In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array
Abstract
:1. Introduction
2. Materials and Methods
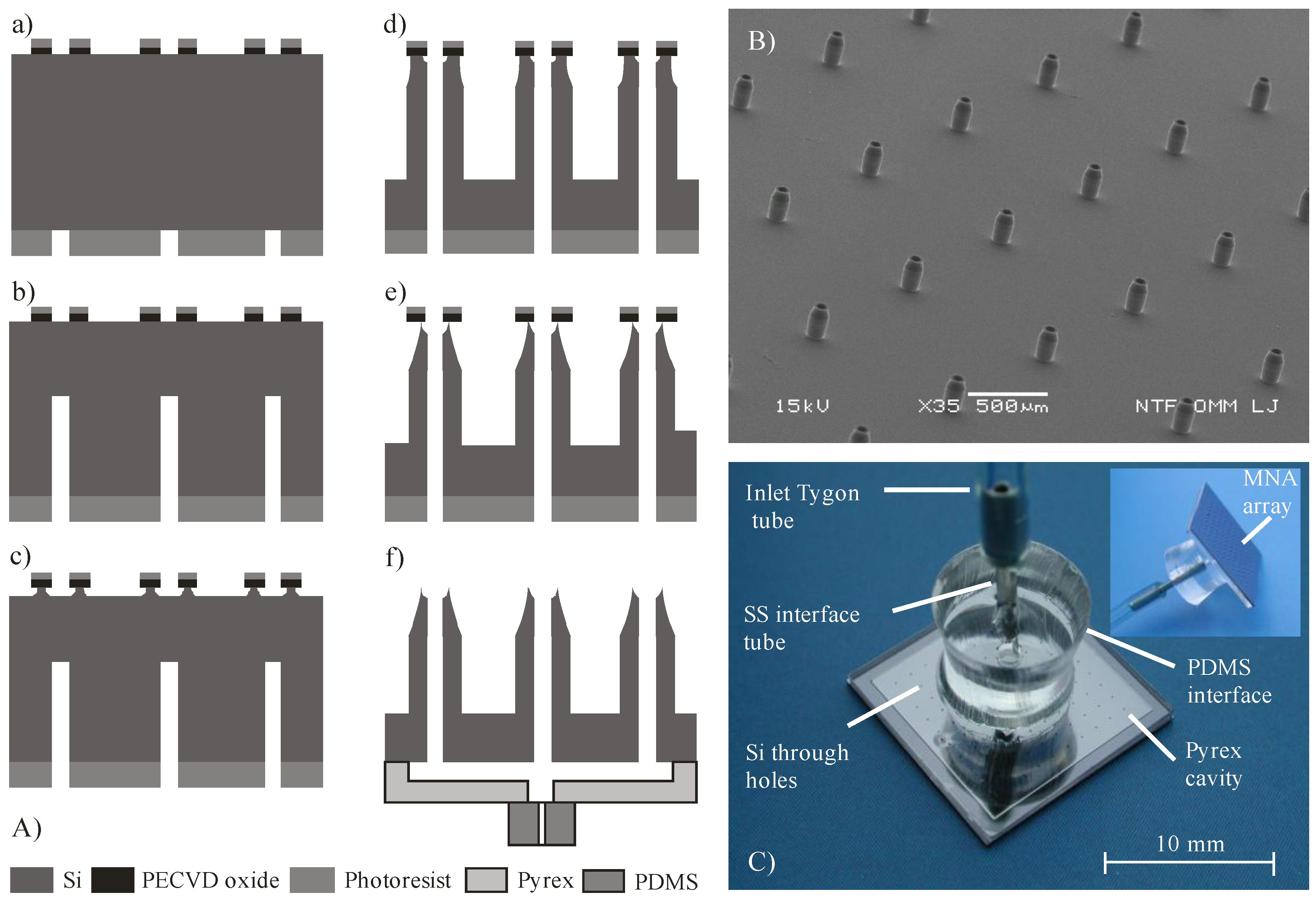
2.1. Design and Fabrication of MNA
2.2. MNA Carrier and Skin Preparation
2.3. Saline and Insulin Delivery Protocol
3. Results
3.1. Methylene Blue Dye Delivery into the Skin by MNA
3.2. Saline Delivery into the Skin by MNA
3.2.1. Transfer Efficiency η
3.2.2. The Influence of Flow Rate and MNA Array Size on Transfer Efficiency
3.2.3. Influence of Delivered Dose on Transfer Efficiency
3.2.4. Backpressure versus Applied Force and Correlation with η
3.3. Insulin Control Delivery by Subcutaneous Route
3.4. Microinjection of Insulin through Hollow MNA
4. Discussion
4.1. Skin Penetration Mechanism by Hollow MNA
4.2. Saline Delivery into the Skin
4.3. Insulin Delivery into the Skin
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Purdon, C.H.; Azzi, C.G.; Zhang, J.; Smith, E.W.; Maibach, H.I. Penetration enhancement of transdermal delivery—Current permutations and limitations. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 97–132. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Al-Qallaf, B.; Das, D.B. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 2008, 86, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, F.S.; Dumitrescu-Ionescu, D.; Petrescu, M.; Iliescu, C. A review on transdermal drug delivery using microneedles: Current research and perspective. Ann. Acad. Rom. Sci. 2014, 7, 7–34. [Google Scholar]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N. Microneedles: An innovative approach to transdermal delivery—A review. Int. J. Pharm. Pharm. Sci. 2014, 6, 18–25. [Google Scholar]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Poh, A.; Fan, S.; Ho, P.; Chan, Y.; Chan, S. Reversible effects of permeation enhancers on human skin. Eur. J. Pharm. Biopharm. 2007, 67, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wei, J.; Iliescu, C. Sonophoretic enhanced microneedles array (SEMA)—Improving the efficiency of transdermal drug delivery. Sens. Actuators B Chem. 2010, 145, 54–60. [Google Scholar] [CrossRef]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, J.S.; Anbalagan, P.; Shelar, S.B.; Neo, J.K.; Iliescu, C.; Kang, L. Direct microneedle array fabrication off a photomask to deliver collagen through skin. Pharm. Res. 2014, 31, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Das, D.B. Permeability enhancement for transdermal delivery of large molecule using low-frequency sonophoresis combined with microneedles. J. Pharm. Sci. 2013, 102, 3614–3622. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.; Rao, M.; Turner, K.; Meinhart, C.; MacDonald, N. Bulk micromachined titanium microneedles. J. Microelectromech. Syst. 2007, 16, 289–295. [Google Scholar] [CrossRef]
- Ji, J.; Tay, F.E.; Miao, J.; Iliescu, C. Microfabricated microneedle with porous tip for drug delivery. J. Micromech. Microeng. 2006, 16, 958. [Google Scholar] [CrossRef]
- Kathuria, H.; Kochhar, J.S.; Fong, M.H.M.; Hashimoto, M.; Iliescu, C.; Yu, H.; Kang, L. Polymeric microneedle array fabrication by photolithography. J. Vis. Exp. 2015, 105, e52914. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.-H.; Chen, M.-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Kerimoglu, O. Novel use of pectin as a microneedle base. Chem. Pharm. Bull. 2015, 63, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.J.; Kim, T.; Park, S.J.; Kim, D.S. Simple and cost-effective fabrication of solid biodegradable polymer microneedle arrays with adjustable aspect ratio for transdermal drug delivery using acupuncture microneedles. J. Micromech. Microeng. 2014, 24, 115015. [Google Scholar] [CrossRef]
