A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications
Abstract
:1. Background and Introduction
1.1. Folate-Receptor Tissue Distribution
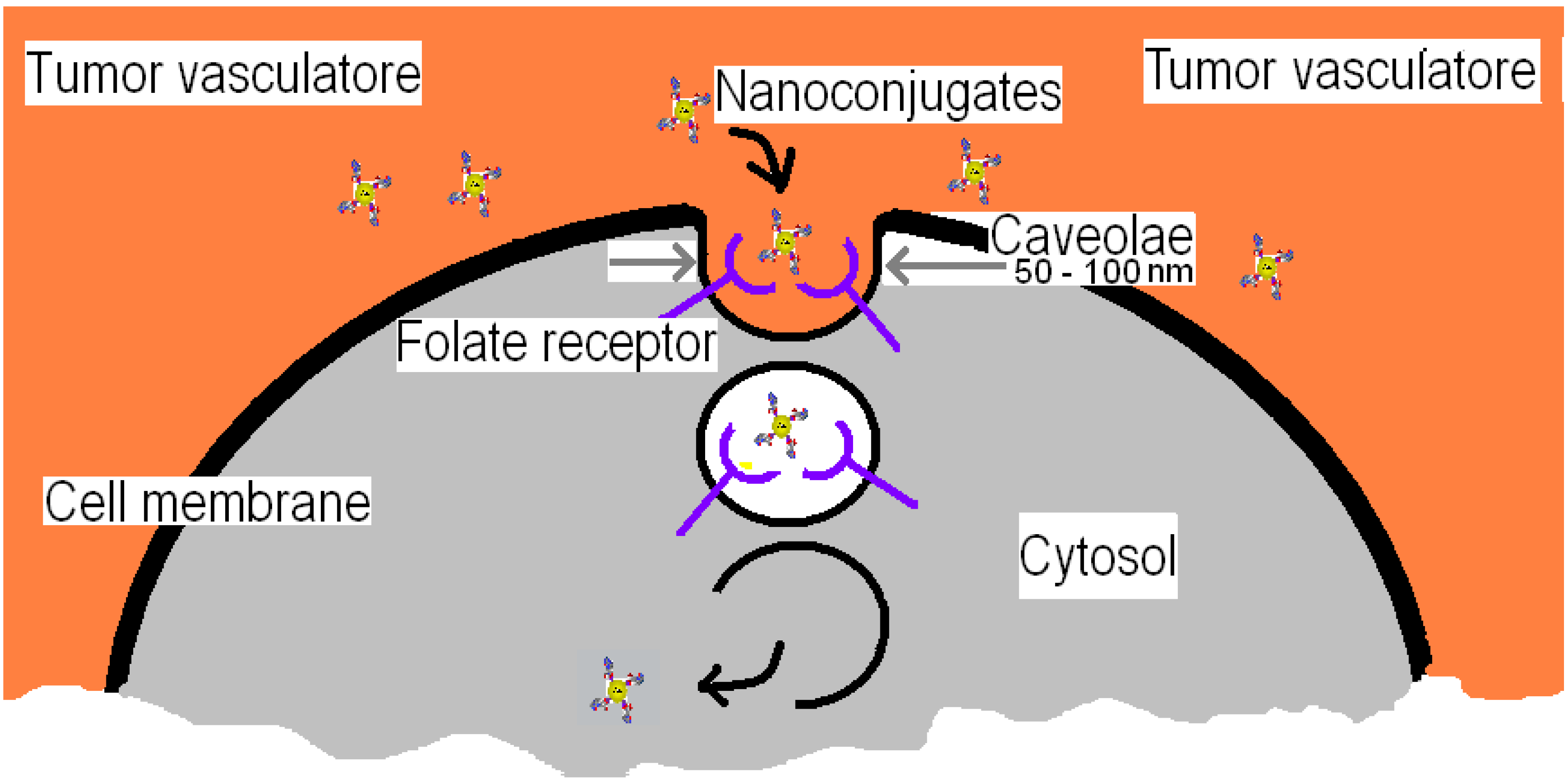
1.2. Folate-Receptor Endocytosis for Targeted Nanotechnology

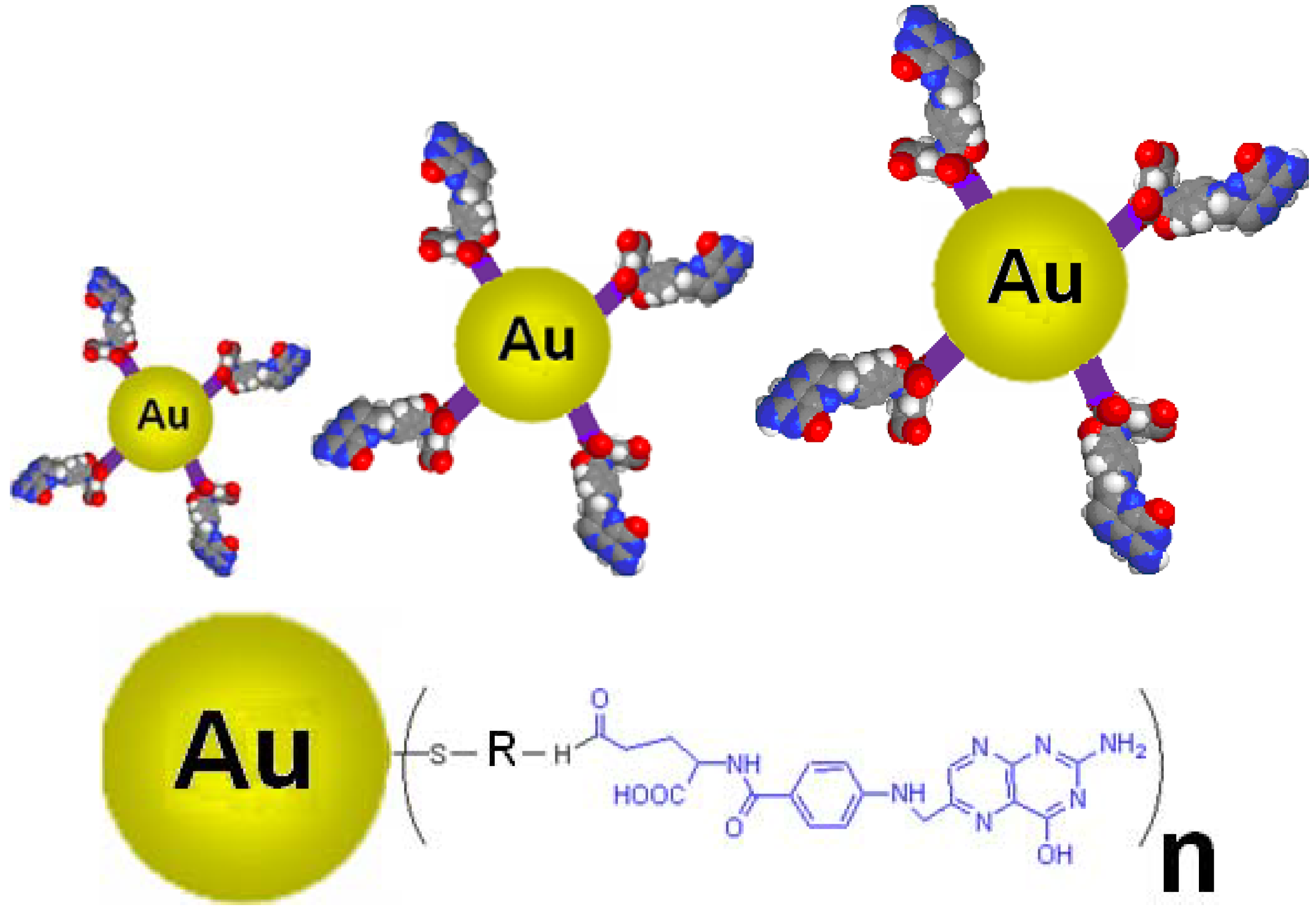
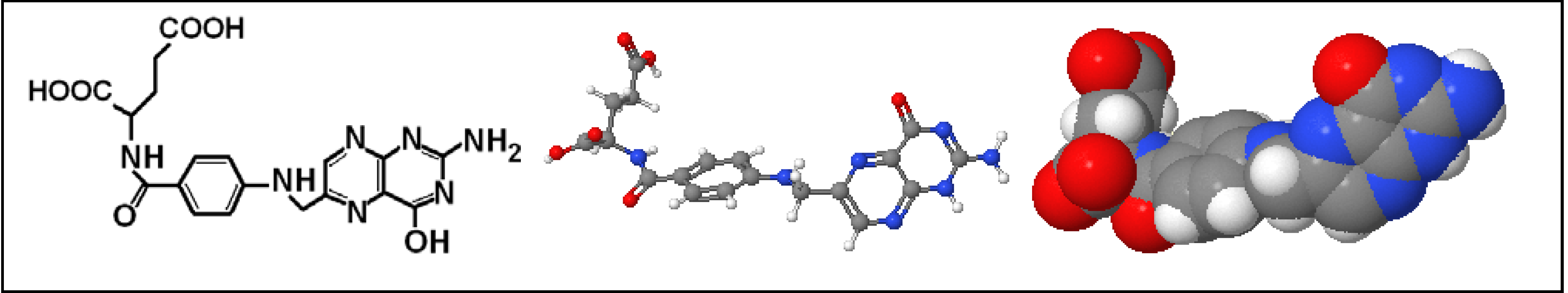
1.3. Nanoconjugates Synthesis and Characterization
2. Materials and Methods
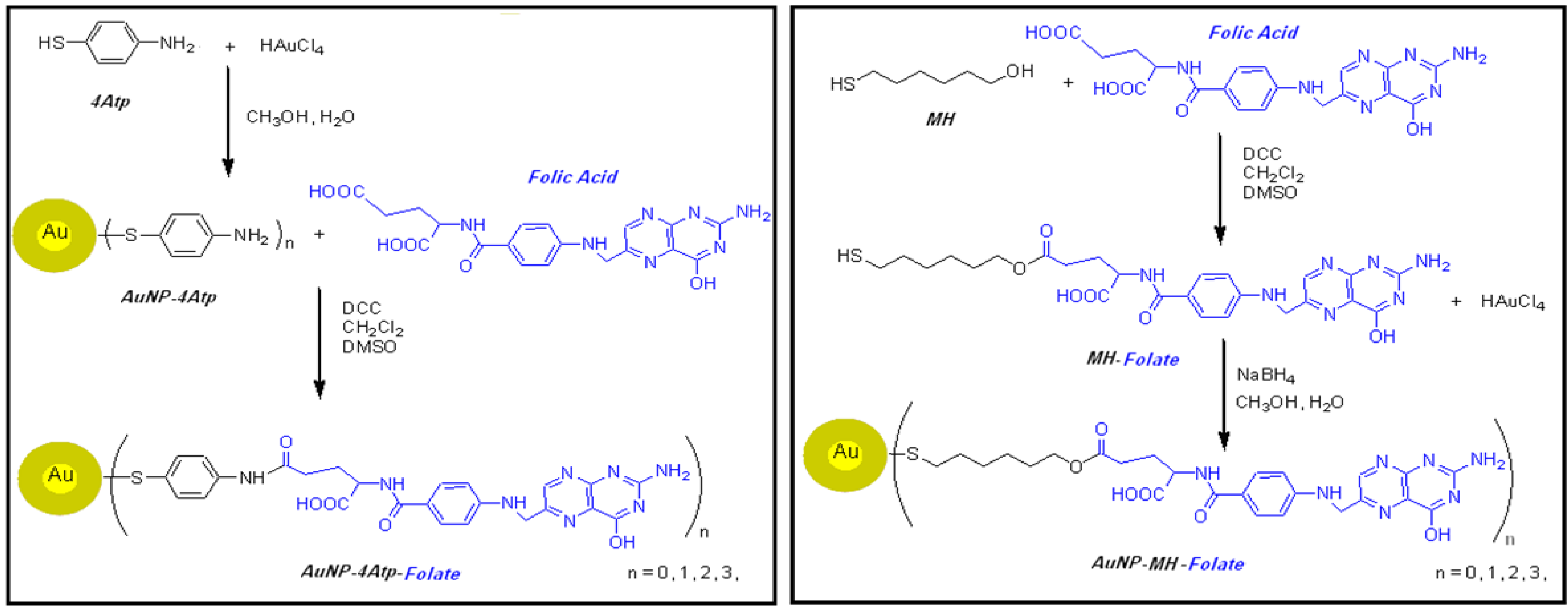
2.1. Characterization of Nanoconjugates
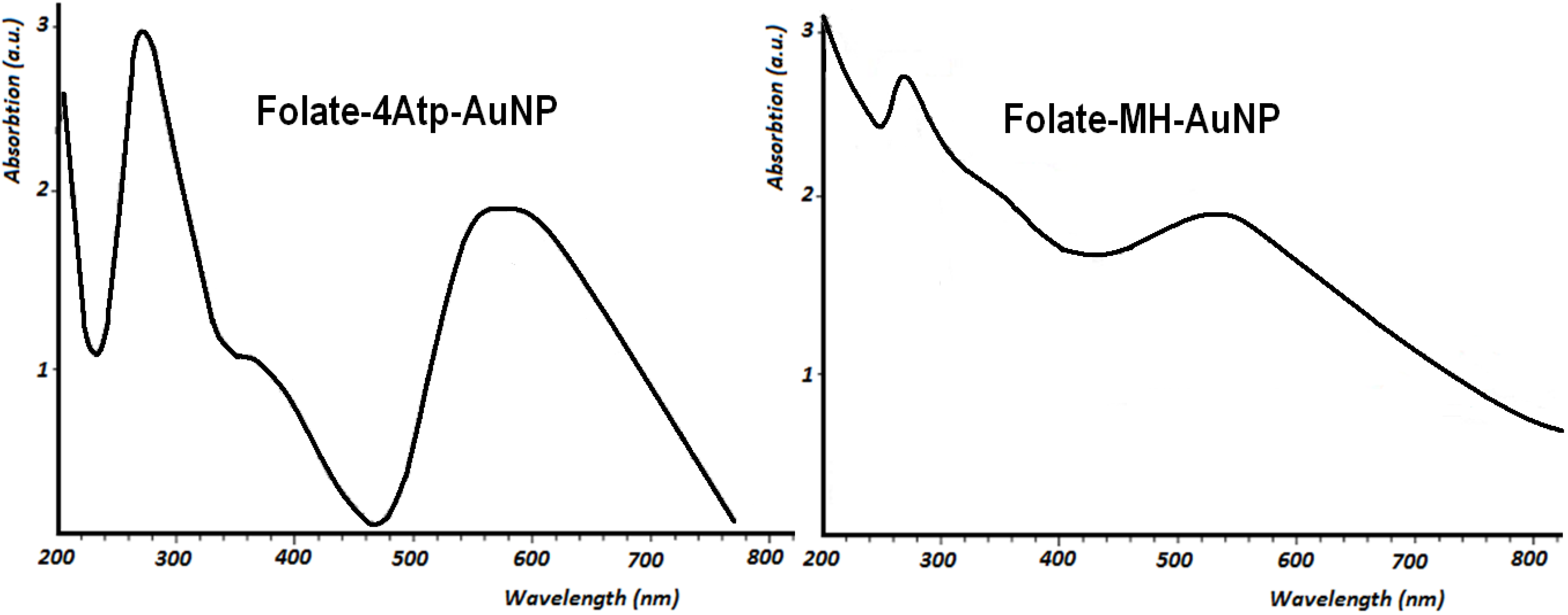
2.1.1. UV-Vis Spectroscopy
2.1.2. Fourier Transform Infra Red Spectroscopy

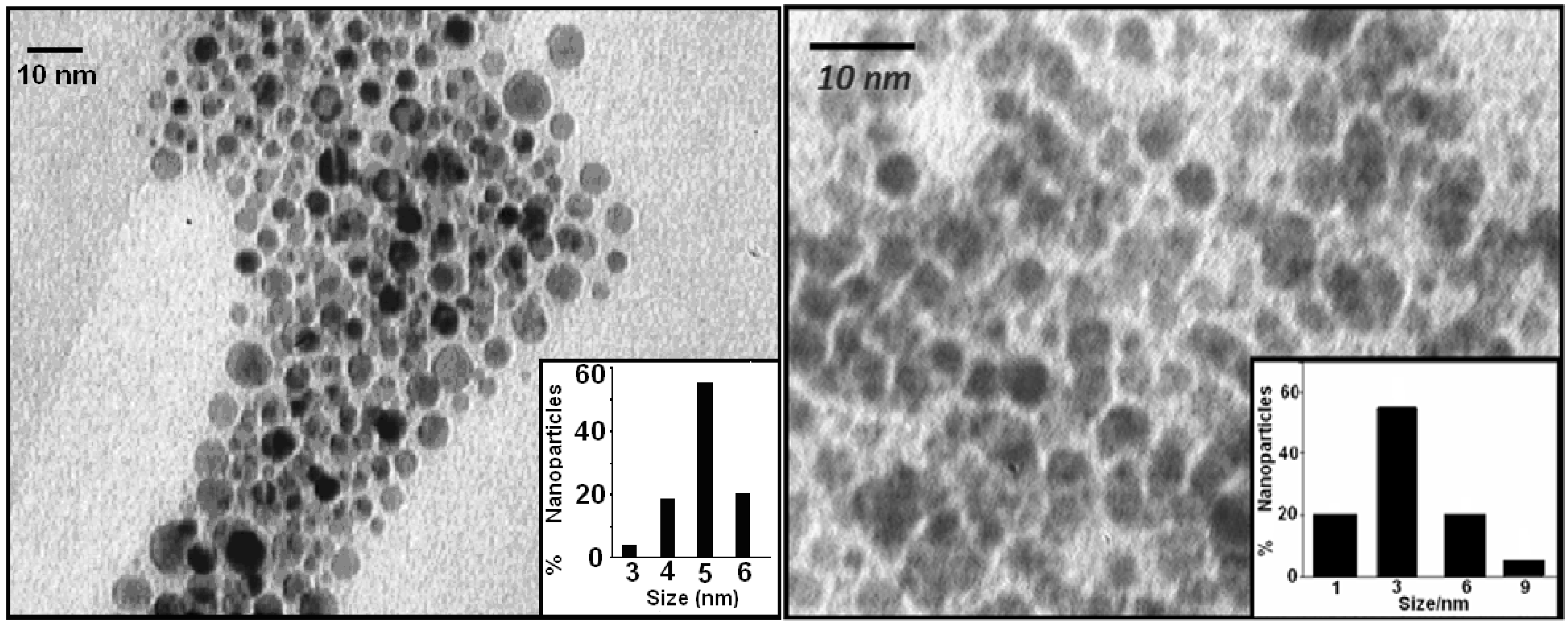
2.1.3. Transmission Electron Microscopy
2.1.4. X-Ray Diffraction
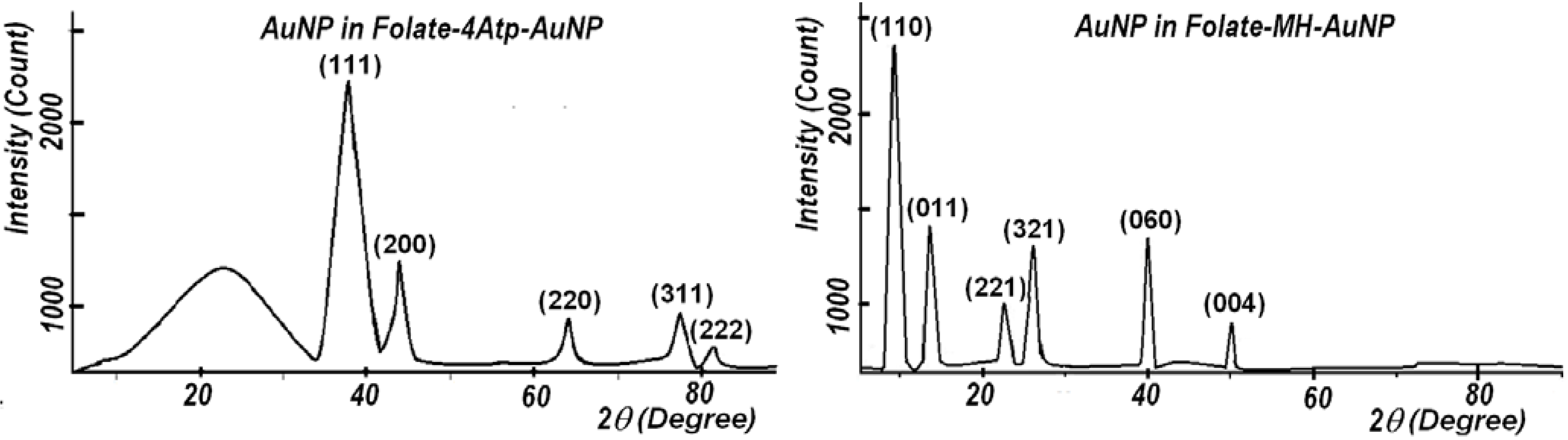
2.1.5. Elemental Analysis
| Elements→ | [Au] | [C] | [H] | [N] | [S] | [O] | Total | [C]:[H] | [S]:[H] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nanoconjugate ↓ | ||||||||||
| Folate-4Atp-AuNP | Expt’l [25,26,27] | 41.3 | 28.7 | 2.5 | 13.3 | 3.5 | 10.7 | 99.9 | 11.48 | 1.4 |
| Stochiometric | 26.5 | 40.3 | 3.1 | 15 | 4.3 | 10.8 | 100 | 13 | 1.39 | |
| Folate-MH-AuNP | Expt’l [28,29] | 32 | 38.2 | 3.8 | 11.2 | 3.6 | 11.2 | 100 | 10.03 | 0.95 |
| Stochiometric | 26.2 | 39.8 | 4 | 13 | 4.2 | 12.6 | 100 | 9.95 | 1.05 | |
