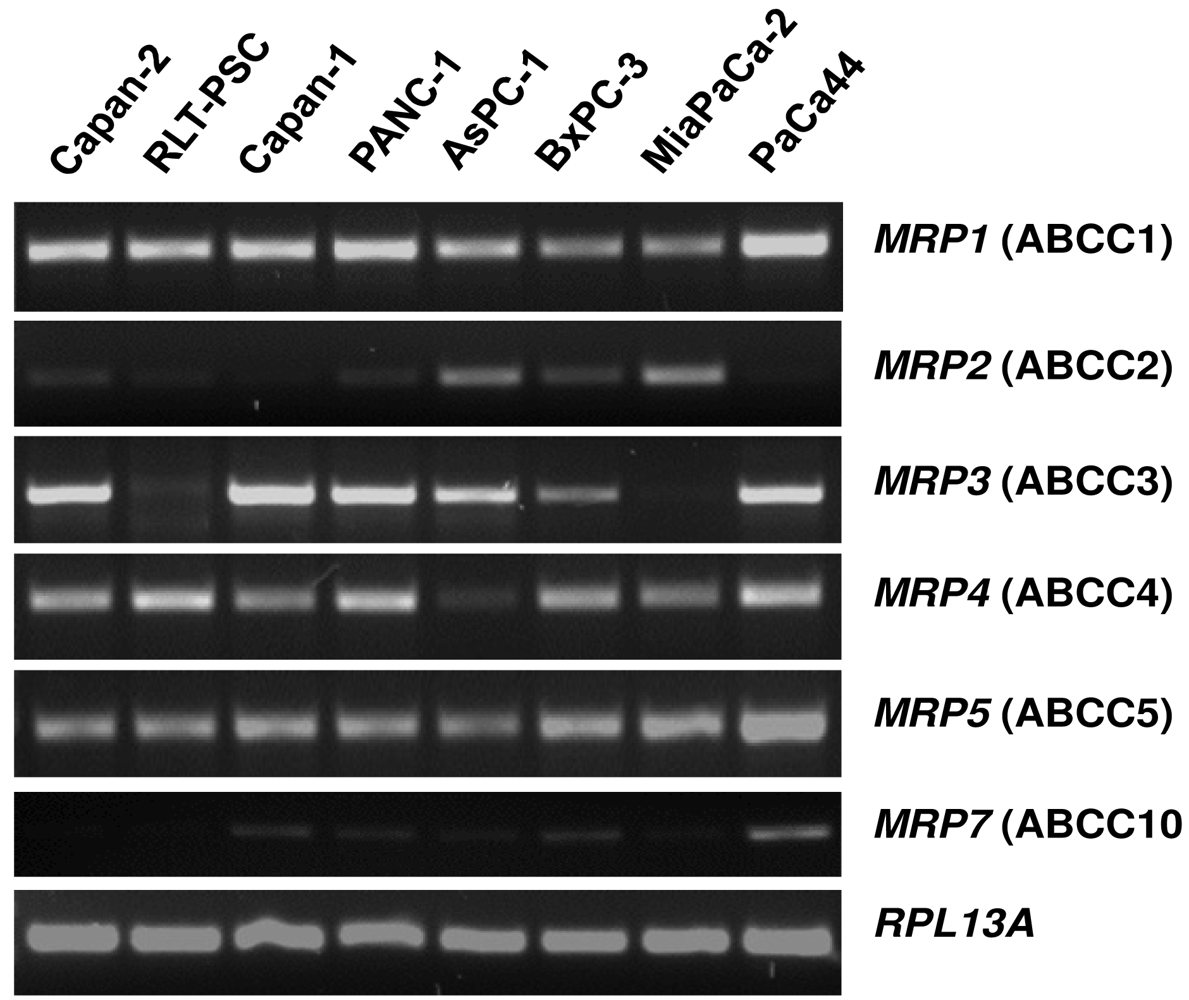
The mRNA expression profile of MRP family members in human pancreatic carcinoma cell lines (Figure 2) closely resembles the pattern found in normal pancreatic duct cells [26]. MRP1, MRP3, MRP4, and MRP5 mRNAs are strongly expressed in most of these cancer cells, while MRP2 and MRP7 are only weakly expressed (Figure 2), and MRP6, MRP8, and MRP9 mRNAs were not detected in any of these pancreatic cancer cell lines (data not shown). For comparison, the human pancreatic stellate cell line, RLT-PSC [67], shows strong expression of MRP1, MRP4, and MRP5 mRNA, but very low expression of MRP2 and MRP3 mRNA (Figure 2); this finding may be relevant in view of the discussed role of this pancreatic cell type in the development of chronic pancreatitis and pancreatic cancer [68-71].
2.3. Altered MRP Expression Profile in 5-FU-Resistant Pancreatic Cancer Cells
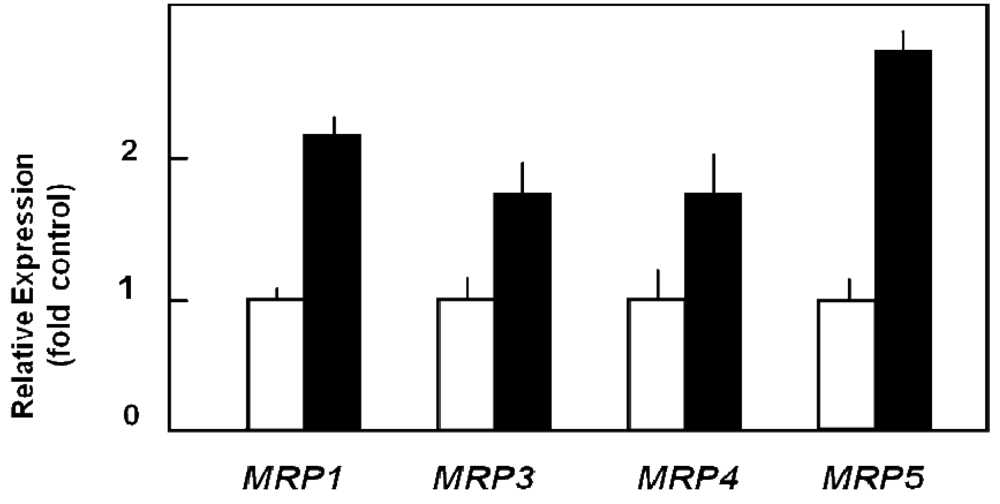
Capan-1 cells are characterized by relatively pronounced sensitivity to the cytotoxic action of 5-FU [72,73], but can be adapted to acquire high resistance to this drug by prolonged exposure to it. Such 5-FU-resistant Capan-1 cells, which possess a 27-fold increased LD50 value for 5-FU as compared to parental Capan-1 cells [74], show indeed an altered MRP expression profile with about two-fold upregulation of several MRP mRNAs (Figure 3) [26]. This increased MRP expression in 5-FU resistant cells is also reflected in the MRP protein level [26]. Most importantly, this elevated ABC transporter protein level in 5-FU resistant cells holds true for the relatively highly expressed MRP3, MRP4, and MRP5 proteins, of which at least MRP5 and MRP8 have been demonstrated to confer drug resistance to 5-FU [31,75]. This suggested role of MRP5 in mediating 5-FU chemoresistance was tested in pancreatic cancer cells by specific RNA interference silencing MRP5 expression. Indeed, such MRP5-silenced Capan-1 cells in which MRP5 mRNA expression was downregulated to about 25% of that in control cells [26] showed an increased sensitivity to 5-FU as compared to parental or vector-transfected Capan-1 cells (Table 1) [26]. The contribution of MRP5 to 5-FU resistance was confirmed in a more recent study using Patu-02 pancreatic cancer cells where knock down of MRP5 also significantly increased cellular cytotoxicity of 5-FU [76].