- Liu, G.; Shen, C.; Yang, Z.; Cai, X.; Zhang, H. A disposable piezoelectric micropump with high performance for closed-loop insulin therapy system. Sens. Actuators A Phys. 2010, 163, 291–296. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, E.; Collins, S.; Isseroff, R.R.; Smith, R. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens. Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Chua, B.; Desai, S.P.; Tierney, M.J.; Tamada, J.A.; Jina, A.N. Effect of microneedles shape on skin penetration and minimally invasive continuous glucose monitoring in vivo. Sens. Actuators A Phys. 2013, 203, 373–381. [Google Scholar] [CrossRef]
- Chaudhri, B.P.; Ceyssens, F.; De Moor, P.; Van Hoof, C.; Puers, R. A high aspect ratio SU-8 fabrication technique for hollow microneedles for transdermal drug delivery and blood extraction. J. Micromech. Microeng. 2010, 20, 064006. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.-B. High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst. Technol. 2007, 13, 231–235. [Google Scholar] [CrossRef]
- Wang, P.-C.; Paik, S.-J.; Chen, S.; Rajaraman, S.; Kim, S.-H.; Allen, M.G. Fabrication and characterization of polymer hollow microneedle array using UV lithography into micromolds. J. Microelectromech. Syst. 2013, 22, 1041–1053. [Google Scholar] [CrossRef]
- Mansoor, I.; Hafeli, U.O.; Stoeber, B. Hollow out-of-plane polymer microneedles made by solvent casting for transdermal drug delivery. J. Microelectromech. Syst. 2012, 21, 44–52. [Google Scholar] [CrossRef]
- Mansoor, I.; Liu, Y.; Häfeli, U.; Stoeber, B. Arrays of hollow out-of-plane microneedles made by metal electrodeposition onto solvent cast conductive polymer structures. J. Micromech. Microeng. 2013, 23, 085011. [Google Scholar] [CrossRef]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion using hollow microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Stoeber, B.; Wu, G.C.; Zhai, H.; Liepmann, D.; Maibach, H. Clinical microneedle injection of methyl nicotinate: Stratum corneum penetration. Skin Res. Technol. 2005, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Felner, E.I.; Prausnitz, M.R. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol. Ther. 2009, 11, 329–337. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Gardeniers, H.J.; Luttge, R.; Berenschot, E.J.; De Boer, M.J.; Yeshurun, S.Y.; Hefetz, M.; van’t Oever, R.; van den Berg, A. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 2003, 12, 855–862. [Google Scholar] [CrossRef]
- Teo, M.A.L.; Shearwood, C.; Ng, K.C.; Lu, J.; Moochhala, S. In vitro and in vivo characterization of MEMS microneedles. Biomed. Microdevices 2005, 7, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, L.; Roxhed, N.; Griss, P.; Stemme, G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: An initial comparison with subcutaneous administration. Pharm. Res. 2007, 24, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Johnson, D.; Westerman, R. Aspect ratio dependent etching lag reduction in deep silicon etch processes. J. Vac. Sci. Technol. A 2006, 24, 1283–1288. [Google Scholar] [CrossRef]