2.1.6. Stability Comparison
2.2. In Vitro Tests of Nanoconjugates on Cancer Cells
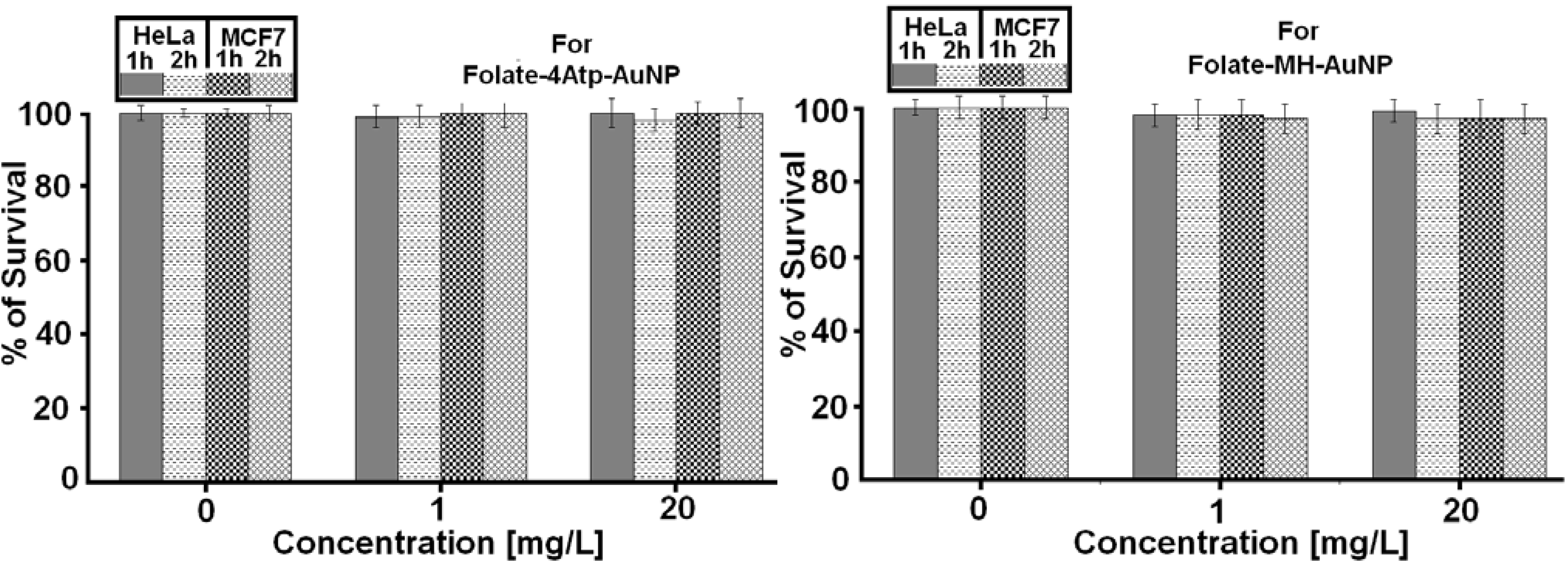
2.2.1. Nanoparticle Cytotoxicity
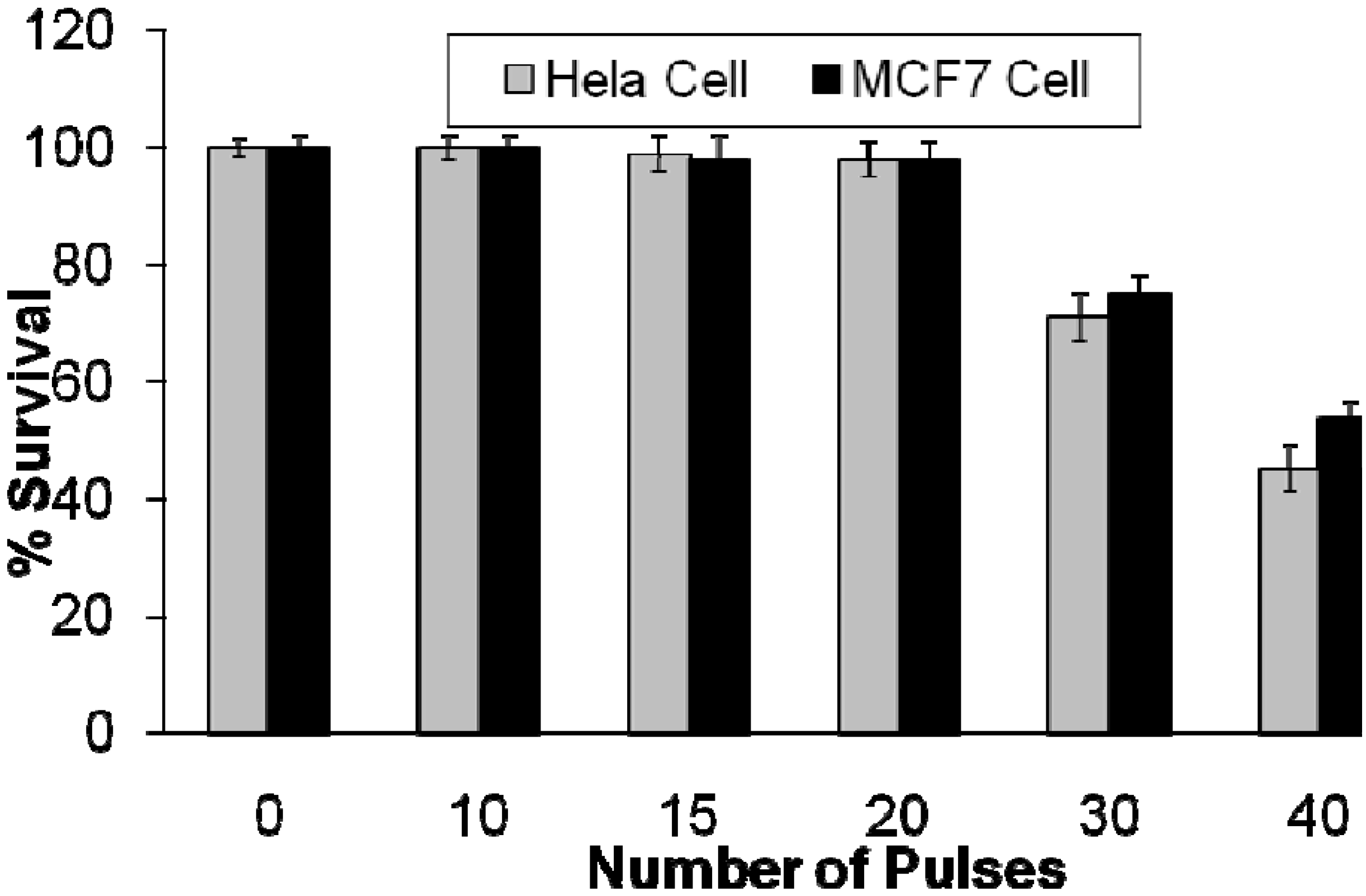
2.2.2. Effects of Intense Pulsed Light (IPL) Exposure to Cells
2.2.3. Photothermal Studies
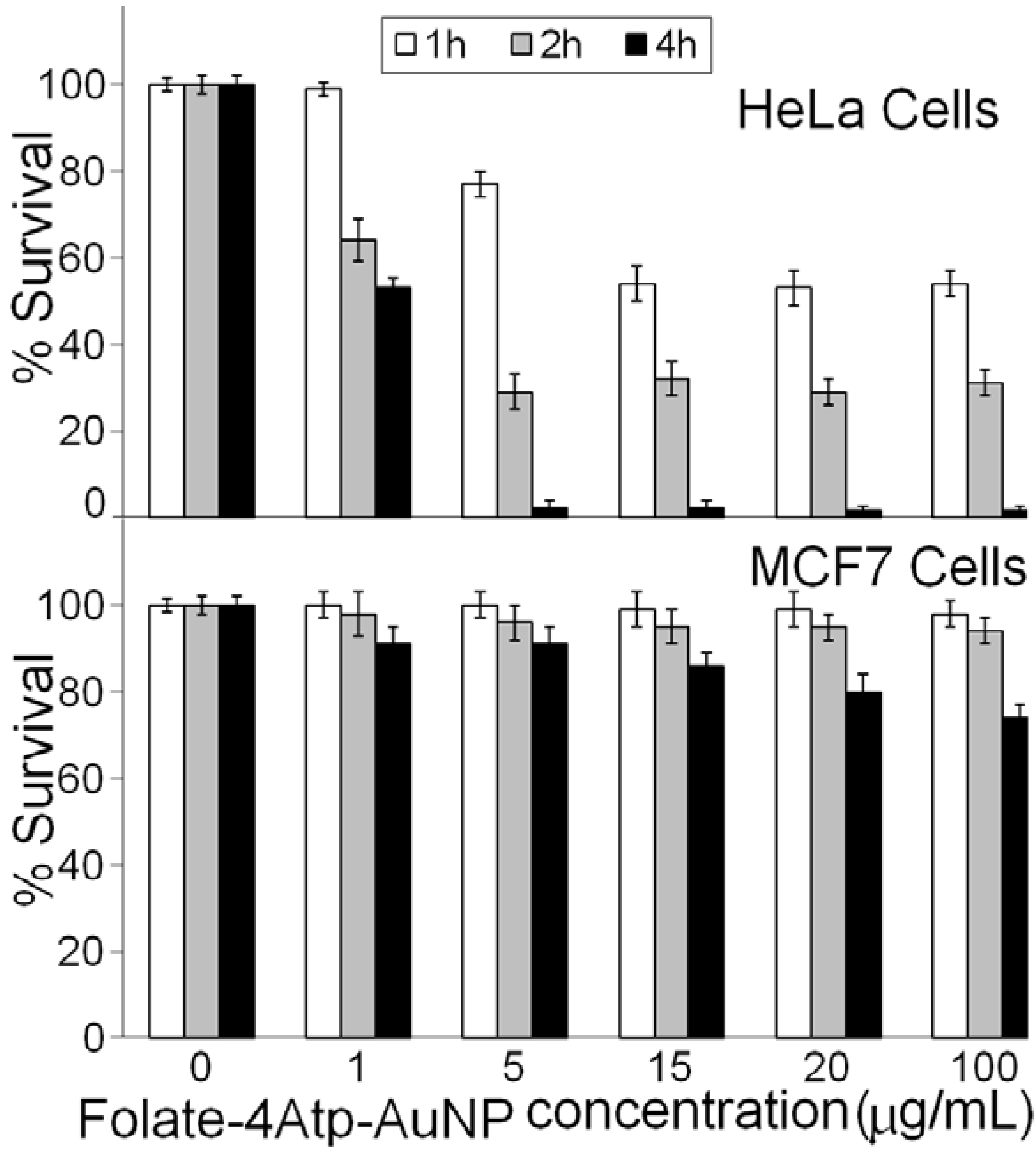
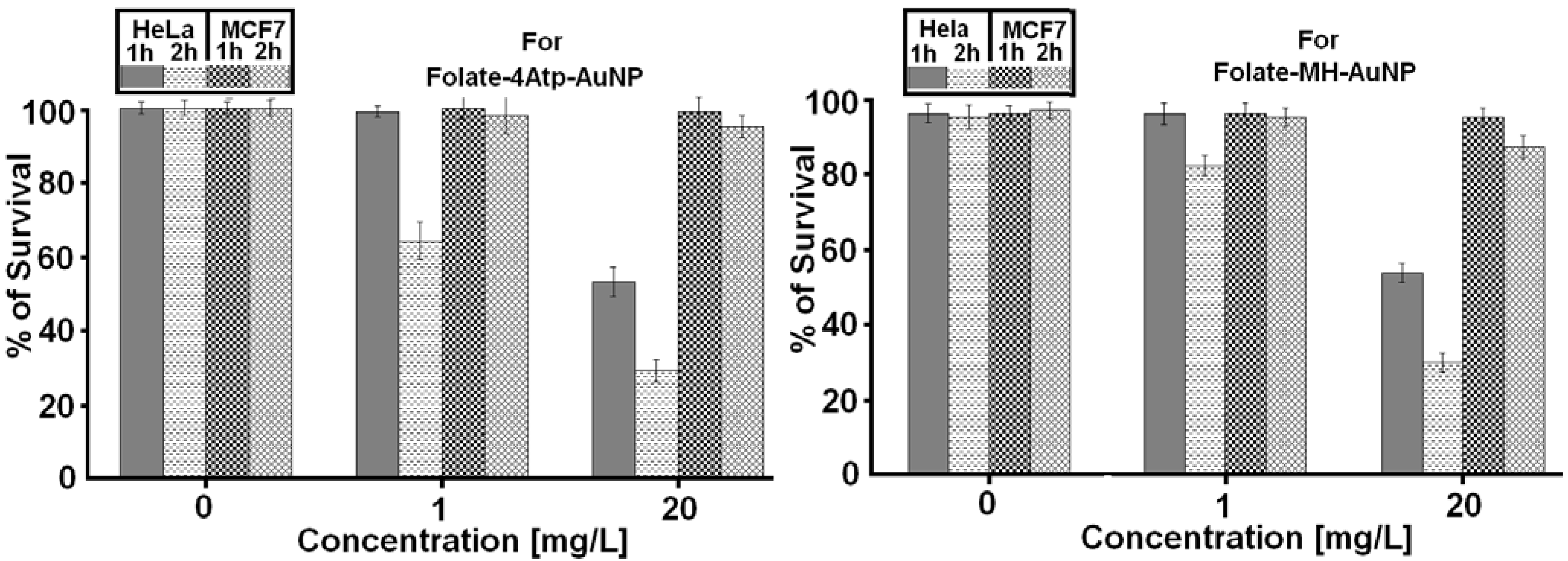
3. Discussion
4. Future Work
5. Conclusions
Acknowledgements
References
- Mansoori, G.A.; Mohazzabi, P.; McCormack, P; Jabbari, S. Nanotechnology in cancer prevention, detection and treatment: bright future lies ahead. WRSTSD 2007, 4, 226–257. [Google Scholar] [CrossRef]
- Mansoori, G.A. Principles of Nanotechnology: Molecular Based Study of Condensed Matter in Small Systems; World Sci. Pub. Co.: Hackensack, NJ, USA, 2005. [Google Scholar]
- Mansoori, G.A.; George, T.F.; Assoufid, L.; Zhang, G. Molecular Building Blocks for Nanotechnology: From Diamondoids to Nanoscale Materials and Applications, Topics in Applied Physics; Springer: New York, NY, USA, 2007; Volume 109. [Google Scholar]
- National Cancer Institute. Available online: http://www.cancer.gov (accessed on 18 November 2010).
