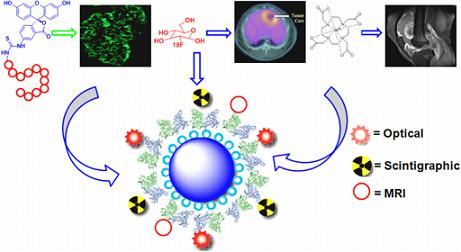
Exogenous Molecular Probes for Targeted Imaging in Cancer: Focus on Multi-modal Imaging
Abstract
:1. Introduction
| Modality | Advantages | Disadvantages |
|---|---|---|
| Optical imaging |
|
|
| Scintigraphic |
|
|
| Magnetic resonance imaging |
|
|
| Ultrasound |
|
|
| Detection ligand | Advantages | Disadvantages |
|---|---|---|
 |
|
|
| (Antibody) | ||
 |
|
|
| (Antibody fragments) | ||
 |
|
|
| (Peptides) | ||
 |
|
|
| (Small molecules) | ||
 |
|
|
| (Nanoparticles) |
2. Scope of the Review
3. Imaging Modalities
3.1. Optical Imaging
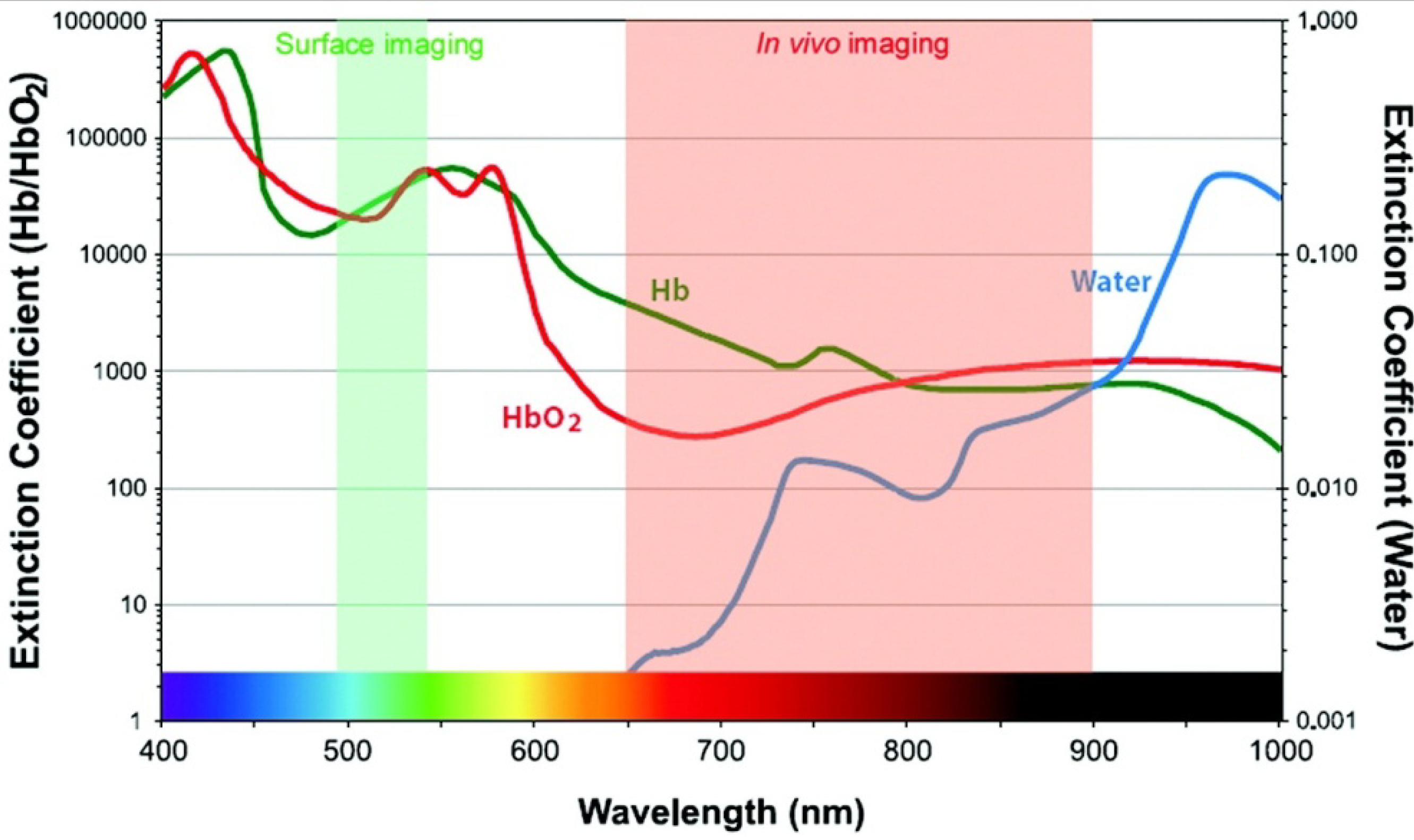
3.2. Scintigraphic Imaging
3.3. Magnetic Resonance Imaging (MRI)
3.4. Ultrasound
3.5. Multi-Modal Imaging Platform
4. Molecular Probes for Optical Imaging
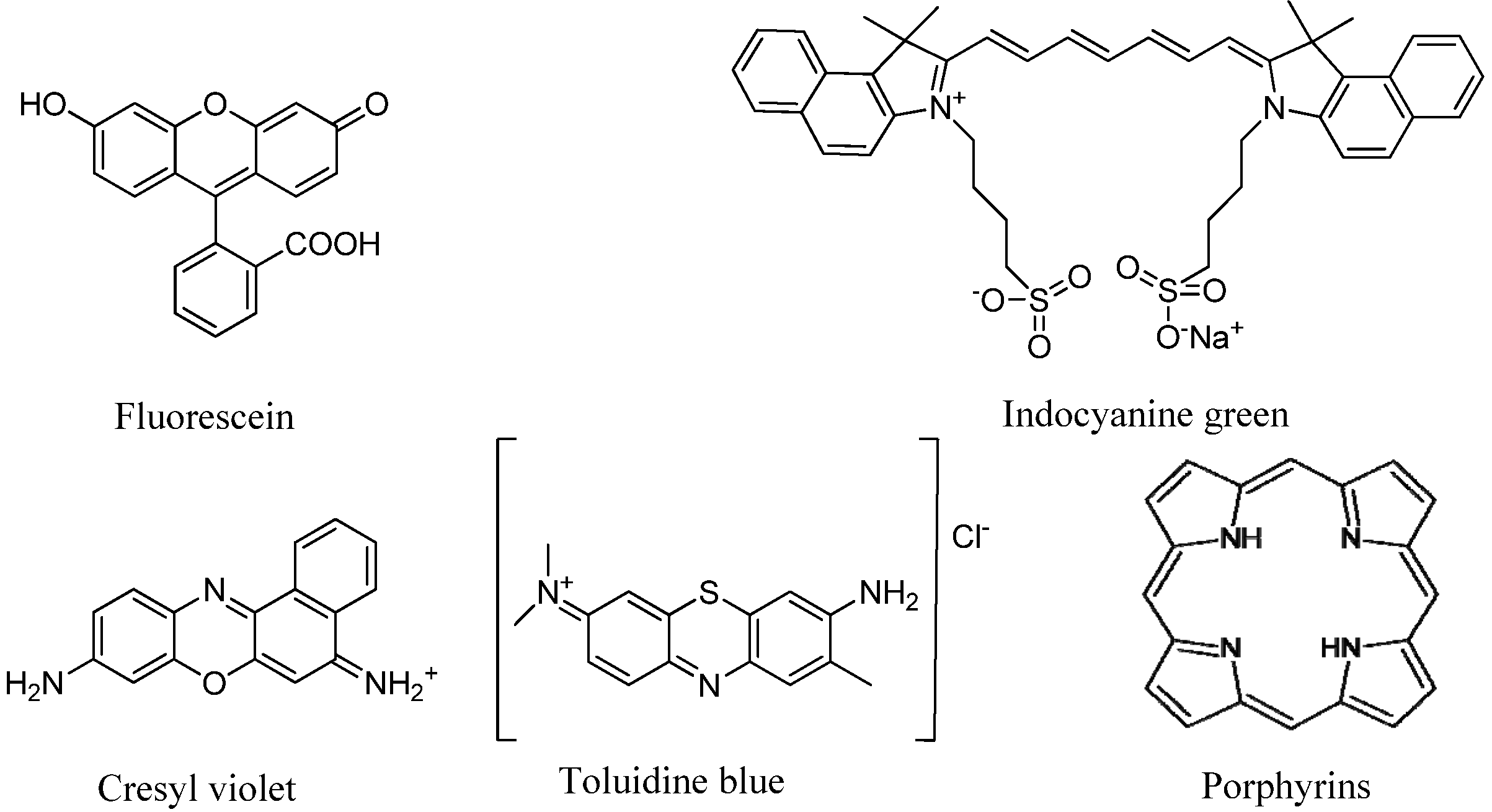
4.1.Non-specific Optical Contrast Agents
4.2. Target Specific Molecular Probes
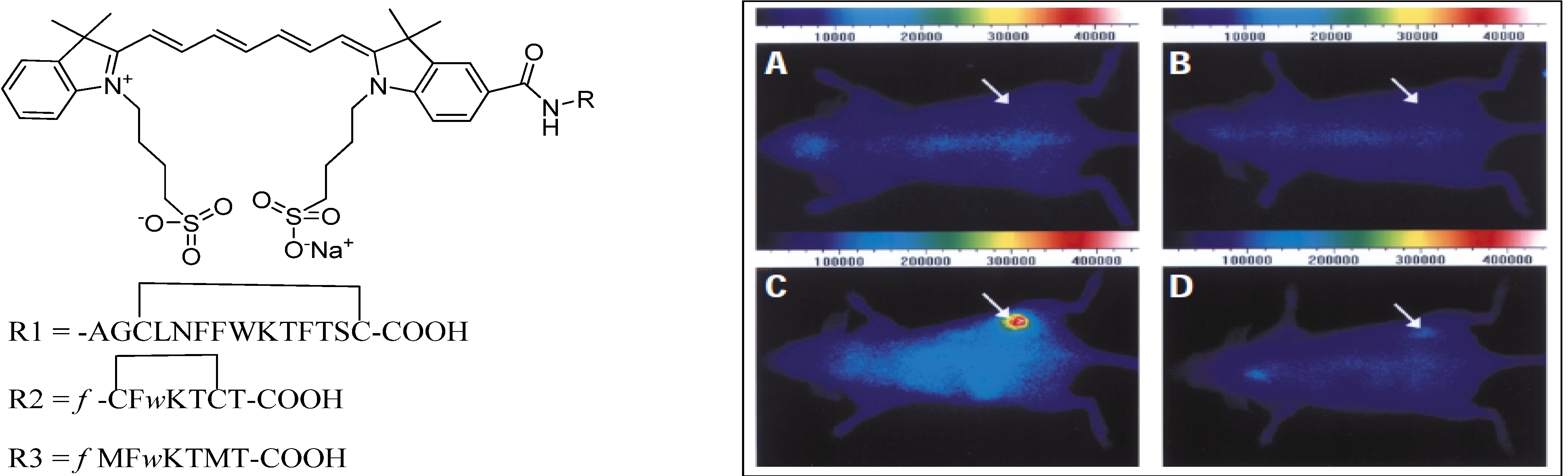
4.2.1. Conjugates with Peptide Ligands
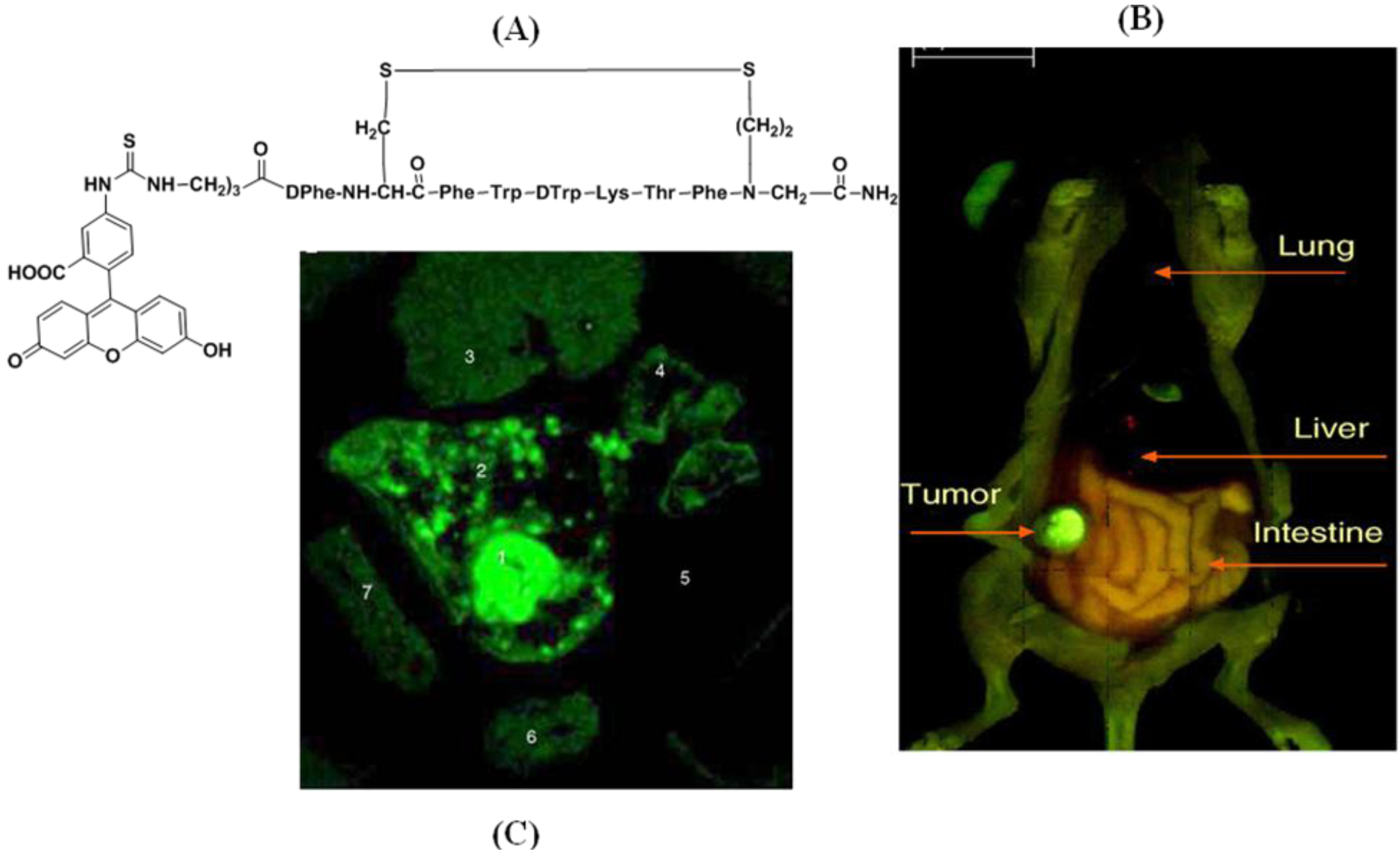
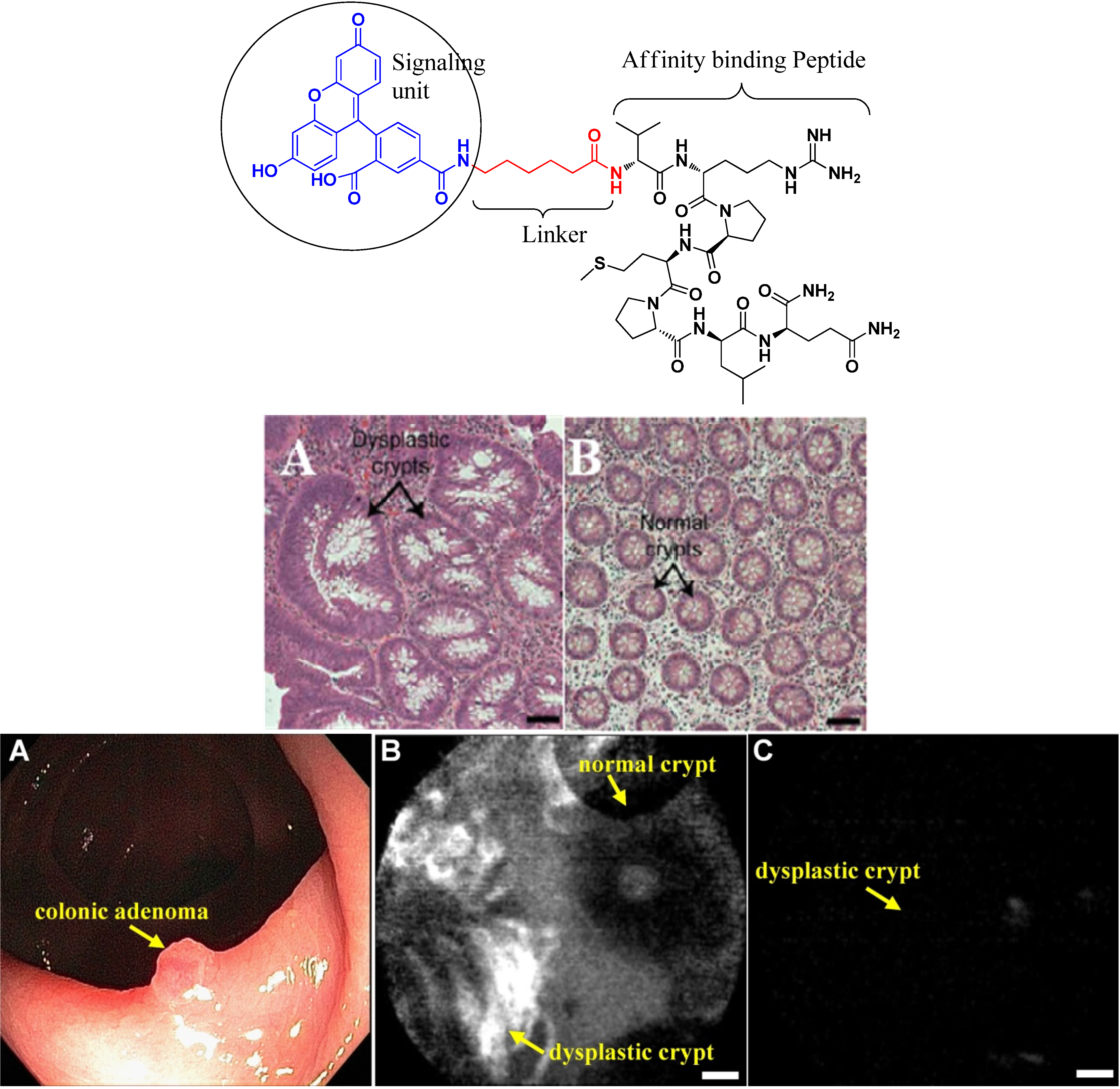
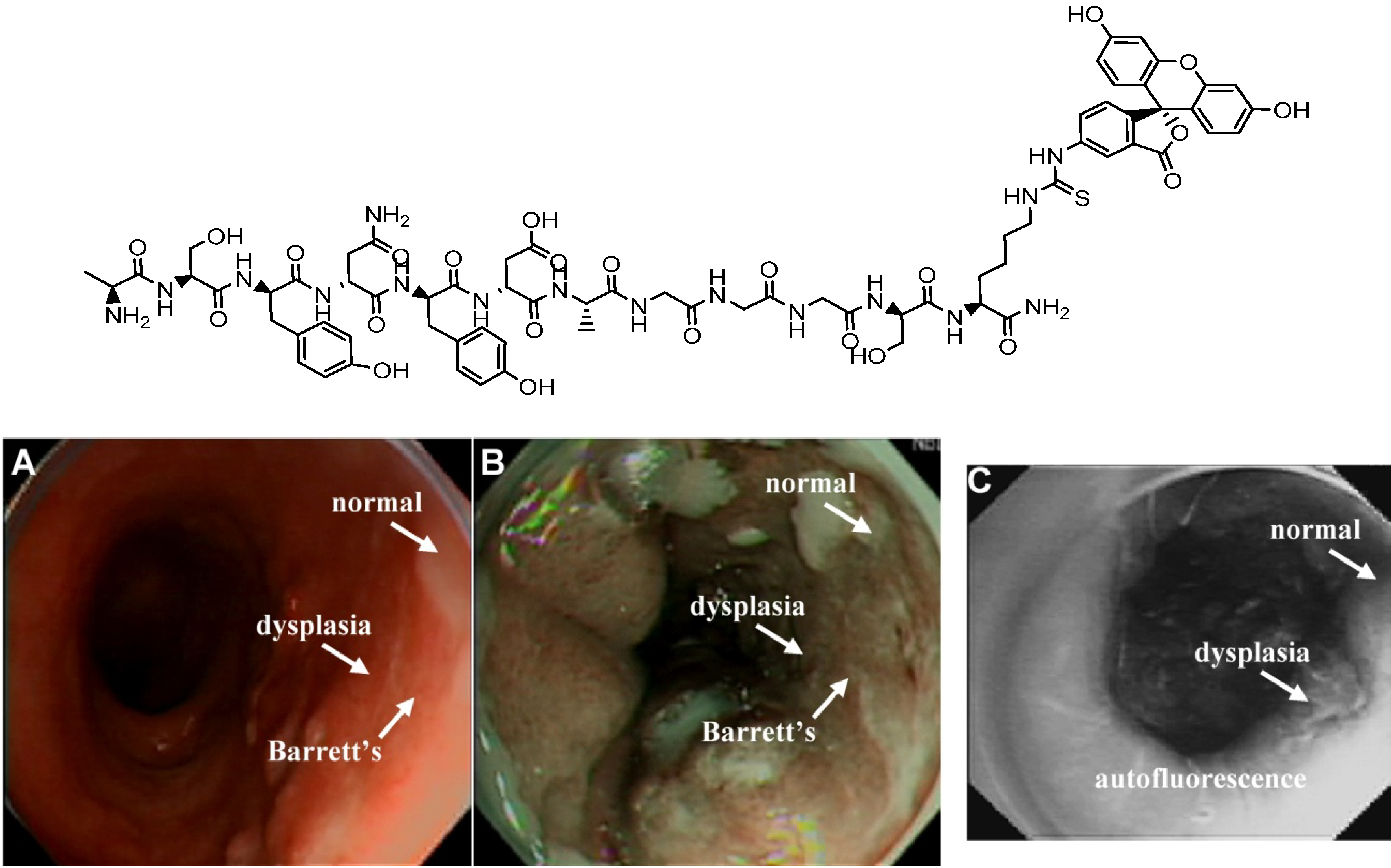
4.2.2. Conjugates with Antibody Ligands
4.2.3. Conjugates with Small Molecule Ligands
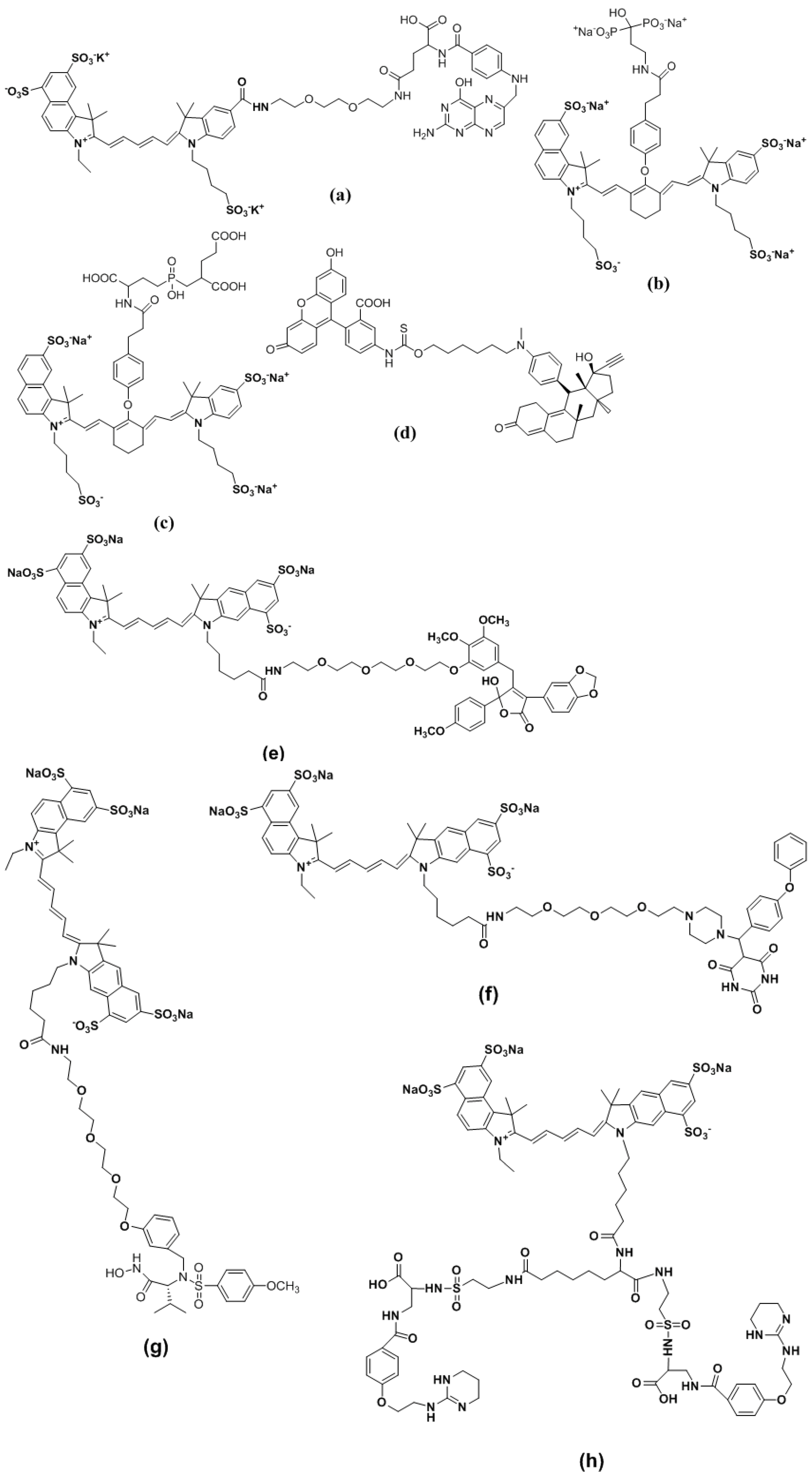
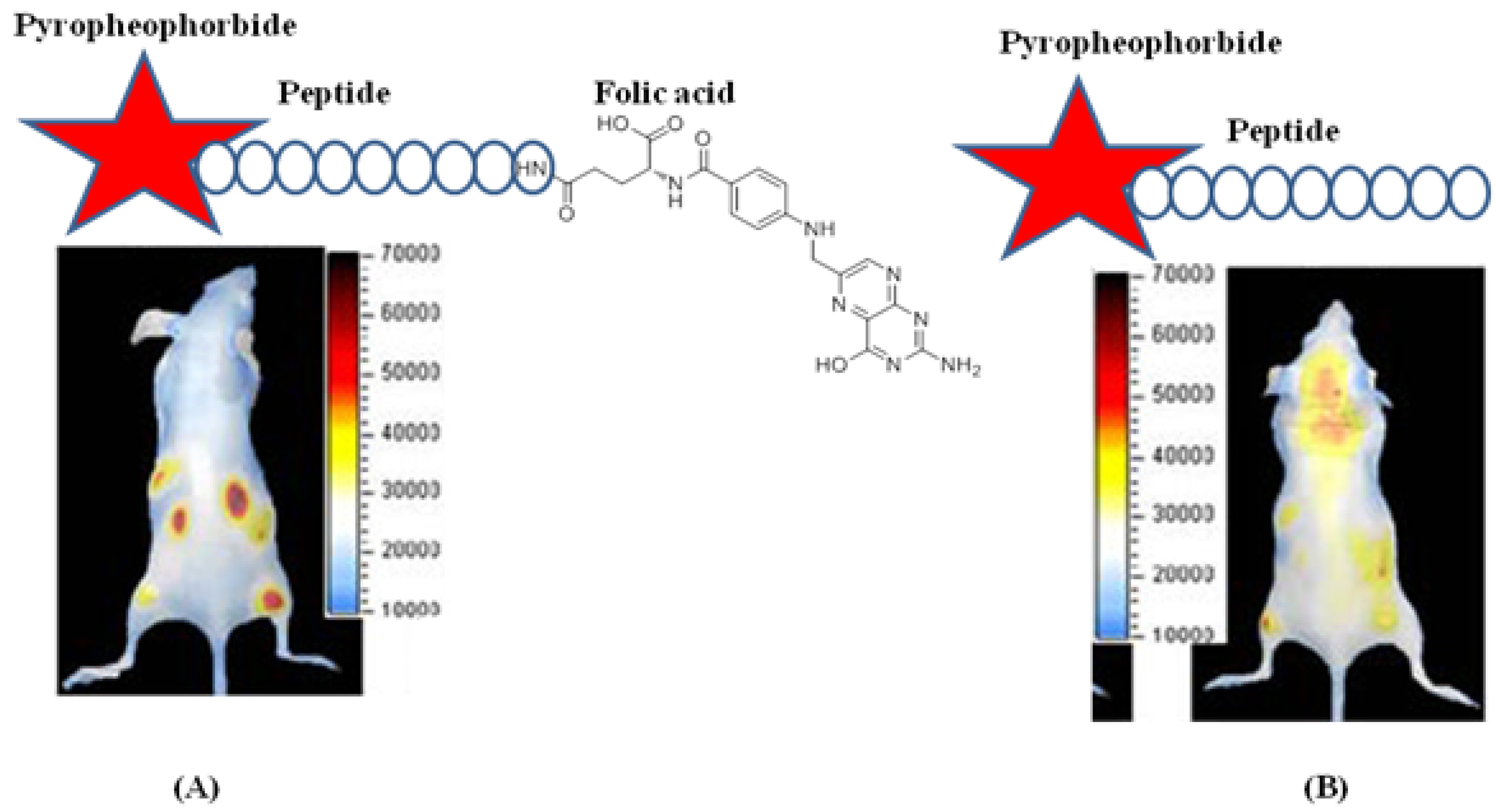
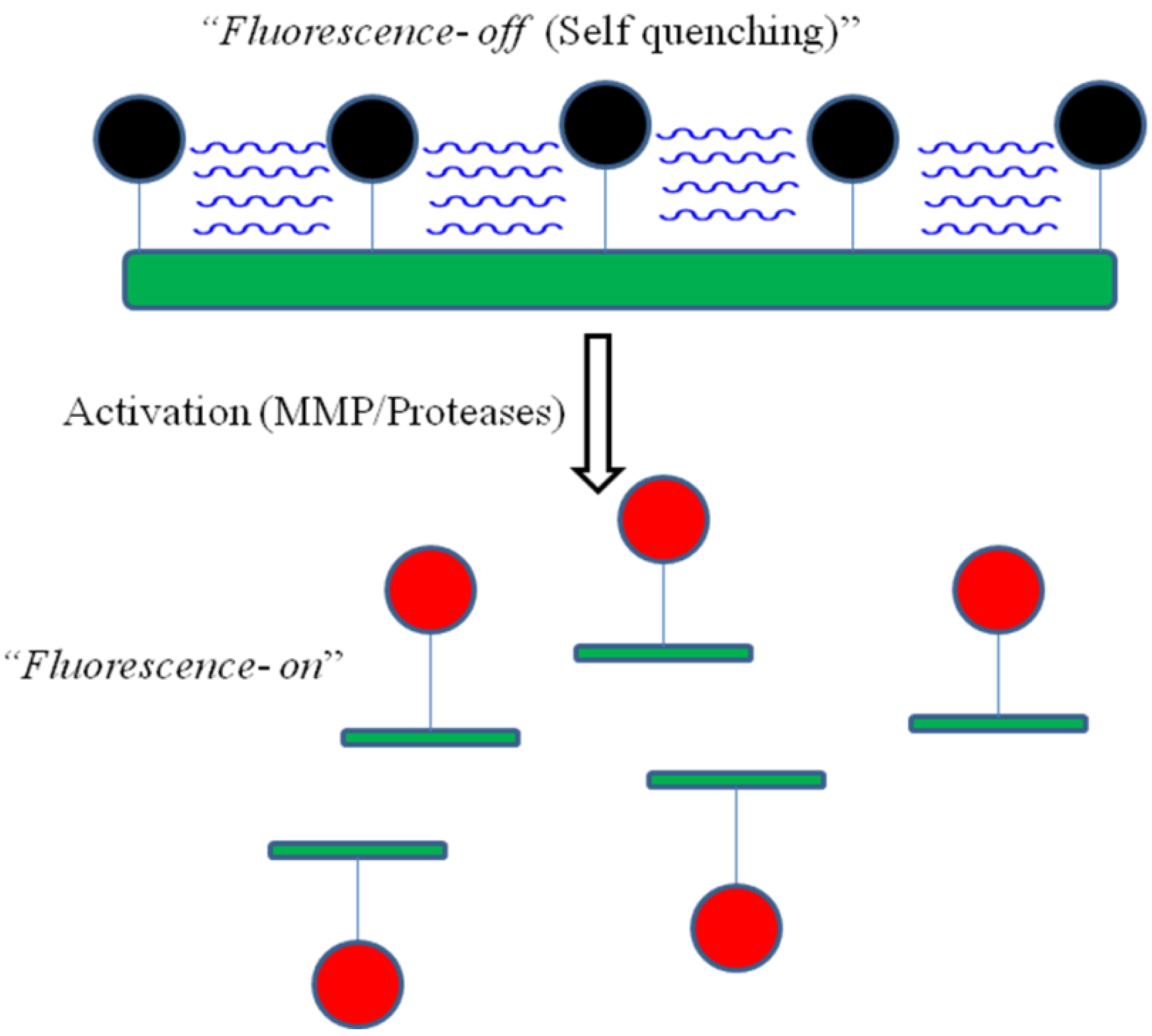
4.3. Activatable Probes
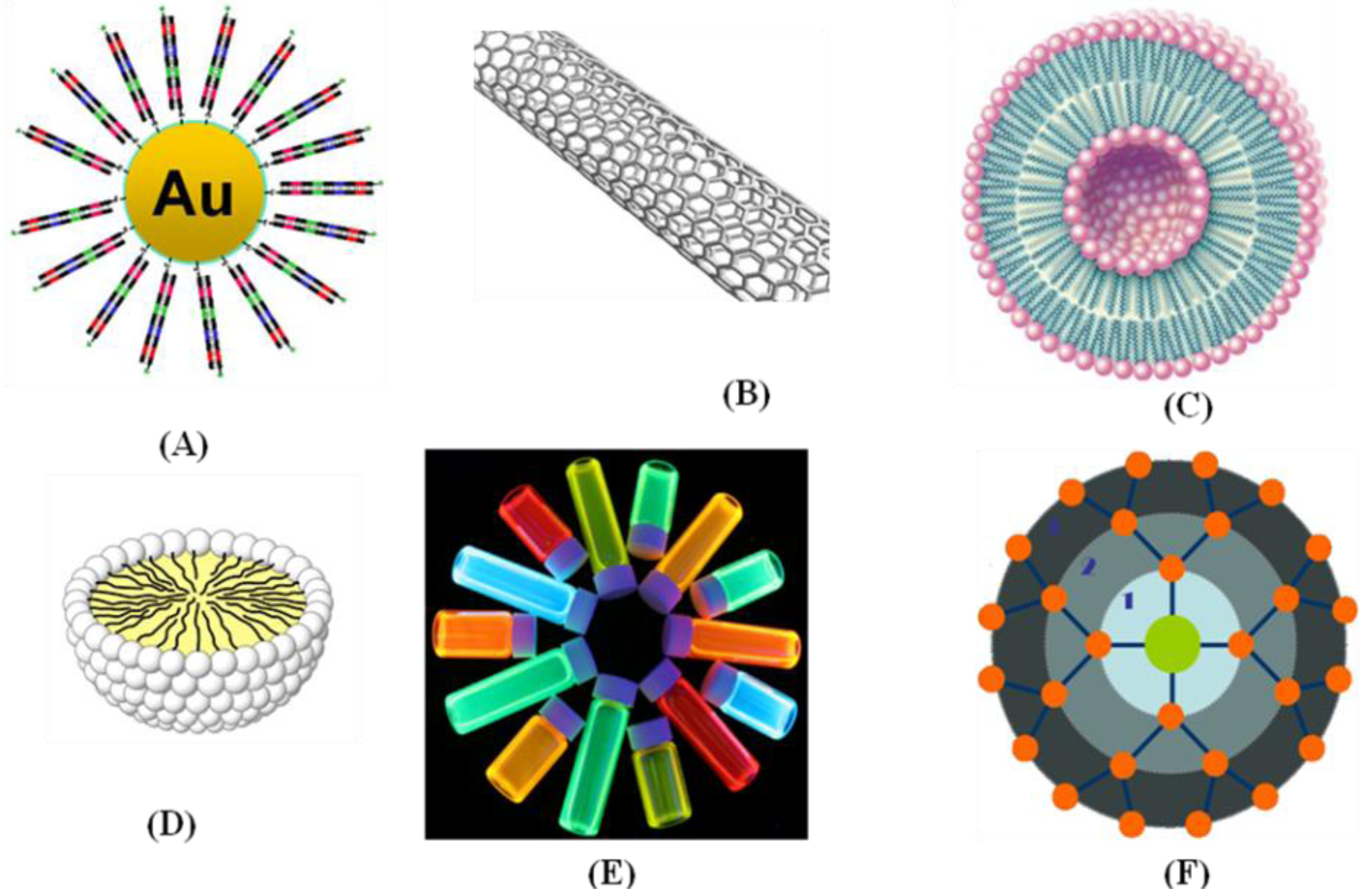
4.4. Optical Probes Based on Nanoparticles
5. Molecular Probes for Scintigraphic Imaging
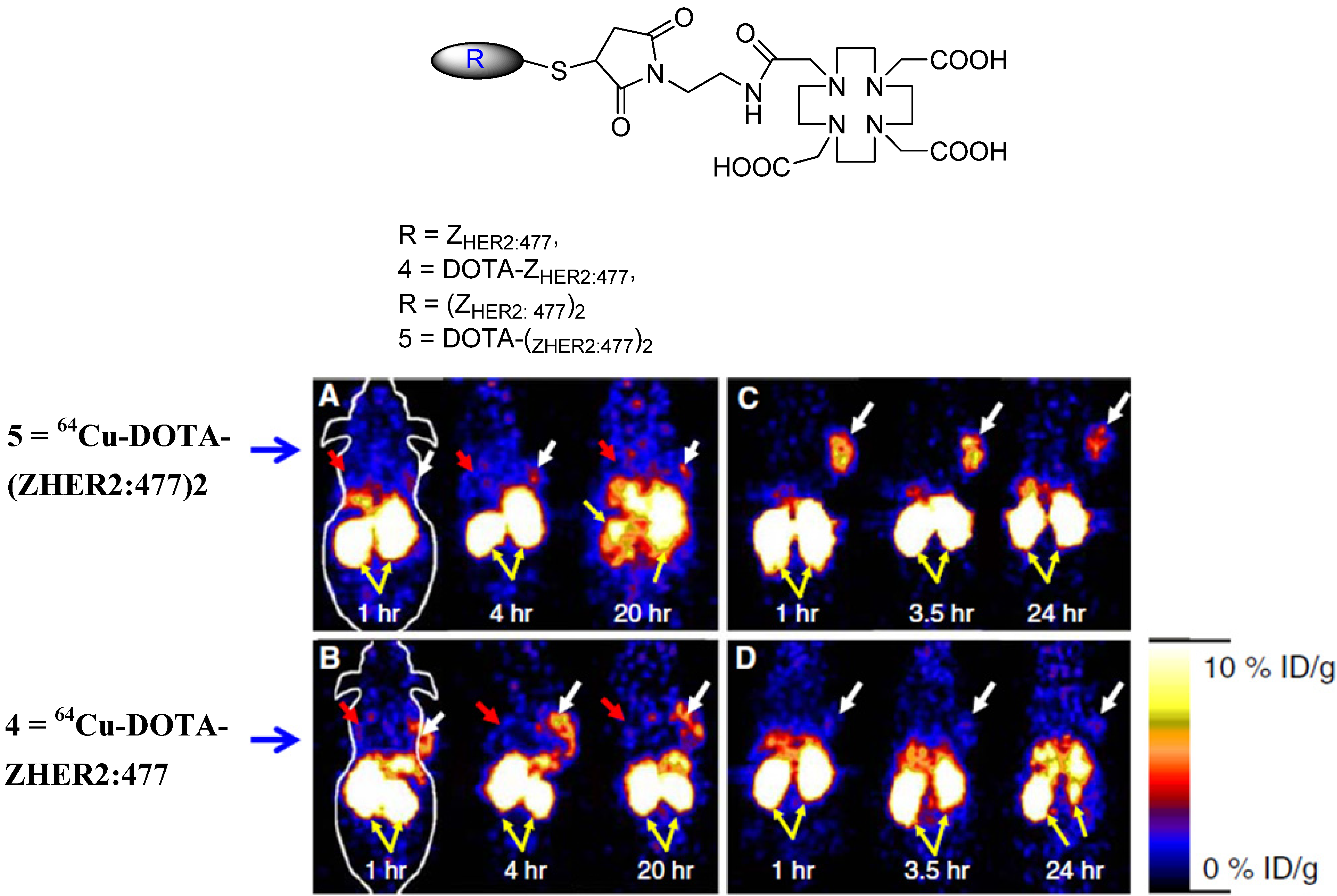
6. Molecular Probes for MRI
7. Molecular Probes for US
8. Dual Labeled Probe for Multi-Modality Imaging
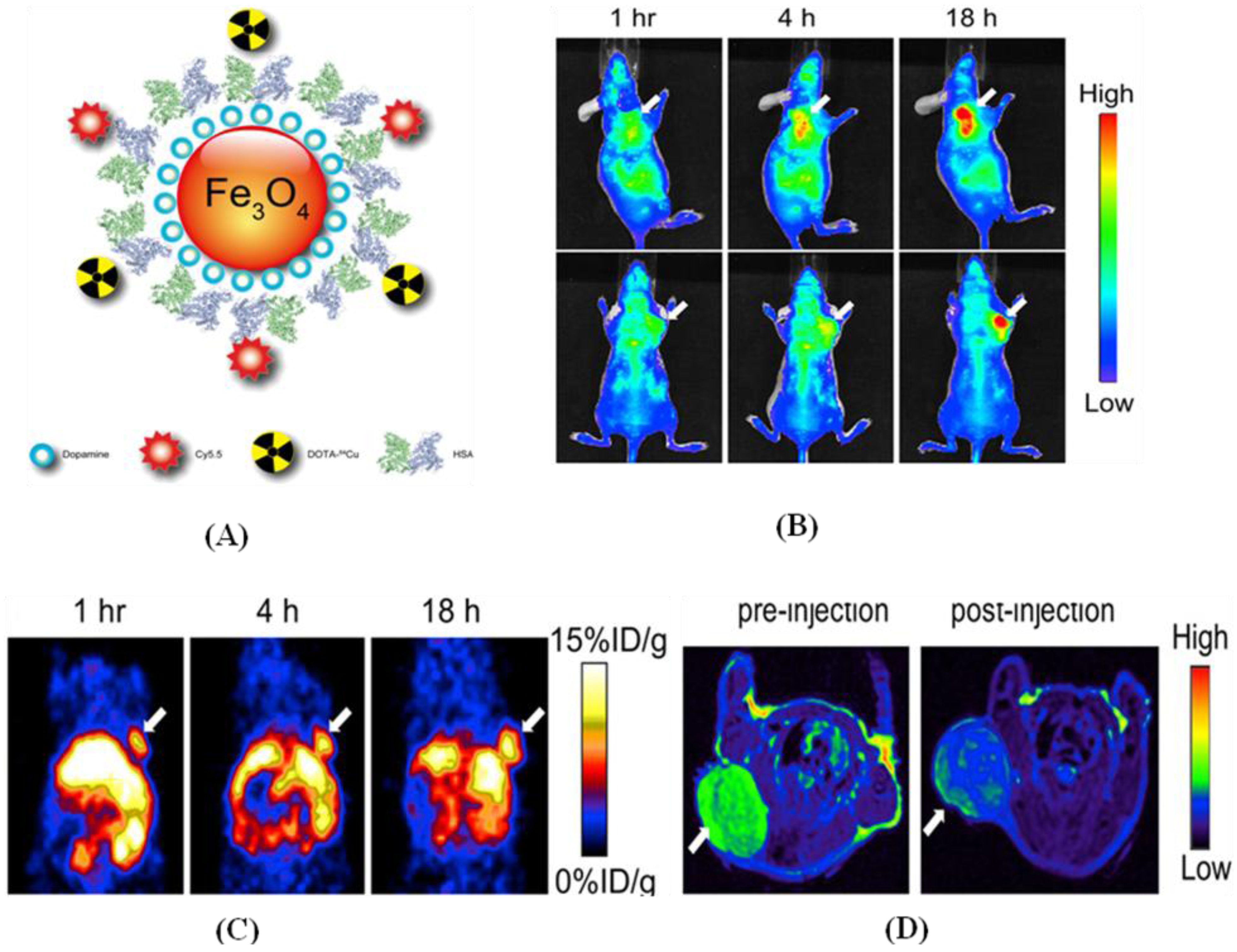
9. Summary and Future Prospects
Abbreviations
| PET | Positron Emission Tomography |
| SPECT | Single Photon Emission Computed Tomography |
| US | Ultrasound |
| CT | Computed Tomography |
| OCT | Optical Coherence Tomography |
| MRI | Magnetic Resonance Imaging |
| NIR | Near Infra Red |
| PL | Polylysine |
| MPEG | Methoxypolyethylene Glycol |
| FITC | Fluorescein Isothiocyanate |
| ICG | Indocyanine Green |
| ITCC | Indotricarbocyanine |
| AMC | 7-Amino 4-methyl coumarin |
| ETAR | Endothelial A receptor |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
| EGFR | Epidermal Growth Factor Receptor |
| MMPs | Matrix Metallo Proteinases |
| GRPR | Gastrin Releasing Peptide Receptor |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| SPARC | Spectral Protein Acidic and Rich in Cystein |
| HAS | Human Serum Albumin |
| IONPs | Iron Oxide Nanoparticles |
| MW | Molecular Weight |
| CA | Cholenic Acid |
| BODIPY | Borondipyrromethane |
| PEG | Polyethylene Glycol |
| GSH | Glutathione |
| CdSe-ZnS | Cadmium Selenide-Zinc Sulfide |
| PDT | Photodynamic Therapy |
| VIP | Vasoactive Intestinal Peptide |
| SFB | Succinimidy Fluorobenzote |
| SCLC | Small Cell Lung Cancer |
| NSCLC | Non-Small Cell Lung Cancer |
| HYNIC | Hydrazidonicotinamide |
| QD | Quantum Dot |
| IRB | Institutional Review Board |
| H&E | Hematoxylin and Eosin |
| TAMRA | Tetramethylrhodamine |
| FDA | Food and Drug Administration |
| FDG | Fludeoxyglucose |
| FLT | Deoxyfluorothymidine |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| PAMAM | Polyaminoamine |
Acknowledgements
References
- Weissleder, R.; Reimer, P.; Lee, A.S.; Wittenberg, J.; Brady, T.J. MR Receptor Imaging: Ultrasmall Iron Oxide Particles Targeted to Asialoglycoprotein Receptors. Am. J. Roentgenol. 1990, 155, 1161–1167. [Google Scholar] [CrossRef]
- Louie, A.Y.; Huber, M. M.; Ahrens, E.T.; Rothbacher, U.; Moats, R.; Jacobs, R.E.; Fraser, S.E.; Meade, T.J. In Vivo Visualization of Gene Expression Using Magnetic Resonance Imaging. Nat. Biotechnol. 2000, 18, 321–325. [Google Scholar]
- Gambhir, S.S.; Barrio, J.R.; Phelps, M.E.; Iyer, M.; Namavari, M.; Satyamurthy, N.; Wu, L.; Green, L.A.; Bauer, E.; MacLaren, D.C.; Nguyen, K.; Berk, A.J.; Cherry, S.R.; Herschman, H.R. Imaging Adenoviral-directed Reporter Gene Expression in Living Animals with Positron Emission Tomography. Proc. Natl. Acad. Sci. USA 1999, 96, 2333–2338. [Google Scholar] [CrossRef]
- Mayerhofer, R.; Araki, K.; Szalay, A.A. Monitoring of Spatial Expression of Firefly Luciferase in Transformed Zebrafish. J. Biolumin. Chemilumin. 1995, 10, 271–275. [Google Scholar] [CrossRef]
- Rehemtulla, A.; Stegman, L.D.; Cardozo, S.J.; Gupta, S.; Hall, D.E.; Contag, C.H.; Ross, B.D. Rapid and Quantitative Assessment of Cancer Treatment Response Using In Vivo Bioluminescence Imaging. Neoplasia 2000, 2, 491–495. [Google Scholar]