2.4. Cellular Protein Pattern in 5-FU-Resistant Pancreatic Cancer Cells
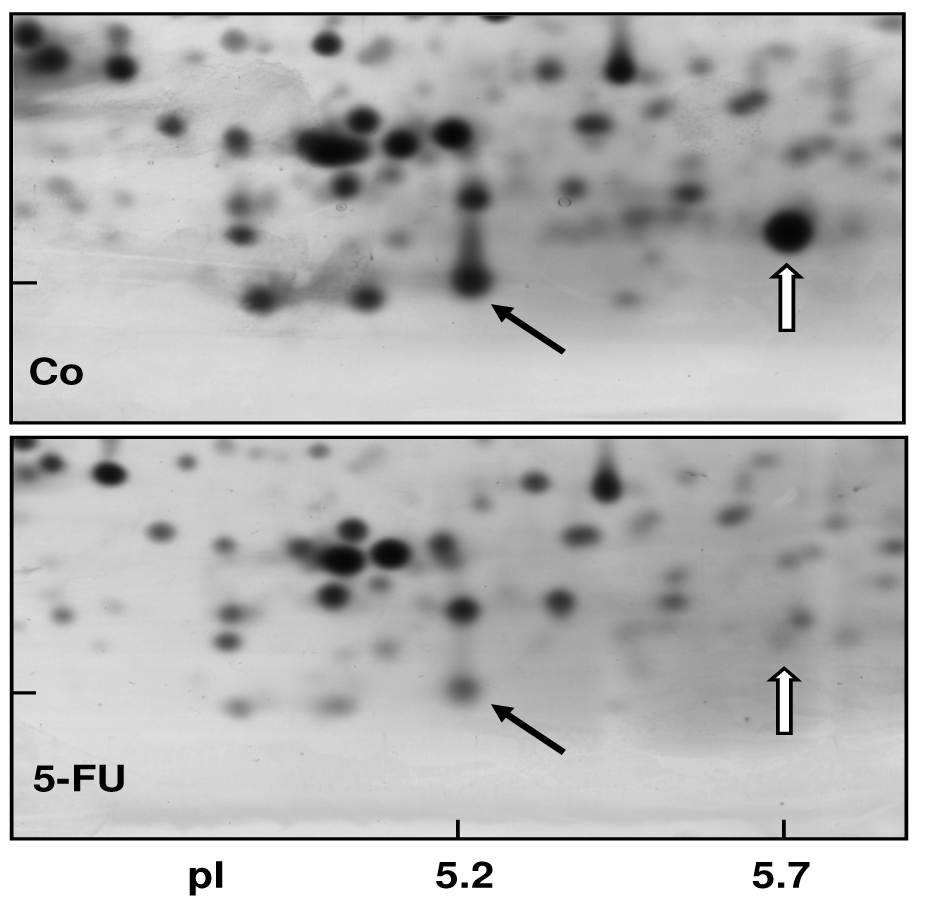
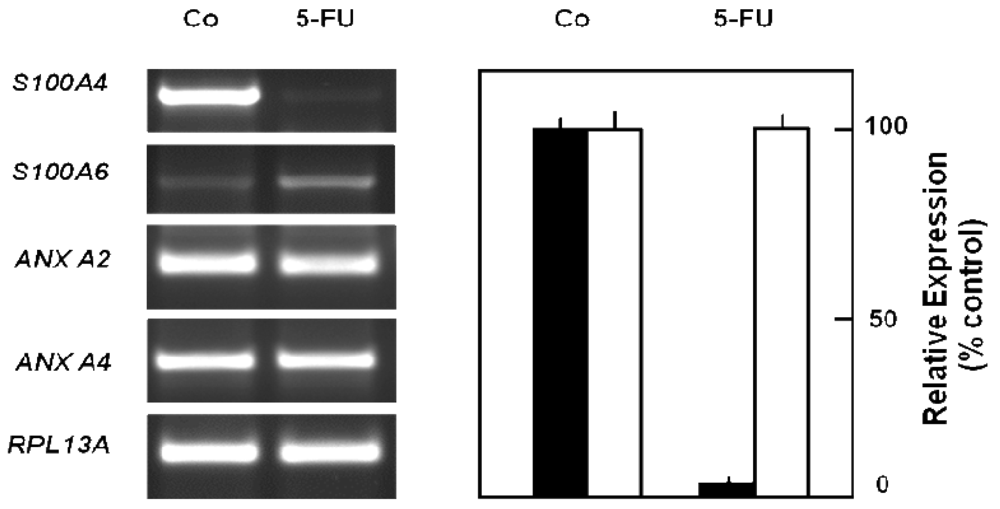
In order to obtain a direct fingerprint of cellular drug response, we analyzed the protein expression profiles of 5-FU resistant cells compared to parental pancreatic Capan-1 cancer cells by two-dimensional fluorescence difference gel electrophoresis (2D-DIGE). Among the approximately 1,200 separated proteins, the resistant cells showed altered expression of several proteins (cut-off: at least two-fold up-or down-regulated expression), for example, Annexin A2 and A4, and also the S100A6 protein spot which was diminished to about 30% of the amount in parental Capan-1 cells (Table 2). However, 5-FU resistant cells particularly showed massively reduced expression of a protein identified by ESI-MS/MS and confirmed by Western blot as the Ca-binding protein S100A4 (Figure 4). Interestingly, this S100A4 protein is discussed to have a role in cancer metastasis and chemoresistance [77-79]. Recently, S100A4 knockdown was reported to increase sensitivity of pancreatic cancer cells towards gemcitabine [80]. It remains to be clarified whether the apparently contradictory relation between S100A4 expression and chemoresistance is due to the different drugs used (5-FU, gemcitabine), different cell lines, or different experimental setups (acute toxicity vs. acquired resistance). Our novel finding of a loss of S100A4 expression in 5-FU resistant cells was confirmed at the mRNA level (Figure 5). In contrast, the mRNA expression levels of S100A6 and of two members of the annexin family (ANX A2, ANX A4) were demonstrated to be practically unaltered in 5-FU resistant Capan-1 cells (Figure 5). Further studies including RNA interference strategies are underway to clarify which direct or indirect role S100A4 may play in the 5-FU resistance phenotype of pancreatic cancer cells.