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the Folate Receptor GP38 in Normal and Malignant Cell Lines and Tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- Leamon, C.P.; Reddy, J.A. Folate-targeted Chemotherapy. Adv. Drug Deliv. Rev. 2004, 56, 1127–1141. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R. Nanomaterials for Cancer Therapy (Nanotechnologies for the Life Sciences), ibid, Nanomaterials for Cancer Diagnosis (Nanotechnologies for the Life Sciences); Wiley-VCH: Berlin, German, 2007. [Google Scholar]
- Doucette, M.M.; Stevens, V.L. Folate Receptor Function Is Regulated in Response to Different Cellular Growth Rates in Cultured Mammalian Cells. J. Nutr. 2001, 131, 2819–2825. [Google Scholar]
- Kamen, B.A.; Smith, A.K. A Review of Folate Receptor Alpha Cycling and 5-methyltetrahydrofolate Accumulation with an Emphasis on Cell Models in vitro. Adv. Drug Deliv. Rev. 2004, 56, 1085–1097. [Google Scholar] [CrossRef]
- Hong, S.; Leroueil, P.R.; Majoros, I.J.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. The Binding Avidity of a Nanoparticle-Based Multivalent Targeted Drug Delivery Platform. Chem. Biol. 2007, 14, 107–115. [Google Scholar] [CrossRef]
- Leamon, C.P.; Low, P.S. Delivery of macromolecules into living cells: A Method that Exploits Folate Receptor Endocytosis. Proc. Natl. Acad. Sci. USA 1991, 88, 5572–5576. [Google Scholar] [CrossRef]