- Halpern, J. Contrast-enhanced Ultrasound Imaging of Prostate Cancer. Rev. Urol. 2006, 8, S29–S37. [Google Scholar]
- Contag, C.H.; Bachmann, M.H. Advances in in Vivo Bioluminescence Imaging of Gene Expression. Annu. Rev. Biomed. Eng. 2002, 4, 235–260. [Google Scholar] [CrossRef]
- Soling, A.; Rainov, N.G. Bioluminescence Imaging in Vivo -application to Cancer Research. Expert Opin. Biol. Ther. 2003, 3, 1163–1172. [Google Scholar]
- Nahar, M.; Dutta, T.; Murugesan, S.; Asthana, A.; Mishra, D.; Rajkumar, V.; Tare, M.; Saraf, S.; Jain, N. K. Functional Polymeric Nanoparticles: an Efficient and Promising Tool for Active Delivery of Bioactives. Crit. Rev. Ther. Drug Carrier Syst. 2006, 23, 259–318. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, K.; Nam, H.Y.; Lee, S.; Kim, K.; Kwon, K.C. Polymers for Bioimaging. Prog. Polym. Sci. 2007, 32, 1031–1053. [Google Scholar] [CrossRef]
- Hama, Y.; Urano, Y.; Koyama, Y.; Kamiya, M.; Bernardo, M.; Paik, R.S.; Shin, I.S.; Paik, C.H.; Choyke, P.L.; Kobayashi, H. A Target Cell-Specific Activatable Fluorescence Probe for in vivo Molecular Imaging of Cancer Based on a Self-quenched Avidin-rhodamine Conjugate. Cancer Res. 2007, 67, 2791–2799. [Google Scholar] [CrossRef]
- Bremer, C.; Tung, C.H.; Bogdanov, A., Jr.; Weissleder, R. Imaging of Differential Protease Expression in Breast Cancers for Detection of Aggressive Tumor Phenotypes. Radiology 2002, 222, 814–818. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Deng, X.; Sun, H.; Shi, Z.; Gu, Z.; Liu, Y.; Zhao, Y. Biodistribution of Carbon Single-Wall Carbon Nanotubes in Mice. J. Nanosci. Nanotechnol. 2004, 4, 1019–1024. [Google Scholar] [CrossRef]
- Singh, R.; Pantarotto, D.; Lacerda, L.; Pastorin, G.; Klumpp, C.; Prato, M.; Bianco, A.; Kostarelos, K. Tissue Biodistribution and Blood Clearance Rates of Intravenously Administered Carbon Nanotube Radiotracers. Proc. Natl. Acad. Sci. USA 2006, 103, 3357–3362. [Google Scholar] [CrossRef]
- Villa, C.H.; McDevitt, M.R.; Escorcia, F.E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Scheinberg, D.A. Synthesis and Biodistribution of Oligonucleotide-functionalized, Tumor-targetable Carbon Nanotubes. Nano Lett. 2008, 8, 4221–4228. [Google Scholar] [CrossRef]
- Benaron, D.A. The Future of Cancer Imaging. Cancer Metastasis Rev. 2002, 21, 45–78. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Houston, J.P.; Gurfinkel, M. Fluorescence-enhanced, Near Infrared Diagnostic Imaging with Contrast Agents. Curr. Opin. Chem. Biol. 2002, 6, 642–650. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar]
- Goetz, M.; Wang, T.D. Molecular Imaging in Gastrointestinal Endoscopy. Gastroenterology 2010, 138, 828–833. [Google Scholar] [CrossRef]
- De Rosales, R.T.M.; Arstad, E.; Blower, P.J. Nuclear Imaging of Molecular Processes in Cancer. Targ. Oncol. 2009, 4, 183–197. [Google Scholar] [CrossRef]
- Wester, H.J. Nuclear Imaging Probes: from Bench to Bedside. Clin. Cancer Res. 2007, 13, 3470–3481. [Google Scholar] [CrossRef]
- Ross, B.D.; Chenevert, T.L.; Rehemtulla, A. Magnetic Resonance Imaging in Cancer Research. Eur. J. Cancer 2002, 38, 2147–2156. [Google Scholar] [CrossRef]
- Chan, K.W.; Wong, W.T. Small Molecular Gadolinium (III) Complexes as MRI Contrast Agents for Diagnostic Imaging. Coord. Chem. Rev. 2007, 251, 2428–2451. [Google Scholar] [CrossRef]
- Cosgrove, D. Ultrasound Contrast Agents: An Overview. Eur. J. Radiol. 2006, 60, 324–330. [Google Scholar] [CrossRef]
- Lin, Y.; Weissleder, R.; Tung, C.H. Novel Near-Infrared Cyanine Fluorochromes: Synthesis, Properties, and Bioconjugation. Bioconjugate Chem. 2002, 13, 605–610. [Google Scholar] [CrossRef]
- Pierce, M.C.; Javier, D.J.; Kortum, R.R. Optical Contrast Agents and Imaging Systems for Detection and Diagnosis of Cancer. Int. J. Cancer 2008, 123, 1979–1990. [Google Scholar] [CrossRef]
- Ganguly, B.N.; Mondal, N.N.; Nandy, M.; Roesch, F. Some Physical Aspects of Positron Annihilation Tomography: a Critical Review. J. Radioanal. Nucl. Chem. 2009, 279, 685–698. [Google Scholar]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, Limitations and Challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Patton, J.A.; Townsend, D.W.; Hutton, B.F. Hybrid Imaging Technology: from Dreams and Vision to Clinical Devices. Semin. Nucl. Med. 2009, 39, 247–263. [Google Scholar] [CrossRef]
- Devous, M.D.; Lowe, J.L., Sr.; Payne, J.K. Dual-Isotope Brain SPECT Imaging with Technetium and Iodine-123: Validation by Phantom Studies. J. Nucl. Med. 1992, 33, 2030–2035. [Google Scholar]