2.5. Transporter Expression and Resistance in Gemcitabine Treated Pancreatic Cancer Cells
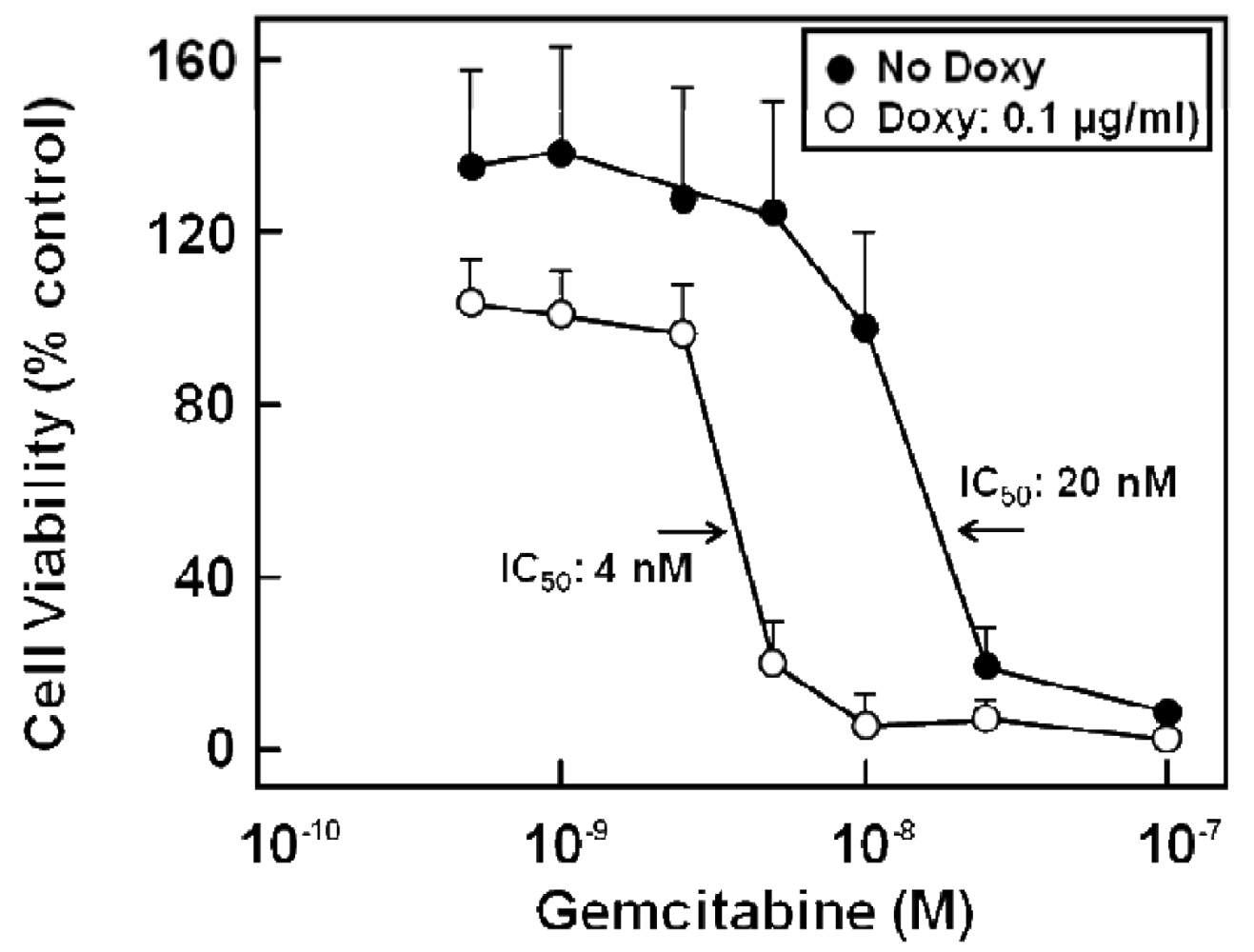
Various chemotherapy regimens using gemcitabine together with other drugs have been developed, some combining gemcitabine with 5-FU and resulting in improved benefit [81-83]. In addition, studies with human pancreatic cancer cells in vitro [39] or in a murine xenograft model [84] showed a better therapeutic effect when 5-FU was administered before gemcitabine. Furthermore, chemotherapeutic drug treatment of cells can alter the expression of nucleoside and ABC transporters [39,85], and drug export via MRP transporters contributes to cellular resistance against various chemotherapeutic compounds [23,25,26,28,30-32]. Indeed, gemcitabine alone or in combination with 5-FU treatment has been demonstrated to alter the expression of various MRPs, but also of uptake transporters of the CNT and ENT families [27]: While 5-FU or gemcitabine alone also elicited the enhanced expression of MRP5 and ENT1 mRNA, this upregulation was highest in all investigated pancreatic cancer cells after combined drug administration and was most prominent in Capan-1 and PANC-1 cells, with MRP5 increasing 33-fold, and ENT1 increasing 52-fold in Capan-1 cells [27]. Interestingly, the expression of the nucleoside transporter CNT3 increased more than 100-fold in 5-FU/gemcitabine-treated Capan-1 cells [27]. In addition, MRP5 had been suggested to affect chemoresistance against gemcitabine [32,86], and this role of MRP5 in chemoresistance, which we earlier demonstrated for 5-FU [23], is evident also from results obtained with stably MRP5-silenced pancreatic cancer cells. In these experiments, MRP5 mRNA expression was down to about 50% of controls as determined by QRT-PCR, and the cells possessed a markedly increased sensitivity to gemcitabine (Figure 6) [27].
3. Experimental Section
3.1. Cells and Drugs
Human pancreatic carcinoma cell lines (Capan-1, Capan-2, PANC-1, AsPC-1, BxPC-3, MiaPaCa-2, PaCa44) and immortalized pancreatic stellate cells (RLT-PSC) [67,87] were cultured at 37 °C, 5% CO2, and 95% humidity as reported [26]. Gemcitabine was obtained from Eli Lilly (Indianapolis, IN, U.S.), 5-FU from Teva (Kirchzarten, Germany), doxycycline from Sigma-Aldrich (St. Louis, MO, U.S.), and tetracycline-free fetal bovine serum from Clontech (Mountain View, CA, U.S.).
3.2. Generation of Resistant and Knockdown Cell Lines
Capan-1 cells with acquired resistance towards 5-FU (Capan-1/5-FU cells) were established as by adaptation of parental Capan-1 cells to increasing concentrations of 5-FU reaching 15.3 μM 5-FU (2 μg/mL) within 14 passages; these 5-FU resistant Capan-1 cells were subsequently maintained in medium containing 15.3 μM 5-FU. Silencing of endogenous MRP5 was achieved in two cell lines: in Capan-1 cells, RNA interference was performed using the BLOCK-iT™ Pol II miR RNAi expression vector kit (Invitrogen) with the pcDNA™6.2-GW/miR vector containing one of three miRNAs directed against different regions of the MRP5 mRNA (base position 266–286, 330–350, or 433–453), which were stably transfected into Capan-1 cells using Fugene HD (Roche, Mannheim, Germany). For control, Capan-1 cells were transfected with pcDNA™6.2-GW/miR-neg vector (Invitrogen) expressing pre-miRNA, the mature miRNA of which does not target any known vertebrate gene. Stable clones were selected using blasticidin (5 μg/mL) and analyzed by RT-PCR, QRT-PCR, and agarose gel electrophoresis for their relative expression of MRP5/RPL13A mRNA as reported [26]. Targeted MRP5 knockdown in PANC-1 cells was achieved using the pSingle-tTS-shRNA vector (Clontech) containing one of three doxycycline-inducible short hairpin RNAs (shRNAs) targeting MRP5 mRNA (oligos for bases 158–176 (target sense and antisense sequence in italics): 5′-TCGAGG-CCGTGAAGATTCCAAGTTC-TCAAGAGA-GAACTTGGAATCTTCACGG-CTTTTT TACGCGTA-3′ and 3′-CCGGCACTTCTAAGGTTCAAGAAGTTCTCTCTTGAACC-TTAGAAGTGCCGAAAAAATGCGCATTCGA-5′; for bases 368-386: 5′-TCGAGG-AGCTCAGAATCC TGGATGA-TTCAAGAGA-TCATCCAGGATTCTGAGCT-CTTTTTTACGCGT-A-3′ and 3′-CCTCGAGTCTTAGGACCT ACTAAGTTCTCTAGTAGGTCCTAAGACTC-GAGAAAAAATGCGCA TTCGA-5′; for bases 547–565: 5′-TCGAGG-CTCTCAATGGAAGACGTGT-TTCAAGAGA-ACACGTCTTCCATTGA GAG-CTTTTTTACGCGT-A-3′ and 3′-CCGAGAGTTACCTTCTGCACAAAGTTCTCTTGTGCAGAA GGTAACTCTCG-AAAAAATGCGCATTCGA-5′). Selected clones of these PANC- 1/shMRP5 cells were kept under geneticin (800 μg/mL) and were cultured in tetracycline-free medium. MRP5 silencing was checked by QRT-PCR using PANC-1/shMRP5 clones treated without or with doxycycline for 3-6 days with replenishment of doxycycline every 48 h.