- Elnakat, E.; Ratnam, M. Distribution, functionality and Gene Regulation of Folate Receptor Isoforms: Implications in Targeted Therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef]
- Kamen, B.A.; Wang, M.; Streckfuss, A.J.; Peryea, X.; Anderson, R.G.W. Delivery of Folates to the Cytoplasm of MA104 Cells Is Mediated by a Surface Membrane Receptor that Recycles. J. Biol. Chem. 1988, 263, 13602–13609. [Google Scholar]
- Rothberg, K.G.; Ying, Y.; Kolhouse, J.F.; Kamen, B.A.; Anderson, R.G.W. The Glycophospholipid-linked Folate Receptor Internalizes Folate Without Entering the Clathrin-coated Pit Endocytic Pathway. J. Cell Biol. 1990, 110, 637–649. [Google Scholar] [CrossRef]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Mandelbaum-Shavit, F.; Qazen, M.M.; Zalipsky, S. Targeting Folate Receptor with Folate Linked to Extremities of Poly(ethylene glycol)-Grafted Liposomes: In Vitro Studies. Bioconjugate Chem. 1999, 10, 289–298. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Horowitz, A.T.; Zalipsky, S. Tumor Cell Targeting of Liposome-Entrapped Drugs with Phospholipid-Anchored Folic Acid-PEG conjugates. Adv. Drug Deliv. Rev. 2004, 56, 1177–1192. [Google Scholar] [CrossRef]