- Raymond, K.N.; Pierre, V.C. Next Generation, High Relaxivity Gadolinium MRI Agents. Bioconjugate Chem. 2005, 16, 3–8. [Google Scholar] [CrossRef]
- Weller, G.E.R.; Wong, M.K.K.; Modzelewski, R.A.; Lu, E.; Klibanov, A.L.; Wagner, W.; Villanueva, F.S. Ultrasonic Imaging of Tumor Angiogenesis Using Contrast Microbubbles Targeted via the Tumor-binding Peptide Arginine-Arginine-Leucine. Cancer Res. 2005, 65, 533–539. [Google Scholar]
- Gao, Z.; Kennedy, A.M.; Christensen, D.A.; Rapoport, N.Y. Drug-Loaded Nano/Microbubbles for Combining Ultrasonography and Targeted Chemotherapy. Ultrasonics 2008, 48, 260–270. [Google Scholar] [CrossRef]
- Blodgett, T.M.; Meltzer, C.C.; Townsend, D.W. PET/CT: Form and Function. Radiology 2007, 242, 360–385. [Google Scholar] [CrossRef]
- Bullok, K.E.; Dyszlewski, M.; Prior, J.L.; Pica, C.M.; Sharma, V.; Piwnica-Worms, D. Characterization of Novel Histidine-Tagged Tat-Peptide Complexes Dual-Labeled with (99m)Tc-Tricarbonyl and Fluorescein for Scintigraphy and Fluorescence Microscopy. Bioconjugate Chem. 2002, 13, 1226–1237. [Google Scholar] [CrossRef]
- Houston, J.P.; Ke, S.; Wang, W.; Li, C.; Sevick-Muraca, E.M. Quality Analysis of In Vivo Near-Infrared Fluorescence and Conventional Gamma Images Acquired Using a Dual-Labeled Tumor-Targeting Probe. J. Biomed. Opt. 2005, 10, 054010. [Google Scholar] [CrossRef]
- Huber, M.M.; Staubli, A.B.; Kustedjo, K.; Gray, M.H.; Shih, J.; Fraser, S.E.; Jacobs, R.E.; Meade, T.J. Fluorescently Detectable Magnetic Resonance Imaging Agents. Bioconjugate Chem. 1998, 9, 242–249. [Google Scholar] [CrossRef]
- Kircher, M.F.; Mahmood, U.; King, R.S.; Weissleder, R.; Josephson, L. A Multimodal Nanoparticle for Preoperative Magnetic Resonance Imaging and Intraoperative Optical Brain Tumor Delineation. Cancer Res. 2003, 63, 8122–8125. [Google Scholar]
- Talanov, V.S.; Regino, C.A.; Kobayashi, H.; Bernardo, M.; Choyke, P.L.; Brechbiel, M.W. Dendrimer-Based Nanoprobe for Dual Modality Magnetic Resonance and Fluorescence Imaging. Nano Lett. 2006, 6, 1459–1463. [Google Scholar] [CrossRef]
- Hoffman, R.M. The Multiple Uses of Fluorescent Proteins to Visualize Cancer In Vivo. Nat. Rev. Cancer 2005, 5, 796–806. [Google Scholar] [CrossRef]
- Hama, Y.; Koyama, Y.; Urano, Y.; Choyke, P.L.; Kobayashi, H. Simultaneous Two-Color Spectral Fluorescence Lymphangiography with Near Infrared Quantum Dots to Map Two Lymphatic Flows from the Breast and the Upper Extremity. Breast Cancer Res. Treat. 2007, 103, 23–28. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hama, Y.; Koyama, Y.; Barrett, T.; Regino, C.A.; Urano, Y.; Choyke, P.L. Simultaneous Multicolor Imaging of Five Different Lymphatic Basins Using Quantum Dots. Nano Lett. 2007, 7, 1711–1716. [Google Scholar] [CrossRef]
- Polglase, A.L.; McLaren, W.J.; Skinner, S.A.; Kiesslich, R.; Neurath, M.F.; Delaney, P.M. Fluorescence Confocal Endomicroscope for In Vivo Microscopy of the Upper and Lower Gastrointestinal Tract. Gastrointest. Endosc. 2005, 62, 686–695. [Google Scholar] [CrossRef]
- Lam, S.; Standish, B.; Baldwin, C.; McWilliams, A.; leRiche, J.; Gazdar, A.; Vitkin, A.I.; Yang, V.; Ikeda, N.; MacAulay, C. In Vivo Optical Coherence Tomography Imaging of Preinvasive Bronchial Lesions. Clin. Cancer Res. 2008, 14, 2006–2011. [Google Scholar] [CrossRef]
- Jacobs, R.E.; Cherry, S.R. Complementary Emerging Techniques: High-resolution PET and MRI. Curr. Opin. Neurobiol. 2001, 11, 621–629. [Google Scholar] [CrossRef]
- Te Velde, E.A.; Veerman, T.; Subramaniam, V.; Ruers, T. The Use of Fluorescent Dyes and Probes in Surgical Oncology. EJSO 2010, 36, 6–15. [Google Scholar] [CrossRef]
- Kusano, M.; Tajima, Y.; Yamazaki, K.; Kato, M.; Watanabe, M.; Miwa, M. Sentinel Node Mapping Guided by Indocyanine Green Fluorescence Imaging: a New Method for Sentinel Node Navigation Surgery in Gastrointestinal Cancer. Dig. Surg. 2008, 25, 103–108. [Google Scholar] [CrossRef]
- Klohs, J.; Wunder, A.; Licha, K. Near-Infrared Fluorescent Probes for Imaging Vascular Pathophysiology. Basic Res. Cardiol. 2008, 103, 144–151. [Google Scholar] [CrossRef]
- Licha, K.; Olbrich, C. Optical Imaging in Drug Discovery and Diagnostic Applications. Adv. Drug Delivery Rev. 2005, 57, 1087–1108. [Google Scholar] [CrossRef]
- Lee, S.; Park, K.; Kim, K.; Choi, K.; Kwon, I.C. Activatable Imaging Probes with Amplified Fluorescent Signals. Chem. Commun. 2008, 28, 4250–4260. [Google Scholar]