3.3. Drug Treatment of Cells
Cells were incubated with gemcitabine (20 μM) for 1 h, then medium was replaced with fresh medium containing no gemcitabine, and RNA was isolated 72 h later. In experiments comparing the effects of single or combined treatment of cells, the following schedules were applied: (a) 5-FU (30 μM, 24 h), then fresh drug-free medium; (b) gemcitabine (20 μM, 1 h), then fresh drug-free medium; (c) 5-FU (30 μM, 24 h), then gemcitabine (20 μM, 1 h), then fresh drug-free medium. RNA isolation was performed 4 days after 5-FU addition or 3 days after gemcitabine treatment.
3.4. Cytotoxicity Studies
Cytotoxicity of 5-FU was performed as reported [26] with at least triplicate biological and technical replicates for each condition. Cell viability was determined after 3 days of continued drug treatment using the CytoScan WST-1 cell cytotoxicity assay (G-Biosciences, St.Louis, MO, U.S.). For determination of gemcitabine cytotoxicity in stably MRP5-silenced PANC-1/shMRP5 cells, cells were treated for 6 days without or with doxycycline (100 ng/mL), trypsinized, seeded into 96-well plates (3,000 cells/well), and exposed to gemcitabine for another 6 days before WST-1 assay; during the whole experiment, doxycycline was replenished every 48 h.
3.5. RT-PCR and QRT-PCR Analyses
mRNAs were detected or quantified by RT-PCR or QRT-PCR, respectively, with these specific sense and anti-sense primers: ENT1 (gene symbol: SLC29A1; accession: NM_004955), 5′ -AGTGGCTCGGAGCTATCAGA-3′ and 5′ GTGCTCGAA-GACCACAGTCA-3′ (588 bp fragment, bases 918-1505); CNT3 (gene symbol: SLC28A3; accession: NM_022127), 5′ -ATGAATTCAGCCCTGTCCTG-3′ and 5′-AAACGTGATGGCAGT-TGATG-3′ (484 bp fragment, bases 1482-1965). Annexin A2 (gene symbol: ANXA2; accession: NM_001002857), 5′ -AAAGTACGGCAAGTCCCTGT-3′ and 5′ -TTGGGGGTAATGCTAAC-GTC-3′ (223 bp fragment, bases 1063-1285); Annexin A4 (gene symbol: ANXA4; accession: NM_001153), 5′-TGGAAAGTCTCTGTACTCGTTCAT-3′ and 5′-GCTTTGAAATGCA-AGTACAGCTT-3′ (294 bp fragment, bases 952-1245); S100A4 (accession: NM_019554), 5′-CTTGCACACGCTGTTGCTAT-3′ and 5′-AACTTGCTCAGCATCAAGCA-3′ (467 bp fragment, bases 73-539); S100A6 (accession: NM_014624), 5′-CGACCGCTATAAGGCCAGT-3′ and 5′- CCCACCACTGGATTTGACTC-3′ (437 bp fragment, bases 214-650); OATP-A (gene symbol: SLCO1A2; accession: NM_021094), 5′-CCCACATAGGATGTTGGTTATCC-3′ and 5′-ATGTATGTAATCCCACACCAAGG-3′ (495 bp fragment, bases 1163-1657); OATP-B (gene symbol: SLCO2B1; accession: NM_007256), 5′- CGACTCAACGTGCAGCCATC-3′ and 5′- CCGACACTAGCAATTGCTGCT-3′ (437 bp fragment, bases 1659-2095); OATP-C (gene symbol: SLCO1B1; accession: NM_006446), 5′ -TGCACTTGGAGGCACCTCAC-3′ and 5′-CTTCATCCATGACACTTCCATT-3′ (359 bp fragment, bases 1644-2002); OATP-D (gene symbol: SLCO3A1; accession: NM_013272), 5′-GTTGGGCTTCATCCCTCCAC-3′ and 5′-TTAGTCACTATAAAACGGACT-3′ (392 bp fragment, bases 1749-2140); OATP-E (gene symbol: SLCO4A1; accession: NM_016354), 5′-GAGACTGTAGCTGTATCCCTC-3′ and 5′-GCGGTGGTCAGACGCTGCT-3′ (531 bp fragment, bases 1646-2176); OATP-H (gene symbol: SLCO4C1; accession: NM_180991), 5′-CTCCTATAACTGTGTCTATCCT-3′ (395 bp fragment, bases 1787-2181); OATP-8 (gene symbol: SLCO1B3; accession: NM_019844), 5′ -TCATAAACTCTTTGTTCTCTGC-3′ and 5′-GTTGGCAGCAGCATTGTCTTG-3′ (482 bp fragment, bases 1625–2106). The mRNA expression analyses of MRP isoforms and of RPL13A were performed as reported [26].
3.6. DIGE and Mass Spectrometry Analyses
Parental Capan-1 and 5-FU resistant Capan-1 cells [26] were analyzed following lysis (9.5 M urea, 2% CHAPS, 0.8% Pharmalyte, 1% DTT and 5 mM Pefabloc SC plus) and centrifugation at 13000× g for 60 min at 15 °C. Protein labeling for DIGE was conducted as described in the Ettan DIGE user manual. 50 μg of each protein sample were labeled with 400 pmol of CyDye DIGE Fluor minimal dyes Cy2, Cy3, and Cy5. Internal standard for DIGE was generated by pooling parental and resistant Capan-1 cell samples. Individual parental and resistant Capan-1 cell samples were labeled with Cy3 or Cy5, while the internal standard was always labeled with Cy2. First dimensional electrophoresis was performed as reported [88] on linear immobilized pH gradient strips using 50 μg total protein/strip. The second dimension (SDS-PAGE) used 12.5% gels running for about 22 h in TGS electrode buffer at 10 °C and 85 V, and the protein spots were visualized on the Typhoon 9400 variable mode imager using optimal emission wavelength for each DIGE flours. DeCyder image analysis software was used to analyze the DIGE images as described in the Ettan DIGE user manual.
For mass spectrometric analysis, Coomassie stained gel pieces containing the proteins of interest were manually excised from a corresponding preparative gel and subjected to in-gel tryptic digestion [88]. Proteins were identified by nanoLC-ESI-MS/MS on an LCQ mass spectrometer as described [89].