- Hilgenbrink, A.R.; Low, P.S. Folate Receptor—Mediated Drug Targeting: From Therapeutics to Diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Mansoori, G.A. Diamondoid Molecules. Adv. Chem. Phys. 2007, 136, 207–258. [Google Scholar] [CrossRef]
- Lu, Y.; Sega, E.; Leamon, C.P.; Low, P.S. Folate Receptor-targeted Immunotherapy of Cancer: Mechanism and Therapeutic Potential. Adv. Drug Deliv. Rev. 2004, 56, 1161–1176. [Google Scholar] [CrossRef]
- Dixit, V.; Van den Bossche, J.; Sherman, D.M.; Thompson, D.H.; Andres, R.P. Synthesis and Grafting of Thioctic Acid—PEG—Folate Conjugates onto Au Nanoparticles for Selective Targeting of Folate Receptor—Positive Tumor Cells. Bioconjugate Chem. 2006, 17, 603–609. [Google Scholar] [CrossRef]
- Manohar, S.; Rayavarapu, R.; Petersen, W.; van Leeuwen, T.G. Cell viability studies of PEG-thiol treated gold nanorods as optoacoustic contrast agents. Proc. SPIE 2009, 7177. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence Enhancement by Au Nanostructures: Nanoshells and Nanorods. ACS Nano. 2009, 3, 744–752. [Google Scholar] [CrossRef]
- Brandenburg, K.S.; Kent, M.; Swan, D. (G.A. Mansoori, Faculty supervisor); Development of a Theoretical Nanocomposite to Selectively Target and Destroy Malignant Cancer Cells. UIC Engineering EXPO: Chicago, IL, USA, April 2006. [Google Scholar]