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M. Semiconductor Quantum Dots and Metal Nanoparticles: Syntheses, Optical Properties, and Biological Applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo Near-infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Becker, A.; Hessenius, C.; Licha, K.; Ebert, B.; Sukowski, U.; Semmler, W.; Wiedenmann, B.; Grotzinger, C. Receptor-targeted Optical Imaging of Tumors with Near-infrared Fluorescent Ligands. Nat. Biotechnol. 2001, 19, 327–331. [Google Scholar]
- Kostenich, G.; Livnah, N.; Bonasera, T.A.; Yechezkel, T.; Salitra, Y.; Litman, P.; Kimel, S.; Orenstein, A. Targeting Small Cell Lung Cancer with Novel Fluorescent Analogs of Somatostatin. Lung Cancer 2005, 50, 319–328. [Google Scholar] [CrossRef]
- Kostenich, G.; Oron-Herman, M.; Kimel, S.; Livnah, N.; Tsarfaty, I.; Orenstein, A. Diagnostic Targeting of Colon Cancer Using a Novel Fluorescent Somatostatin Conjugate in a Mouse Xenograft Model. Int. J. Cancer 2008, 122, 2044–2049. [Google Scholar] [CrossRef]
- Ma, L.; Yu, P.; Veerendra, B.; Rold, T.L.; Retzloff, L.; Prasanphanich, A.; Sieckman, G.; Hoffman, T.J.; Volkert, W.A.; Smith, C.J. In Vitro and In Vivo Evaluation of Alexa Fluor 680-Bombesin-[7-14]NH2 Peptide Conjugate, a High-affinity Fluorescent Probe with High Selectivity for the Gastrin-releasing Peptide Receptor. Mol. Imaging 2007, 6, 171–180. [Google Scholar]
- Chen, X.; Conti, P.S.; Moats, R.A. In vivo Near-infrared Fluorescence Imaging of Integrin αvβ3 in Brain Tumor Xenografts. Cancer Res. 2004, 64, 8009–8014. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, Y.; Xiong, Z.; Gambhir, S.S.; Chen, X. Near-infrared Fluorescent RGD Peptides for Optical Imaging of Integrin αvβ3 Expression in Living Mice. Bioconjugate Chem. 2005, 16, 1433–1441. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, W.; Chen, X. Near-infrared Fluorescence Imaging of Tumor Integrin αvβ3 Expression with Cy7-labeled RGD Multimers. Mol. Imaging Biol. 2006, 8, 226–236. [Google Scholar] [CrossRef]
- Kelly, K.A.; Waterman, P.; Weissleder, R. In vivo Imaging of Molecularly Targeted Phage. Neoplasia 2006, 8, 1011–1018. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Brekken, R.A.; Sivridis, E.; Gatter, K.C.; Harris, A.L.; Sage, E.H. Enhanced Expression of SPARC/Osteonectin in the Tumor-Associated Stroma of Non-small Cell Lung Cancer is Correlated with Markers of Hypoxia/acidity and with Poor Prognosis of Patients. Cancer Res. 2006, 63, 5376–5380. [Google Scholar]
- Cybulsky, M.I.; Gimbrone, M.A., Jr. Endothelial Expression of a Mononuclear Leukocyte Adhesion Molecule During Atherogenesis. Science 1991, 251, 788–791. [Google Scholar]
- Hsiung, P.L.; Hardy, J.; Friedland, S.; Soetikno, R.; Du, C.B.; Wu, A.P.; Sahbaie, P.; Crawford, J.M.; Lowe, A.W.; Contag, C.H.; Wang, T.D. Detection of Colonic Dysplasia In Vivo Using a Targeted Heptapeptide and Confocal Microendoscopy. Nat. Med. 2008, 14, 454–458. [Google Scholar] [CrossRef]
- Folli, S.; Westermann, P.; Braichotte, D.; Pelegrin, A.; Wagnieres, G.; van den Bergh, H.; Mach, J.P. Antibody-indocyanin Conjugates for Immunophotodetection of Human Squamous Cell Carcinoma in Nude Mice. Cancer Res. 1994, 54, 2643–2649. [Google Scholar]
- Ballou, B.; Fisher, G.W.; Waggoner, A.S.; Farkas, D.L.; Reiland, J.M.; Jaffe, R.; Mujumdar, R.B.; Mujumdar, S.R.; Hakala, T.R. Tumor Labeling In Vivo Using Cyanine-conjugated Monoclonal Antibodies. Cancer Immunol. Immunother. 1995, 41, 257–263. [Google Scholar] [CrossRef]
- Birchler, M.; Neri, G.; Tarli, L.; Halin, C.; Viti, F.; Neri, D. Infrared Photodetection for the In Vivo Localisation of Phage-derived Antibodies Directed Against Angiogenic Markers. J. Immunol. Methods 1999, 231, 239–248. [Google Scholar] [CrossRef]
- Matter, C.M.; Schuler, P.K.; Alessi, P.; Meier, P.; Ricci, R.; Zhang, D.; Halin, C.; Castellani, P.; Zardi, L.; Hofer, C.K.; Montani, M.; Neri, D.; Luscher, T.F. Molecular Imaging of Atherosclerotic Plaques Using a Human Antibody Against the Extra-domain B of Fibronectin. Circ. Res. 2004, 95, 1225–1233. [Google Scholar] [CrossRef]
- Birchler, M.; Viti, F.; Zardi, L.; Spiess, B.; Neri, D. Selective Targeting and Photocoagulation of Ocular Angiogenesis Mediated by a Phage-Derived Human Antibody Fragment. Nat. Biotechnol. 1999, 17, 984–988. [Google Scholar] [CrossRef]
- Ke, S.; Wen, X.; Gurfinkel, M.; Charnsangarvej, C.; Wallace, S.; Sevick-Muraca, E.M.; Li, C. Near-infrared Optical Imaging of Epidermal Growth Factor Receptor in Breast Cancer Xenografts. Cancer Res. 2003, 63, 7870–7875. [Google Scholar]