4. Conclusions
In summary, since the overall benefit of radio- and chemotherapeutic treatment of pancreatic cancer is at present still regrettably low, the overview on the current experimental evidence of drug-induced alterations in relevant transporter expression and on the contribution of these specific cellular membrane factors towards chemoresistance stresses the importance of integrating the role of uptake and export transporters of the nucleoside and MRP families into strategies aimed at developing improved chemotherapies against pancreatic carcinoma. To this aim, experimental studies using in vivo models resembling human pancreatic cancer are required to proof a causal connection between expression and activity of transporters such as MRP5 and tumor resistance to 5-FU and gemcitabine. It will further be interesting to study in more detail the possible connection between drug transporters and cell adhesion-mediated drug resistance (CAM-DR) [5,8990] as well as cell signaling pathways such as the sonic hedgehog pathway, which has recently be demonstrated to contribute to gemcitabine resistance in mice in vivo [91]. Such in vivo studies will certainly also increase our understanding of the role of interacting mechanisms between pancreatic stellate cells and pancreatic cancer cells in chemoresistance (for review see [92]).
| 5-FU (μg/ml) | Cytotoxicity (%) ± S.D. | |
|---|---|---|
| Controls | MRP5-silenced Cells | |
| 0 | 0 | 0 |
| 0.06 | 0.6 ± 0.7 | 43.8 ± 0.9 |
| 0.13 | 3.5 ± 6.3 | 44.9 ± 0.7 |
| 0.25 | 11.2 ± 3.6 | 43.2 ± 0.7 |
| 0.50 | 21.3 ± 3.2 | 48.6 ± 2.0 |
| Protein Name | Acc. No. | Expression | Peptides Matched | Sequence Coverage | MW (th./exp.) | pi (th./exp.) |
|---|---|---|---|---|---|---|
| Cathepsin D (heavy chain) | 4503143 | only in 5-FU resistant cells | 5 | 12% | 26.7/30.8 | 5.5/5.2 |
| Glyoxalase I | 15030212 | 2-fold up | 6 | 22% | 21.0/24.7 | 5.1/5.1 |
| Isocitrate dehydrogenase 3 /alpha enolase | 48256839 | 2-fold up | 6 | 17% | 40.0/37.6 | 6.4/6.0 |
| Tropomyosin 3 | 24119203 | 2-fold up | 7 | 23% | 29.2/31.1 | 4.7/4.7 |
| Peroxiredoxin 3 | 32483377 | 2-fold up | 6 a | 44% | 26.1/26.7 | 7.0/6.2 |
| 26S Protease reg. subunit 6A | 20532406 | 2-fold down | 4 | 12% | 49.4/47.2 | 5.1/5.2 |
| Annexin A2 (fragment) | 16306978 | 3-fold down | 13 | 41% | 38.8/28.9 | 7.6/5.6 |
| Cofilin 1 | 5031635 | down (only in parental cells) | 7 | 48% | 18.7/14.6 | 8.2/5.7 |
| Dynactin 2 | 5453629 | 3-fold down | 5 | 19% | 44.9/50.2 | 5.1/5.2 |
| Gelsolin-like capping protein | 63252913 | 3-fold down | 4 | 21% | 38.8/39.4 | 5.8/6.0 |
| Keratin 19 (4 spots) | 24234699 | 3 fold down | 23 | 53% | 44.1/40.0 | 4.9/4.9–5.1 |
| Lectin, galactose-bindg., soluble | 4504981 | 3 fold down | 4 | 33% | 15.0/15.0 | 5.3/5.1 |
| S100A4 (Calvasculin) | 4506765 | down (only in parental cells) | 2 b | 19.8% | 11.7/12.7 | 5.8/5.7 |
| S100 A6 (Calcyclin) | 30582769 | 3-fold down | 2 b | 16% | 10.2/11.9 | 5.3/5.2 |
| S100A11 (Calgizzarin) | 5032057 | 3-fold down | 4 | 33% | 11.8/13.4 | 6.6/6.1 |
| Stratifin; 14-3-3 sigma | 5454052 | 2-fold down | 7 b | 26% | 27.9/29.2 | 4.7/4.6 |
| Triosephosphate isomerase 1 | 4507645 | 2-fold down | 6 | 29% | 26.9/28.3 | 6.5/6.3 |
| UMP-CMP kinase | 12644008 | 2-fold down | 1 a | 22.2/24.2 | 5.4/5.7 |
MW: molecular weight (kDa); th.: theoretical; exp.: experimental; pI: isoelectric point.aconfirmed by Post-Source Decay (PSD) MALDI-MS;bidentified by ESI-MS/MS.
Acknowledgements
We acknowledge the excellent technical help of Anette Funk, Silke Wandschneider, Uwe Warnken, Swetlana Sander-Naderi, Stephanie Dräger, and Vera Mickler. This study was supported by a grant from the Hans and Lore Graf Stiftung (M.L.).
References
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar]
- Lohr, M. Is it possible to survive pancreatic cancer? Nat. Clin. Pract. Gastroenterol Hepatol. 2006, 3, 236–237. [Google Scholar]
- Merl, M.Y.; Abdelghany, O.; Li, J.; Saif, M.W. First-line treatment of metastatic pancreatic adenocarcinoma: Can we do better? In JOP, Highlights from the “2010 ASCO Annual Meeting”, Chicago, IL, USA, 4–8 June 2010; 2010; 11, pp. 317–320. [Google Scholar]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar]
- Hazlehurst, L.A.; Dalton, W.S. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001, 20, 43–50. [Google Scholar]
- Ghaneh, P.; Smith, R.; Tudor-Smith, C.; Raraty, M.; Neoptolemos, J.P. Neoadjuvant and adjuvant strategies for pancreatic cancer. Eur. J. Surg. Oncol. 2008, 34, 297–305. [Google Scholar]
- Hilbig, A.; Oettle, H. Adjuvant therapy of pancreatic cancer. Expert. Rev. Anticancer Ther. 2010, 10, 485–491. [Google Scholar]
- Mackey, J.R.; Mani, R.S.; Selner, M.; Mowles, D.; Young, J.D.; Belt, J.A.; Crawford, C.R.; Cass, C. E. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998, 58, 4349–4357. [Google Scholar]
- Mackey, J.R.; Yao, S.Y.; Smith, K.M.; Karpinski, E.; Baldwin, S.A.; Cass, C.E.; Young, J.D. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J. Natl. Cancer Inst. 1999, 91, 1876–1881. [Google Scholar]
- Rius, M.; Keller, D.; Brom, M.; Hummel-Eisenbeiss, J.; Lyko, F.; Keppler, D. Vectorial transport of nucleoside analogs from the apical to the basolateral membrane in double-transfected cells expressing the human concentrative nucleoside transporter hCNT3 and the export pump ABCC4. Drug Metab. Dispos. 2010, 38, 1054–1063. [Google Scholar]
- Ritzel, M.W.; Ng, A.M.; Yao, S.Y.; Graham, K.; Loewen, S.K.; Smith, K.M.; Hyde, R.J.; Karpinski, E.; Cass, C.E.; Baldwin, S.A.; Young, J.D. Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: Identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). Mol. Membr. Biol. 2001, 18, 65–72. [Google Scholar]
- Andersson, R.; Aho, U.; Nilsson, B.I.; Peters, G.J.; Pastor-Anglada, M.; Rasch, W.; Sandvold, M.L. Gemcitabine chemoresistance in pancreatic cancer: Molecular mechanisms and potential solutions. Scand. J. Gastroenterol. 2009, 44, 782–786. [Google Scholar]