- Mansoori, G.A. Synthesis of Nanoparticles by Fungi. US Patent 20100055199, 2010. [Google Scholar]
- Shakeri-Zadeh, A.; Ghasemifard, M.; Mansoori, G.A. Structural and optical characterization of folate-conjugated gold-nanoparticles. Phys. E: Low-dim. Sys. Nanostr. 2009. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Eshghi, H.; Mansoori, G.A.; Hashemian, A.R. Gold Nanoparticles Conjugated with Folic Acid using Mercaptohexanol as the Linker. J. Nanotech. Prog. Intl. (JONPI). 2009, 1, 13–23. [Google Scholar]
- Hashemian, A.R.; Eshghi, H.; Mansoori, G.A.; Shakeri-Zadeh, A. Folate-Conjugated Gold Nanoparticles (Synthesis, characterization and design for cancer cells nanotechnology-based targeting). Intl. J. Nanosci. Nanotech. 2010, 5, 25–33. [Google Scholar]
- Shakeri-Zadeh, A.; Mansoori, G.A. Cancerous Cells Targeting and Destruction Using Folate Conjugated Gold Nanoparticles. Dynamic Biochem. Proc. Biotech. Mol. Biol. 2010, 4. In press. [Google Scholar]
- Eshghi, H.; Hashemian, A.R.; Shakeri-Zadeh, A.; Sazgarnia, A.; Mansoori, G.A. Targeting, and Photo-Activated Destruction of Cancer Cells, through a New Folate Conjugated Gold Nanoparticle. Int. J. Nanotech. 2010. In press. [Google Scholar]