- Citrin, D.; Scott, T.; Sproull, M.; Menard, C.; Tofilo, P.J.; Camphausen, K. In Vivo Tumor Imaging Using a Near-infrared-labeled Endostatin Molecule. Int. J. Radiation Oncology Biol. Phys. 2004, 58, 536–541. [Google Scholar] [CrossRef]
- Licha, K.; Debus, N.; Ebert, B.; Vollmer-Emig, S.; Hasbach, M.; Sydow, S.; Stibenz, D.; Semmler, W.; Buhrer, C.; Schirner, M.; Tauber, R. Optical Molecular Imaging of Lymph Nodes Using a Targeted Vascular Contrast Agent. J. Biomed. Optics 2005, 10. doi:041205-1-041205-6. [Google Scholar]
- Bando, T.; Muguruma, N.; Ito, S.; Musashi, Y.; Inayama, K.; Kusaka, Y.; Tadatsu, M.; Ii, K.; Irimura, T.; Shibamura, S.; Takesako, K. Basic Studies on a Labeled Anti-mucin Antibody Detectable by Infrared-fluorescence Endoscopy. J. Gastroenterol. 2002, 37, 260–269. [Google Scholar] [CrossRef]
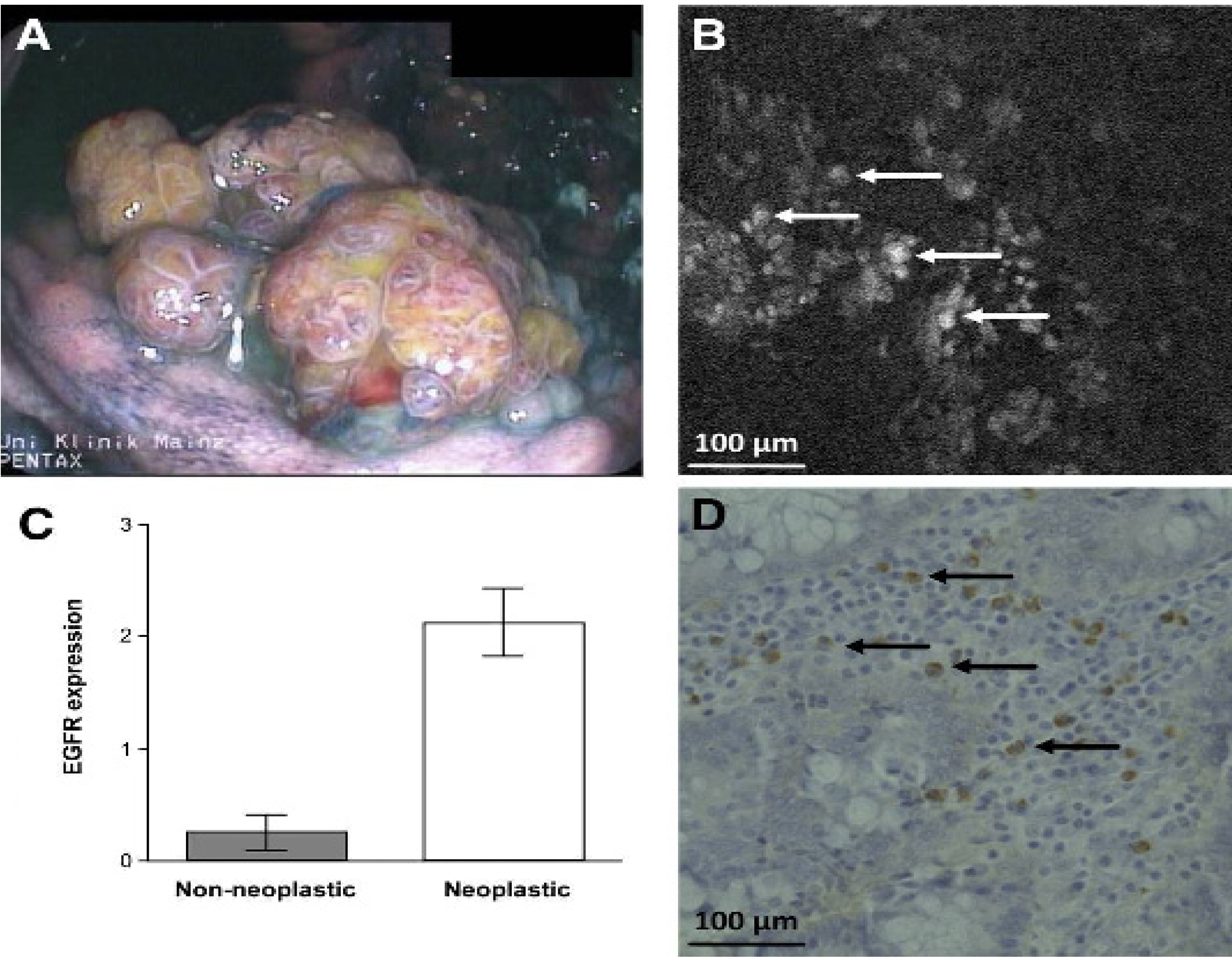
- Goetz, M.; Ziebart, A.; Foersch, S.; Vieth, M.; Waldner, M.J.; Delaney, P.; Galle, P.R.; Neurath, M.F.; Kiesslich, R. In vivo Molecular Imaging of Colorectal Cancer with Confocal Endomicroscopy of Epidermal Growth Factor Receptor. Gastroenterology 2010, 138, 435–446. [Google Scholar] [CrossRef]
- Tung, C.H.; Lin, Y.; Moon, W.K.; Weissleder, R. A Receptor-targeted Near-infrared Fluorescence Probe for In Vivo Tumor imaging. Chembiochem 2002, 3, 784–786. [Google Scholar] [CrossRef]
- Moon, W.K.; Lin, Y.; O’Loughlin, T.; Tang, Y.; Kim, D.E.; Weissleder, R.; Tung, C.H. Enhanced Tumor Detection Using a Folate Receptor-targeted Near-infrared Fluorochrome Conjugate. Bioconjugate Chem. 2003, 14, 539–545. [Google Scholar] [CrossRef]
- Chen, Y.; Gryshuk, A.; Achilefu, S.; Ohulchansky, T.; Potter, W.; Zhong, T.; Morgan, J.; Chance, B.; Prasad, P.N.; Henderson, B.W.; Oseroff, A.; Pandey, R.K. A Novel Approach to a Bifunctional Photosensitizer for Tumor Imaging and Phototherapy. Bioconjugate Chem. 2005, 16, 1264–1274. [Google Scholar] [CrossRef]
- Stefflova, K.; Li, H.; Chen, J.; Zheng, G. Peptide-Based Pharmacomodulation of a Cancer-Targeted Optical Imaging and Photodynamic Therapy Agent. Bioconjugate Chem. 2007, 18, 379–388. [Google Scholar] [CrossRef]
- Holtke, C.; von Wallbrunn, A.; Kopka, K.; Schober, O.; Heindel, W.; Schäfers, M.; Bremer, C. Fluorescent Photoprobe for the Imaging of Endothelin Receptors. Bioconjugate. Chem. 2007, 18, 685–694. [Google Scholar] [CrossRef]
- Faust, A.; Waschkau, B.; Waldeck, J.; Holtke, C.; Breyholz, H.J.; Wagner, S.; Kopka, K.; Heindel, W.; Schäfers, M.; Bremer, C. Synthesis and Evaluation of a Novel Fluorescent Photoprobe for Imaging Matrix Metalloproteinases. Bioconjugate Chem. 2008, 19, 1001–1008. [Google Scholar] [CrossRef]
- Faust, A.; Waschkau, B.; Waldeck, J.; Holtke, C.; Breyholz, H.J.; Wagner, S.; Kopka, K.; Schober, O.; Heindel, W.; Schäfers, M.; Bremer, C. Synthesis and Evaluation of a Novel Hydroxamate Based Fluorescent Photoprobe for Imaging of Matrix Metalloproteinases. Bioconjugate Chem. 2009, 20, 904–912. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Jas, G.S.; Zhang, J.; Qin, G.; Xing, J.; Cotes, C.; Zhao, H.; Wang, X.; Diaz, L.A.; Shi, Z.Z.; Lee, D.Y.; Li, K.C.; Li, Z. Synthesis and Evaluation of a Near-infrared Fluorescent Non-peptidic Bivalent Integrin αvβ3 Antagonist for Cancer Imaging. Bioconjugate Chem. 2010, 21, 270–278. [Google Scholar]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bognanov, A. In Vivo Imaging of Tumors with Protease-activated Near-infrared Fluorescent Probes. Nat. Biotech. 1999, 17, 375–378. [Google Scholar]
- Ogawa, M.; Kosaka, N.; Longmire, M.R.; Urano, Y.; Choyke, P.L.; Kobayashi, H. Fluorophore-Quencher Based Activatable Targeted Optical Probes for Detecting In Vivo Cancer Metastases. Mol. Pharma. 2009, 6, 386–395. [Google Scholar] [CrossRef]
- Urano, Y.; Asanuma1, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya1, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; Kobayashi, H. Selective Molecular Imaging of Viable Cancer Cells with pH-activatable Fluorescence Probes. Nat. Med. 2009, 8, 104–109. [Google Scholar]
- Cai, W.; Chen, X. Nanoplatforms for Targeted Molecular Imaging in Living Subjects. Small 2007, 3, 1840–1854. [Google Scholar] [CrossRef]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of Gold Nanoparticles in Cancer Nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar]
- Chang, Y.J.; Chang, C.H.; Chang, T.J.; Yu, C.Y.; Chen, L.C.; Jan, M.L.; Luo, T.Y.; Lee, T.W.; Ting, G. Biodistribution, Pharmacokinetics and Micro SPECT/CT Imaging of 188Re-BMEDA-liposome in a C26 Murine Colon Carcinoma Solid Tumor Animal Model. Anticancer Res. 2007, 27, 2217–2225. [Google Scholar]