- Paproski, R.J.; Young, J.D.; Cass, C.E. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3′-deoxy-3′- fluorothymidine. Biochem. Pharmacol. 2010, 79, 587–595. [Google Scholar]
- Garcia-Manteiga, J.; Molina-Arcas, M.; Casado, F. J.; Mazo, A.; Pastor-Anglada, M. Nucleoside Transporter Profiles in Human Pancreatic Cancer Cells: Role of hCNT1 in 2′,2′- Difluorodeoxycytidine- Induced Cytotoxicity. Clin. Cancer Res. 2003, 9, 5000–5008. [Google Scholar]
- Marechal, R.; Mackey, J.R.; Lai, R.; Demetter, P.; Peeters, M.; Polus, M.; Cass, C.E.; Young, J.; Salmon, I.; Deviere, J.; Van Laethem, J.L. Human Equilibrative Nucleoside Transporter 1 and Human Concentrative Nucleoside Transporter 3 Predict Survival after Adjuvant Gemcitabine Therapy in Resected Pancreatic Adenocarcinoma. Clin. Cancer Res. 2009, 15, 2913–2919. [Google Scholar]
- Farrell, J.J.; Elsaleh, H.; Garcia, M.; Lai, R.; Ammar, A.; Regine, W.F.; Abrams, R.; Benson, A.B.; Macdonald, J.; Cass, C.E.; Dicker, A.F.; Mackey, J.R. Human Equilibrative Nucleoside Transporter 1 Levels Predict Response to Gemcitabine in Patients With Pancreatic Cancer. Gastroenterology 2009, 136, 187–195. [Google Scholar]
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17 (Suppl. 5), v7–v12. [Google Scholar]
- Marechal, R.; Mackey, J.R.; Lai, R.; Demetter, P.; Peeters, M.; Polus, M.; Cass, C.E.; Salmon, I.; Deviere, J.; Van Laethem, J.L. Deoxycitidine kinase is associated with prolonged survival after adjuvant gemcitabine for resected pancreatic adenocarcinoma. Cancer 2010, 116, 5200–5206. [Google Scholar]
- Ohhashi, S.; Ohuchida, K.; Mizumoto, K.; Fujita, H.; Egami, T.; Yu, J.; Toma, H.; Sadatomi, S.; Nagai, E.; Tanaka, M. Down-regulation of deoxycytidine kinase enhances acquired resistance to gemcitabine in pancreatic cancer. Anticancer Res. 2008, 28, 2205–2212. [Google Scholar]
- Richards, N.G.; Rittenhouse, D.W.; Freydin, B.; Cozzitorto, J.A.; Grenda, D.; Rui, H.; Gonye, G.; Kennedy, E.P.; Yeo, C.J.; Brody, J.R.; Witkiewicz, A.K. HuR status is a powerful marker for prognosis and response to gemcitabine-based chemotherapy for resected pancreatic ductal adenocarcinoma patients. Ann. Surg 2010, 252, 499–505, discussion 505-496. [Google Scholar]
- Costantino, C.L.; Witkiewicz, A.K.; Kuwano, Y.; Cozzitorto, J.A.; Kennedy, E.P.; Dasgupta, A.; Keen, J.C.; Yeo, C.J.; Gorospe, M.; Brody, J.R. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009, 69, 4567–4572. [Google Scholar]
- Kruh, G.D.; Belinsky, M.G. The MRP family of drug efflux pumps. Oncogene 2003, 22, 7537–7552. [Google Scholar]
- Konig, J.; Nies, A.T.; Cui, Y.; Leier, I.; Keppler, D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophy.s Acta. 1999, 1461, 377–394. [Google Scholar]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. A family of drug transporters: The multidrug resistance-associated proteins. J. Natl. Cancer. Inst. 2000, 92, 1295–1302. [Google Scholar]
- Borst, P.; de Wolf, C.; van de Wetering, K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers. Arch. 2007, 453, 661–673. [Google Scholar]
- Hagmann, W.; Jesnowski, R.; Faissner, R.; Guo, C.; Lohr, J.M. ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology 2009, 9, 136–144. [Google Scholar]
- Hagmann, W.; Jesnowski, R.; Lohr, J.M. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia 2010, 12, 740–747. [Google Scholar]
- Cui, Y.; Konig, J.; Buchholz, J.K.; Spring, H.; Leier, I.; Keppler, D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999, 55, 929–937. [Google Scholar]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar]
- Zelcer, N.; Saeki, T.; Reid, G.; Beijnen, J.H.; Borst, P. Characterization of Drug Transport by the Human Multidrug Resistance protein 3 (ABCC3). J. Biol. Chem. 2001, 276, 46400–46407. [Google Scholar]
- Pratt, S.; Shepard, R.L.; Kandasamy, R.A.; Johnston, P.A.; Perry, W., 3rd; Dantzig, A.H. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol. Cancer. Ther. 2005, 4, 855–863. [Google Scholar]
- Oguri, T.; Achiwa, H.; Sato, S.; Bessho, Y.; Takano, Y.; Miyazaki, M.; Muramatsu, H.; Maeda, H.; Niimi, T.; Ueda, R. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: A role of ABCC5 in gemcitabine sensitivity. Mol. Cancer Ther. 2006, 5, 1800–1806. [Google Scholar]
- Kiuchi, Y.; Suzuki, H.; Hirohashi, T.; Tyson, C.A.; Sugiyama, Y. cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3). FEBS Lett. 1998, 433, 149–152. [Google Scholar]
- Konig, J.; Rost, D.; Cui, Y.; Keppler, D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology 1999, 29, 1156–1163. [Google Scholar]
- Kool, M.; van der Linden, M.; de Haas, M.; Scheffer, G.L.; de Vree, J.M.; Smith, A.J.; Jansen, G.; Peters, G.J.; Ponne, N.; Scheper, R.J.; Elferink, R.P.; Baas, F.; Borst, P. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc. Natl. Acad. Sci. USA 1999, 96, 6914–6919. [Google Scholar]
- Scheffer, G.L.; Kool, M.; de Haas, M.; de Vree, J.M.; Pijnenborg, A.C.; Bosman, D.K.; Elferink, R.P.; van der Valk, P.; Borst, P.; Scheper, R.J. Tissue distribution and induction of human multidrug resistant protein 3. Lab. Invest. 2002, 82, 193–201. [Google Scholar]
- Konig, J.; Hartel, M.; Nies, A.T.; Martignoni, M.E.; Guo, J.; Buchler, M.W.; Friess, H.; Keppler, D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int. J. Cancer. 2005, 115, 359–367. [Google Scholar]
- Nakano, Y.; Tanno, S.; Koizumi, K.; Nishikawa, T.; Nakamura, K.; Minoguchi, M.; Izawa, T.; Mizukami, Y.; Okumura, T.; Kohgo, Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br. J. Cancer 2007, 96, 457–463. [Google Scholar]
- Rauchwerger, D.R.; Firby, P.S.; Hedley, D.W.; Moore, M.J. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000, 60, 6075–6079. [Google Scholar]
- Hong, S.P.; Wen, J.; Bang, S.; Park, S.; Song, S.Y. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer 2009, 125, 2323–2331. [Google Scholar]
- Hong, Y.B.; Kang, H.J.; Kwon, S.Y.; Kim, H.J.; Kwon, K.Y.; Cho, C.H.; Lee, J.M.; Kallakury, B.V.; Bae, I. Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells. Pancreas 2010, 39, 463–472. [Google Scholar]
- Sims-Mourtada, J.; Izzo, J.G.; Ajani, J.; Chao, K.S. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 2007, 26, 5674–5679. [Google Scholar]