- Shakeri-Zadeh, A.; Mansoori, G.A. Cancer Nanotechnology Treatment through Folate Conjugated Gold, Nanoparticles. In Proceedings of WCC 2010 (The 2nd World Congress on Cancer), 2010.
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th edition; John Wiley & Sons: NY, USA, 2007. [Google Scholar]
- Pan, D.; Turner, J.L.; Wooley, K.L. Folic acid-conjugated nanostructured materials designed for cancer cell targeting. Chem. Commun. (Camb) 2003, 7, 2400–2401. [Google Scholar]
- Zhang, Z.; Zhou, F.; Lavernia, E.J. On the analysis of grain size in bulk nanocrystalline materials via x-ray diffraction. Metall. Mater. Trans. A 2003, 34, 1349–1355. [Google Scholar] [CrossRef]
- Sanderson, R.T. Chemical Bonds and Bond Energy; Academic Press: New York, NY, 1976. [Google Scholar]
- Masters, J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Rev. Cancer. 2002, 2, 315–319. [Google Scholar] [CrossRef]
- Chung, K.; Saikawa, Y.; Paik, T.; Dixon, K.H.; Mulligan, T.; Cowan, K.H.; Elwood, P.C. Stable Transfectants of Human MCF-7 Breast Cancer Cells with Increased Levels of the Human Folate Receptor Exhibit an Increases Sensitivity to Antifolates. J. Clin. Invest. 1993, 91, 1289–1294. [Google Scholar] [CrossRef]