- Chang, C.H.; Stabin, M.G.; Chang, Y.J.; Chen, L.C.; Chen, M.H.; Chang, T.J.; Lee, T.W.; Ting, G. Comparative Dosimetric Evaluation of Nanotargeted 188Re-(DXR)-Liposome for Internal Radiotherapy. Cancer Biother. Radiopharm. 2008, 23, 749–758. [Google Scholar] [CrossRef]
- Hamoudeh, M.; Fessi, H.; Mehier, H.; Faraj, A.A.; Canet-Soulas, E. Dirhenium Decacarbonyl-loaded PLLA Nanoparticles: Influence of Neutron Irradiation and Preliminary In Vivo Administration by the TMT Technique. Int. J. Pharm. 2008, 348, 125–136. [Google Scholar]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar]
- Cai, W.; Shin, D.W.; Chen, K.; Gheysens, O.; Cao, Q.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-labeled Near-infrared Quantum Dots for Imaging Tumor Vasculature in Living Subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Hoffman, J.M.; Johnson, B.; Scher, H.I.; Siegel, B.A.; Cheng, E.Y.; Cheson, B.D.; O’Shaughnessy, J.; Guyton, K.Z.; Mankoff, D.A.; Shankar, L.; Larson, S.M.; Sigman, C.C.; Schilsky, R.L.; Sullivan, D.C. Progress and Promise of FDG-PET Imaging for Cancer Patient Management and Oncologic Drug Development. Clin. Cancer Res. 2005, 11, 2785–2808. [Google Scholar] [CrossRef]
- Higashi, K.; Ueda, Y.; Ayabe, K.; Sakurai, A.; Seki, H.; Nambu, Y.; Oguchi, M.; Shikata, H.; Taki, S.; Tonami, H.; Katsuda, S.; Yamamoto, I. FDG PET in the Evaluation of the Aggressiveness of Pulmonary Adenocarcinoma: Correlation with Histopathological Features. Nucl. Med. Commun. 2000, 21, 707–714. [Google Scholar]
- de Geus-Oei, L.F.; Vander Heijden, H.F.; Corstens, F.H.; Oyen, W. Predictive and Prognostic Value of FDG-PET in Non Small-cell Lung Cancer; a Systematic Review. Cancer 2007, 110, 1654–1664. [Google Scholar]
- Ciernik, I.F.; Dizendorf, E.; Baumert, B.G.; Reiner, B.; Burger, C.; Davis, J.B.; Lutolf, U.M.; Steinert, H.C.; Von Schulthess, G.K. Radiation Treatment Planning with an Integrated Positron Emission and Computer Tomography (PET/CT): a Feasibility Study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 853–863. [Google Scholar] [CrossRef]
- Vesselle, H.; Grierson, J.; Muzi, M.; Pugsley, J.M.; Schmidt, R.A.; Rabinowitz, P.; Peterson, L.M.; Vallie`res, E.; Wood, D.E. In vivo Validation of 3’ deoxy-3’-[18F] Fluorothymidine ([18F] FLT) as a Proliferation ImagingT in Humans: Correlation of [18F] FLT) Uptake by Positron Emission Tomography with Ki-67 Immunohistochemistry and Flow Cytometry in Human Lung Tumors. Clin. Cancer Res. 2002, 8, 3315–3323. [Google Scholar]
- Buck, A.K.; Halter, G.; Schirrmeister, H.; Kotzerke, J.; Wurziger, I.; Glatting, G.; Mattfeldt, T.; Neumaier, B.; Reske, S.N.; Hetzel, M. Imaging Proliferation in Lung Tumor with PET 18F-FLT versus 18F-FDG. J. Nucl. Med. 2003, 44, 1426–1431. [Google Scholar]
- Francis, D.L.; Visvikis, D.; Costa, D.C.; Arulampalam, T.H.; Townsend, C.; Luthra, S.K.; Taylor, I.; Ell, P.J. Potential Impact of 18F-3-deoxy-3-fluorothymidine versus 18F-fluoro-2-deoxy-d-glucose in Positron Emission Tomography for Colorectal Cancer. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 988–994. [Google Scholar]
- Leyton, J.V.; Olafsen, T.; Lepin, E.J.; Hahm, S.; Bauer, K.B.; Reiter, R.E.; Wu, A.M. Humanized Radioiodinated Minibody for Imaging of Prostate Stem Cell Antigen-Expressing Tumors. Clin. Cancer Res. 2008, 14, 7488–7496. [Google Scholar]
- Olafsen, T.; Cheung, C.W.; Yazaki, P.J.; Li, L.; Sundaresan, G.; Gambhir, S.S.; Sherman, M.A.; Williams, L.E.; Shively, J.E.; Raubitschek, A.A.; Wu, A.M. Covalent Disulfide-linked Anti-CEA Diabody Allows Site-specific Conjugation and Radiolabeling for Tumor Targeting Applications. Protein Eng. Des. Sel. 2004, 17, 21–27. [Google Scholar] [CrossRef]
- Kobayashi, H.; Han, E.S.; Kim, I.S.; Le, N.; Rajagopal, V.; Kreitman, R.J.; Pastan, I.; Paik, C.H.; Carrasquillo, J.A. Similarities in the Biodistribution of Iodine-labeled Anti-Tac Single-Chain Disulfide-Stabilized Fv Fragment and Anti-Tac Disulfide-Stabilized Fv Fragment- Comparison with its Single-chain analog. Nucl. Med. Biol. 1998, 25, 387–393. [Google Scholar] [CrossRef]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.; Nilsson, M.; Larsson, B.; Hoiden-Guthenberg, I.; Widstrom, C.; Carlsson, J.; Tolmachev, V.; Stahl, S.; Nilsson, F.Y. Tumor Imaging Using a Picomolar Affinity HER2 Binding Affibody Molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar]