- Chun, S.G.; Zhou, W.; Yee, N.S. Combined targeting of histone deacetylases and hedgehog signaling enhances cytoxicity in pancreatic cancer. Cancer Biol. Ther. 2009, 8, 1328–1339. [Google Scholar]
- Cui, D.; Xu, Q.; Wang, K.; Che, X. Gli1 is a potential target for alleviating multidrug resistance of gliomas. J. Neurol. Sci. 2010, 288, 156–166. [Google Scholar]
- Queiroz, K.C.; Ruela-de-Sousa, R.R.; Fuhler, G.M.; Aberson, H.L; Ferreira, C.V.; Peppelenbosch, M.P.; Spek, C.A. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene 2010. Epub ahead of print. [Google Scholar]
- Santisteban, M. ABC Transporters as Molecular Effectors of Pancreatic Oncogenic Pathways: The Hedgehog-GLI Model. J. Gastrointest Cancer 2010, 41, 153–158. [Google Scholar]
- Alvarez, A. I.; Real, R.; Perez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar]
- Borska, S.; Sopel, M.; Chmielewska, M.; Zabel, M.; Dziegiel, P. Quercetin as a potential modulator of P-glycoprotein expression and function in cells of human pancreatic carcinoma line resistant to daunorubicin. Molecules 2010, 15, 857–870. [Google Scholar]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and drug resistance. Cancer Gene Ther. 2010, 17, 523–531. [Google Scholar]
- Giovannetti, E.; Funel, N.; Peters, G.J.; Del Chiaro, M.; Erozenci, L.A.; Vasile, E.; Leon, L.G.; Pollina, L.E.; Groen, A.; Falcone, A.; Danesi, R.; Campani, D.; Verheul, H.M.; Boggi, U. MicroRNA-21 in pancreatic cancer: Correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010, 70, 4528–4538. [Google Scholar]
- Hwang, J. H.; Voortman, J.; Giovannetti, E.; Steinberg, S.M.; Leon, L.G.; Kim, Y.T.; Funel, N.; Park, J.K.; Kim, M.A.; Kang, G.H.; Kim, S.W.; Del Chiaro, M.; Peters, G.J.; Giaccone, G. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One 2010, 5, e10630. [Google Scholar]
- Wang, F.; Xue, X.; Wei, J.; An, Y.; Yao, J.; Cai, H.; Wu, J.; Dai, C.; Qian, Z.; Xu, Z.; Miao, Y. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br. J. Cancer 2010, 103, 567–574. [Google Scholar]
- Haraguchi, N.; Utsunomiya, T.; Inoue, H.; Tanaka, F.; Mimori, K.; Barnard, G.F.; Mori, M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 2006, 24, 506–513. [Google Scholar]
- Yao, J.; Cai, H.H.; Wei, J.S.; An, Y.; Ji, Z.L.; Lu, Z.P.; Wu, J.L.; Chen, P.; Jiang, K.R.; Dai, C.C.; Qian, Z.Y.; Xu, Z.K.; Miao, Y. Side population in the pancreatic cancer cell lines SW1990 and CFPAC-1 is enriched with cancer stem-like cells. Oncol. Rep. 2010, 23, 1375–1382. [Google Scholar]
- Du, Z.; Qin, R.; Wei, C.; Wang, M.; Shi, C.; Tian, R.; Peng, C. Pancreatic Cancer Cells Resistant to Chemoradiotherapy Rich in “Stem-Cell-Like” Tumor Cells. Dig. Dis. Sci. 2010. [Google Scholar] [CrossRef]
- Lonardo, E.; Hermann, P.C.; Heeschen, C. Pancreatic cancer stem cells - update and future perspectives. Mol. Oncol. 2010, 4, 431–442. [Google Scholar]
- Mueller, M. T.; Hermann, P.C.; Heeschen, C. Cancer stem cells as new therapeutic target to prevent tumour progression and metastasis. Front Biosci. (Elite Ed) 2010, 2, 602–613. [Google Scholar]
- Rasheed, Z.A.; Yang, J.; Wang, Q.; Kowalski, J.; Freed, I.; Murter, C.; Hong, S.M.; Koorstra, J.B.; Rajeshkumar, N.V.; He, X.; Goggins, M.; Iacobuzio-Donahue, C.; Berman, D.M.; Laheru, D.; Jimeno, A.; Hidalgo, M.; Maitra, A.; Matsui, W. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J. Natl. Cancer Inst. 2010, 102, 340–351. [Google Scholar]
- Jonckheere, N.; Skrypek, N.; Van Seuningen, I. Mucins and Pancreatic Cancer. Cancers 2010, 2, 1794–1812. [Google Scholar]
- Mimeault, M.; Johansson, S.L.; Senapati, S.; Momi, N.; Chakraborty, S.; Batra, S.K. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010, 295, 69–84. [Google Scholar]
- Hagenbuch, B.; Meier, P.J. Organic anion transporting polypeptides of the OATP/ SLC21 family: Phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004, 447, 653–665. [Google Scholar]
- Konig, J.; Cui, Y.; Nies, A.T.; Keppler, D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J. Biol. Chem. 2000, 275, 23161–23168. [Google Scholar]
- Konig, J.; Cui, Y.; Nies, A.T.; Keppler, D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am. J. Physiol. Gastrointest Liver Physiol. 2000, 278, G156–G164. [Google Scholar]
- Hagenbuch, B.; Meier, P.J. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta. 2003, 1609, 1–18. [Google Scholar]
- Tamai, I.; Nezu, J.; Uchino, H.; Sai, Y.; Oku, A.; Shimane, M.; Tsuji, A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000, 273, 251–260. [Google Scholar]
- Jesnowski, R.; Backhaus, C.; Ringel, J.; Lohr, M. Ribosomal highly basic 23-kDa protein as a reliable standard for gene expression analysis. Pancreatology 2002, 2, 421–424. [Google Scholar]
- Jesnowski, R.; Furst, D.; Ringel, J.; Chen, Y.; Schrodel, A.; Kleeff, J.; Kolb, A.; Schareck, W.D.; Lohr, M. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: Deactivation is induced by matrigel and N-acetylcysteine. Lab Invest. 2005, 85, 1276–1291. [Google Scholar]
- Haber, P.S.; Keogh, G.W.; Apte, M.V.; Moran, C.S.; Stewart, N.L.; Crawford, D.H.; Pirola, R.C.; McCaughan, G.W.; Ramm, G.A.; Wilson, J.S. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 1999, 155, 1087–1095. [Google Scholar]
- Yen, T.W.; Aardal, N.P.; Bronner, M.P.; Thorning, D.R.; Savard, C.E.; Lee, S.P.; Bell, R.H., Jr. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery 2002, 131, 129–134. [Google Scholar]
- Schneiderhan, W.; Diaz, F.; Fundel, M.; Zhou, S.; Siech, M.; Hasel, C.; Moller, P.; Gschwend, J.E.; Seufferlein, T.; Gress, T.; Adler, G.; Bachem, M.G. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J. Cell Sci. 2007, 120, 512–519. [Google Scholar]
- Xu, Z.; Vonlaufen, A.; Phillips, P.A.; Fiala-Beer, E.; Zhang, X.; Yang, L.; Biankin, A.V.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Role of Pancreatic Stellate Cells in Pancreatic Cancer Metastasis. Am. J. Pathol. 2010, 177, 2585–2596. [Google Scholar]