- Kostanski, L.K.; Pope, M.A.; Hrymak, A.N.; Gallant, M.; Whittington, W.; Vesselov, L. Development of novel tunable light scattering coating materials for fiber optic diffusers in photodynamic cancer therapy. J. App. Polymer Sci. 2009, 112, 1516–1523. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Timeline: Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Kayhanian, K.; Mansoori, G.A.; Rahimpour, M. Prospects for Cancer Nanotechnology Treatment by Azurin. Dynamic Biochem. Proc. Biotech. Mol. Biol. 2010, 4. In press. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mansoori, G.A.; Brandenburg, K.S.; Shakeri-Zadeh, A. A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications. Cancers 2010, 2, 1911-1928. https://doi.org/10.3390/cancers2041911
Mansoori GA, Brandenburg KS, Shakeri-Zadeh A. A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications. Cancers. 2010; 2(4):1911-1928. https://doi.org/10.3390/cancers2041911
Chicago/Turabian StyleMansoori, G. Ali, Kenneth S. Brandenburg, and Ali Shakeri-Zadeh. 2010. "A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications" Cancers 2, no. 4: 1911-1928. https://doi.org/10.3390/cancers2041911
APA StyleMansoori, G. A., Brandenburg, K. S., & Shakeri-Zadeh, A. (2010). A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications. Cancers, 2(4), 1911-1928. https://doi.org/10.3390/cancers2041911