- Wu, A.M. Antibodies and Antimatter: The Resurgence of Immuno-PET. J. Nucl. Med. 2009, 50, 2–5. [Google Scholar]
- Cheng, Z.; de Jesus, O.P.; Kramer, D.J.; De, A.; Webster, J.M.; Gheysens, O.; Levi, J.; Namavari, M.; Wang, S.; Park, J.M.; Zhang, R.; Liu, H.; Lee, B.; Syud, F.A.; Gambhir, S.S. 64Cu-Labeled Affibody Molecules for Imaging of HER2 Expressing Tumors. Mol. Imaging Biol. 2009, 25, 1–9. [Google Scholar]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- McAfee, J.G.; Neumann, R.D. Radiolabeled Peptides and Other Ligands for Receptors Overexpressed in Tumor Cells for Imaging Neoplasms. Nucl. Med. Biol. 1996, 23, 673–676. [Google Scholar] [CrossRef]
- Reubi, J.C. Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Weiner, R.E.; Thakur, M.L. Radiolabeled Peptides in the Diagnosis and Therapy of Oncological Diseases. Appl. Radiat. Isot. 2002, 57, 749–763. [Google Scholar] [CrossRef]
- Okarvi, S.M. Peptide-based Radiopharmaceuticals: Future Tools for Diagnostic Imaging of Cancers and Other Diseases. Med. Res. Rev. 2004, 24, 357–397. [Google Scholar] [CrossRef]
- Newton, J.; Deutscher, S.L. Phage Peptide Display. Handb. Exp. Pharmacol. 2008, 185, 145–163. [Google Scholar] [CrossRef]
- Benedetti, E.; Morelli, G.; Accardo, A.; Mansi, R.; Tesauro, D.; Aloj, L. Criteria for the Design and Biological Characterization of Radiolabeled Peptide-based Pharmaceuticals. Bio. Drugs 2004, 18, 279–295. [Google Scholar]
- Aloj, L.; Morelli, G. Design, Synthesis and Preclinical Evaluation of Radiolabeled Peptides for Diagnosis and Therapy. Curr. Pharm. Des. 2004, 10, 3009–3031. [Google Scholar] [CrossRef]
- Chetanneau, A.; Gaucher, L.; Roullier, A. 99mTechnetium-depreotide Thorax Uptake in 21 Patients with Pulmonary Nodule. Med. Nucl. 2003, 27, 265. [Google Scholar]
- Menda, Y.; Kahn, D. Somatostatin Receptor Imaging of Non Small Cell Lung Cancer with 99mTc Depreotide. Sem. Nucl. Med. 2002, 32, 92–96. [Google Scholar] [CrossRef]
- de Jong, M.; Valkema, R.; Jamar, F.; Kvols, L.K.; Kwekkeboom, D.J.; Breeman, W.A.P.; Bakker, W.H.; Smith, C.; Pauwels, S.; Krenning, E.P. Somatostatin Receptor-targeted Radionuclide Therapy of Tumors: Preclinical and Clinical Findings. Semin. Nucl. Med. 2002, 32, 133–140. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Krenning, E.P. Somatostatin Receptor Imaging. Semin. Nucl. Med. 2002, 32, 84–91. [Google Scholar] [CrossRef]
- Dijkgraaf, I.; Boerman, O.C.; Oyen, W.J.; Corstens, F.H.; Gotthardt, M. Development and Application of Peptide-Based Radiopharmaceuticals. Anticancer Agents Med. Chem. 2007, 7, 543–551. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular Imaging and Therapy of Cancer with Radiolabeled Nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef]
- Kehagias, D.T.; Gouliamos, A.D.; Smyrniotis, V.; Vlahos, L.J. Diagnostic Efficacy and Safety of MRI of the Liver with Superparamagnetic Iron Oxide Particles (SHU555A). J. Magn. Reson. Imaging 2001, 14, 595–601. [Google Scholar] [CrossRef]
- Curtet, C.; Maton, F.; Havet, T.; Slinkin, M.; Mishra, A.; Chatal, J.F.; Muller, R.N. Polylysine-Gd-DTPAn and Polylysine-Gd-DOTAn Coupled to Anti-CEA F(ab')2 Fragments as Potential Immunocontrast Agents: Relaxometry, Biodistribution, and Magnetic Resonance Imaging in Nude Mice Grafted With Human Colorectal Carcinoma. Invest. Radiol. 1998, 33, 752–761. [Google Scholar] [CrossRef]
- Sipkins, D.A.; Cheresh, D.A.; Kazemi, M.R.; Nevin, L.M.; Bednarski, M.D.; Li, K.C. Detection of Tumor Angiogenesis In Vivo by αvβ3-targeted Magnetic Resonance Imaging. Nat. Med. 1998, 4, 623–626. [Google Scholar] [CrossRef]
- Kresse, M.; Wagner, S.; Pfefferer, D.; Lawaczeck, R.; Elste, V.; Semmler, W. Targeting of Ultrasmall Superparamagnetic Iron Oxide (USPIO) Particles to Tumor Cells In Vivo by Using Transferrin Receptor Pathways. Magn. Reson. Med. 1998, 40, 236–242. [Google Scholar] [CrossRef]
- Torchilin, V.P. PEG-based Micelles as Carriers of Contrast Agents for Different Imaging Modality. Adv. Drug Deliv. Rev. 2002, 54, 235–252. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, R.; Wen, X.; Li, L.; Li, C. Micelles Based on Biodegradable Poly(L-glutamic acid)-b-polylactide with Paramagnetic Gd Ions Chelated to the Shell Layer as a Potential Nanoscale MRI-visible Delivery. Biomacromolecules 2008, 9, 36–42. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jee, H.W.; Seo, S.M.; Kwak, B.K.; Khang, G.; Cho, S.H. Diethylenetriaminepentaacetic Acid-Gadolinium (DTPA-Gd)-Conjugated Polysuccinimide Derivatives as Magnetic Resonance Imaging Contrast Agents. Bioconjugate Chem. 2006, 17, 700–706. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI Ultrasensitive Drug Delivery Systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef]