- Shi, X.; Liu, S.; Kleeff, J.; Friess, H.; Buchler, M.W. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology 2002, 62, 354–362. [Google Scholar]
- Monti, P.; Marchesi, F.; Reni, M.; Mercalli, A.; Sordi, V.; Zerbi, A.; Balzano, G.; Di Carlo, V.; Allavena, P.; Piemonti, L. A comprehensive in vitro characterization of pancreatic ductal carcinoma cell line biological behavior and its correlation with the structural and genetic profile. Virchows Arch. 2004, 445, 236–247. [Google Scholar]
- Faissner, R. Clinical Cooperation Unit of Molecular Gastroenterology; German Cancer Research Center: Heidelberg, Germany, unpublished data; 2010. [Google Scholar]
- Oguri, T.; Bessho, Y.; Achiwa, H.; Ozasa, H.; Maeno, K.; Maeda, H.; Sato, S.; Ueda, R. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol. Cancer Ther. 2007, 6, 122–127. [Google Scholar]
- Nambaru, P.K.; Hubner, T.; Kock, K.; Mews, S.; Sendler, M.; Grube, M.; Payen, L.; Guitton, J.; Jedlitschky, G.; Rimmbach, C.; Rosskopf, D.; Kowalczyk, D.W.; Kroemer, H.K.; Weiss, F.U.; Mayerle, J.; Lerch, M.M.; Ritter, C.A. Drug efflux transporter MRP5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos 2010, 39, 132–139. [Google Scholar]
- Kikuchi, N.; Horiuchi, A.; Osada, R.; Imai, T.; Wang, C.; Chen, X.; Konishi, I. Nuclear expression of S100A4 is associated with aggressive behavior of epithelial ovarian carcinoma: An important autocrine/paracrine factor in tumor progression. Cancer Sci. 2006, 97, 1061–1069. [Google Scholar]
- Fernandez-Fernandez, M.R.; Veprintsev, D.B.; Fersht, A.R. Proteins of the S100 family regulate the oligomerization of p53 tumor suppressor. Proc. Natl. Acad. Sci. USA 2005, 102, 4735–4740. [Google Scholar]
- Cabezon, T.; Celis, J.E.; Skibshoj, I.; Klingelhofer, J.; Grigorian, M.; Gromov, P.; Rank, F.; Myklebust, J.H.; Maelandsmo, G.M.; Lukanidin, E.; Ambartsumian, N. Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int. J. Cancer 2007, 121, 1433–1444. [Google Scholar]
- Mahon, P.C.; Baril, P.; Bhakta, V.; Chelala, C.; Caulee, K.; Harada, T.; Lemoine, N.R. S100A4 contributes to the suppression of BNIP3 expression, chemoresistance, and inhibition of apoptosis in pancreatic cancer. Cancer Res. 2007, 67, 6786–6795. [Google Scholar]
- Kulke, M.H. Advanced pancreatic cancer: Is there a role for combination therapy? Expert Rev. Anticancer Ther. 2003, 3, 729–739. [Google Scholar]
- El-Rayes, B.F.; Philip, P.A. Systemic therapy for advanced pancreatic cancer. Expert Rev. Anticancer Ther. 2002, 2, 426–436. [Google Scholar]
- Heinemann, V.; Boeck, S.; Hinke, A.; Labianca, R.; Louvet, C. Meta-analysis of randomized trials: Evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008, 8, 82. [Google Scholar]
- Tsujie, M.; Nakamori, S.; Nakahira, S.; Takeda, S.; Takahashi, Y.; Hayashi, N.; Okami, J.; Nagano, H.; Dono, K.; Umeshita, K.; Sakon, M.; Monden, M. Schedule-dependent therapeutic effects of gemcitabine combined with uracil-tegafur in a human pancreatic cancer xenograft model. Pancreas 2006, 33, 142–147. [Google Scholar]
- Pressacco, J.; Mitrovski, B.; Erlichman, C.; Hedley, D.W. Effects of thymidylate synthase inhibition on thymidine kinase activity and nucleoside transporter expression. Cancer Res 1995, 55, 1505–1508. [Google Scholar]
- Reid, G.; Wielinga, P.; Zelcer, N.; De Haas, M.; Van Deemter, L.; Wijnholds, J.; Balzarini, J.; Borst, P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 2003, 63, 1094–1103. [Google Scholar]
- Sipos, B.; Moser, S.; Kalthoff, H.; Torok, V.; Lohr, M.; Kloppel, G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: Towards the establishment of an in vitro research platform. Virchows Arch. 2003, 442, 444–452. [Google Scholar]
- Lohr, J.M.; Faissner, R.; Koczan, D.; Bewerunge, P.; Bassi, C.; Brors, B.; Eils, R.; Frulloni, L.; Funk, A.; Halangk, W.; Jesnowski, R.; Kaderali, L.; Kleeff, J.; Kruger, B.; Lerch, M.M.; Losel, R.; Magnani, M.; Neumaier, M.; Nittka, S.; Sahin-Toth, M.; Sanger, J.; Serafini, S.; Schnolzer, M.; Thierse, H.J.; Wandschneider, S.; Zamboni, G.; Kloppel, G. Autoantibodies against the exocrine pancreas in autoimmune pancreatitis: Gene and protein expression profiling and immunoassays identify pancreatic enzymes as a major target of the inflammatory process. Am. J. Gastroenterol. 2010, 105, 2060–2071. [Google Scholar]
- Schutt, F.; Ueberle, B.; Schnolzer, M.; Holz, F.G.; Kopitz, J. Proteome analysis of lipofuscin in human retinal pigment epithelial cells. FEBS. Lett. 2002, 528, 217–221. [Google Scholar]
- Shain, K.H.; Yarde, D.N.; Meads, M.B.; Huang, M.; Jove, R.; Hazlehurst, L.A.; Dalton, W.S. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: Implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009, 69, 1009–1015. [Google Scholar]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; Frese, K.K.; Denicola, G.; Feig, C.; Combs, C.; Winter, S.P.; Ireland-Zecchini, H.; Reichelt, S.; Howat, W.J.; Chang, A.; Dhara, M.; Wang, L.; Ruckert, F.; Grutzmann, R.; Pilarsky, C.; Izeradjene, K.; Hingorani, S.R.; Huang, P.; Davies, S.E.; Plunkett, W.; Egorin, M.; Hruban, R.H.; Whitebread, N.; McGovern, K.; Adams, J.; Iacobuzio-Donahue, C.; Griffiths, J.; Tuveson, D.A. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar]
- Habisch, H.; Zhou, S.; Siech, M.; Bachem, M.G. Interaction of Stellate Cells with Pancreatic Carcinoma Cells. Cancers 2010, 2, 1661–1682. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hagmann, W.; Faissner, R.; Schnölzer, M.; Löhr, M.; Jesnowski, R. Membrane Drug Transporters and Chemoresistance in Human Pancreatic Carcinoma. Cancers 2011, 3, 106-125. https://doi.org/10.3390/cancers3010106
Hagmann W, Faissner R, Schnölzer M, Löhr M, Jesnowski R. Membrane Drug Transporters and Chemoresistance in Human Pancreatic Carcinoma. Cancers. 2011; 3(1):106-125. https://doi.org/10.3390/cancers3010106
Chicago/Turabian StyleHagmann, Wolfgang, Ralf Faissner, Martina Schnölzer, Matthias Löhr, and Ralf Jesnowski. 2011. "Membrane Drug Transporters and Chemoresistance in Human Pancreatic Carcinoma" Cancers 3, no. 1: 106-125. https://doi.org/10.3390/cancers3010106