- Yang, M.; Zahn, J.D. Microneedle insertion force reduction using vibratory actuation. Biomed. Microdevices 2004, 6, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.; Chen, B.; Miao, J. On the wet etching of Pyrex glass. Sens. Actuators A Phys. 2008, 143, 154–161. [Google Scholar] [CrossRef]
- Roxhed, N.; Gasser, T.C.; Griss, P.; Holzapfel, G.A.; Stemme, G. Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery. J. Microelectromech. Syst. 2007, 16, 1429–1440. [Google Scholar] [CrossRef]
- Gupta, J.; Park, S.S.; Bondy, B.; Felner, E.I.; Prausnitz, M.R. Infusion pressure and pain during microneedle injection into skin of human subjects. Biomaterials 2011, 32, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.S.; Couse, T.; Prausnitz, M.R. Mechanism of fluid infusion during microneedle insertion and retraction. J. Control. Release 2006, 112, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise microinjection into skin using hollow microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Stoeber, B.; Liepmann, D. Arrays of hollow out-of-plane microneedles for drug delivery. J. Microelectromech. Syst. 2005, 14, 472–479. [Google Scholar] [CrossRef]
- Ashraf, M.; Tayyaba, S.; Nisar, A.; Afzulpurkar, N.; Bodhale, D.; Lomas, T.; Poyai, A.; Tuantranont, A. Design, fabrication and analysis of silicon hollow microneedles for transdermal drug delivery system for treatment of hemodynamic dysfunctions. Cardiovasc. Eng. 2010, 10, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, M.; Tarn, T.-J. Dynamic modeling and control of a micro-needle integrated piezoelectric micro-pump for diabetes care. In Proceedings of the IEEE-NANO 2006. Sixth IEEE Conference on Nanotechnology, Cincinnati, OH, USA, 17–20 July 2006; pp. 146–149. [Google Scholar]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Luongo, K.; Strom, J.A.; Bhansali, S. Sharpening of hollow silicon microneedles to reduce skin penetration force. J. Micromech. Microeng. 2010, 20, 045011. [Google Scholar] [CrossRef]
- Shergold, O.A.; Fleck, N.A. Experimental investigation into the deep penetration of soft solids by sharp and blunt punches, with application to the piercing of skin. J. Biomech. Eng. 2005, 127, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Crichton, M.L.; Ansaldo, A.; Chen, X.; Prow, T.W.; Fernando, G.J.; Kendall, M.A. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials 2010, 31, 4562–4572. [Google Scholar] [CrossRef] [PubMed]
- Jepps, O.G.; Dancik, Y.; Anissimov, Y.G.; Roberts, M.S. Modeling the human skin barrier—Towards a better understanding of dermal absorption. Adv. Drug Deliv. Rev. 2013, 65, 152–168. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resnik, D.; Možek, M.; Pečar, B.; Janež, A.; Urbančič, V.; Iliescu, C.; Vrtačnik, D. In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array. Micromachines 2018, 9, 40. https://doi.org/10.3390/mi9010040
Resnik D, Možek M, Pečar B, Janež A, Urbančič V, Iliescu C, Vrtačnik D. In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array. Micromachines. 2018; 9(1):40. https://doi.org/10.3390/mi9010040
Chicago/Turabian StyleResnik, Drago, Matej Možek, Borut Pečar, Andrej Janež, Vilma Urbančič, Ciprian Iliescu, and Danilo Vrtačnik. 2018. "In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array" Micromachines 9, no. 1: 40. https://doi.org/10.3390/mi9010040