- Willmann, J.K.; Kimura, R.H.; Deshpande, N.; Lutz, A.M.; Cochran, J.R.; Gambhir, S.S. Targeted Contrast-Enhanced Ultrasound Imaging of Tumor Angiogenesis with Contrast Microbubbles Conjugated to Integrin-Binding Knottin Peptides. J. Nucl. Med. 2010, 51, 433–440. [Google Scholar] [CrossRef]
- Willmann, J.K.; Paulmurugan, R.; Chen, K.; Gheysens, O.; Rodriguez-Porcel, M.; Lutz, A.M.; Chen, I.Y.; Chen, X.; Gambhir, S.S. US Imaging of Tumor Angiogenesis with Microbubbles Targeted to Vascular Endothelial Growth Factor Receptor Type 2 in Mice. Radiology 2008, 246, 508–518. [Google Scholar] [CrossRef]
- Koch, A.M.; Reynolds, F.; Kircher, M.F.; Merkle, H.P.; Weissleder, R.; Josephson, L. Uptake and Metabolism of a Dual Fluorochrome Tat-nanoparticle in HeLa cells. Bioconjugate Chem. 2003, 14, 1115–1121. [Google Scholar] [CrossRef]
- Huh, Y.M.; Jun, Y.W.; Song, H.T.; Kim, S.; Choi, J.S.; Lee, J.H.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; Cheon, J. In Vivo Magnetic Resonance Detection of Cancer by Using Multifunctional Magnetic Nanocrystals. J. Am. Chem. Soc. 2005, 127, 12387–12391. [Google Scholar] [CrossRef]
- Veiseh, O.; Sun, C.; Gunn, J.; Kohler, N.; Gabikian, P.; Lee, D.; Bhattarai, N.; Ellenbogen, R.; Sze, R.; Hallahan, A.; Olson, J.; Zhang, M. Optical and MRI Multifunctional Nanoprobe for Targeting Gliomas. Nano Lett. 2005, 5, 1003–1008. [Google Scholar] [CrossRef]
- Funovics, M.; Montet, X.; Reynolds, F.; Weissleder, R.; Josephson, L. Nanoparticles for the Optical Imaging of Tumor E-selectin. Neoplasia 2005, 7, 904–911. [Google Scholar] [CrossRef]
- Weissleder, R.; Kelly, K.; Sun, E.Y.; Shtatland, T.; Josephson, L. Cell-specific Targeting of Nanoparticles by Multivalent Attachment of Small Molecules. Nat. Biotechnol. 2005, 23, 1418–1423. [Google Scholar] [CrossRef]
- Quinti, L.; Weissleder, R.; Tung, C.H. A Fluorescent Nanosensor for Apoptotic Cells. Nano Lett. 2006, 6, 488–490. [Google Scholar] [CrossRef]
- Li, G.; Slansky, A.; Dobhal, M.P.; Goswami, L.N.; Graham, A.; Chen, Y.; Kanter, P.; Alberico, R.A.; Spernyak, J.; Morgan, J.; Mazurchuk, R.; Oseroff, A.; Grossman, Z.; Pandey, R.K. Chlorophyll-a Analogs Conjugated with Aminobenzyl-DTPA as Potential Bifunctional Agents for Magnetic Resonance Imaging and Photodynamic Therapy. Bioconjugate Chem. 2005, 16, 32–42. [Google Scholar] [CrossRef]
- Ogawa, M.; Celeste, A.S.; Regino, C.A.S.; Seidel, J.; Green, M.V.; Xi, W.; Williams, M.; Kosaka, N.; Choyke, P.L.; Kobayashi, H. Dual-Modality Molecular Imaging Using Antibodies Labeled with Activatable Fluorescence and a Radionuclide for Specific and Quantitative Targeted Cancer Detection. Bioconjugate Chem. 2009, 20, 2177–2184. [Google Scholar] [CrossRef]
- Liu, S.; Jia, B.; Qiao, R.; Yang, Z.; Yu, Z.; Liu, Z.; Liu, K.; Shi, J.; Ouyang, H.; Wang, F.; Gao, M. A Novel Type of Dual-modality Molecular Probe for MR and Nuclear Imaging of Tumor: Preparation, Characterization and In Vivo Application. Mol. Pharma. 2009, 6, 1074–1082. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI Triple Functional Iron Oxide Nanoparticles. Biomaterials 2010, 11, 3016–3022. [Google Scholar]
- Kimura, R.H.; Miao, Z.; Cheng, Z.; Gambhir, S.S.; Cochran, J.R. A Dual-Labeled Knottin Peptide for PET and Near-Infrared Fluorescence Imaging of Integrin Expression in Living Subjects. Bioconjugate Chem. 2010, 21, 436–444. [Google Scholar] [CrossRef]
- Nam, T.; Park, S.; Lee, S.Y.; Park, K.; Choi, K.; Song, I.C.; Han, M.H.; Leary, J.J.; Yuk, S.A.; Kwon, I.C.; Kim, K.; Jeong, S.Y. Tumor Targeting Chitosan Nanoparticles for Dual-Modality Optical/MR Cancer Imaging. Bioconjugate Chem. 2010, 21, 578–582. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Joshi, B.P.; Wang, T.D. Exogenous Molecular Probes for Targeted Imaging in Cancer: Focus on Multi-modal Imaging. Cancers 2010, 2, 1251-1287. https://doi.org/10.3390/cancers2021251
Joshi BP, Wang TD. Exogenous Molecular Probes for Targeted Imaging in Cancer: Focus on Multi-modal Imaging. Cancers. 2010; 2(2):1251-1287. https://doi.org/10.3390/cancers2021251
Chicago/Turabian StyleJoshi, Bishnu P., and Thomas D. Wang. 2010. "Exogenous Molecular Probes for Targeted Imaging in Cancer: Focus on Multi-modal Imaging" Cancers 2, no. 2: 1251-1287. https://doi.org/10.3390/cancers2021251