Mechanical Vibration Measurement of Solidly Mounted Resonator in Fluid by Atomic Force Microscopy
Abstract
:1. Introduction
2. Experimental Methods
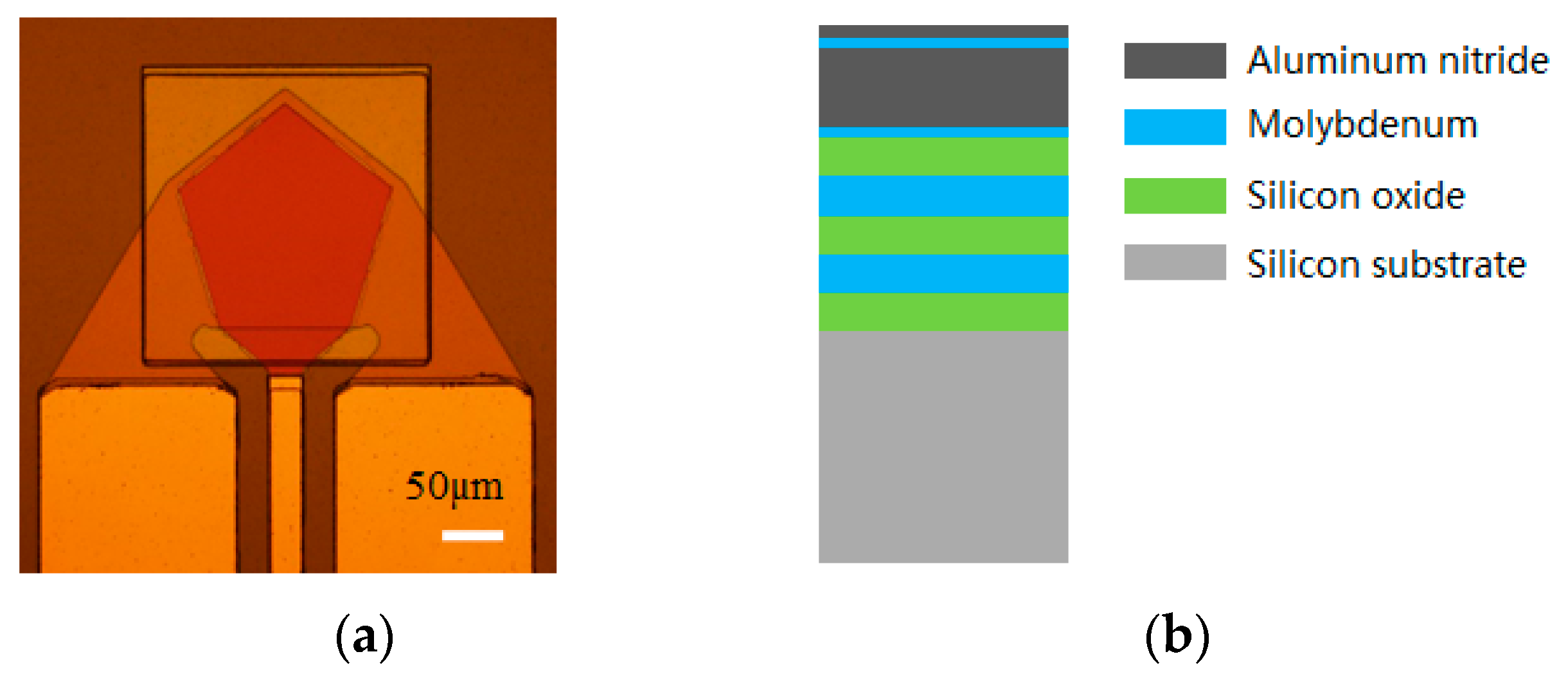
2.1. Structure of the Solidly Mounted Resonator
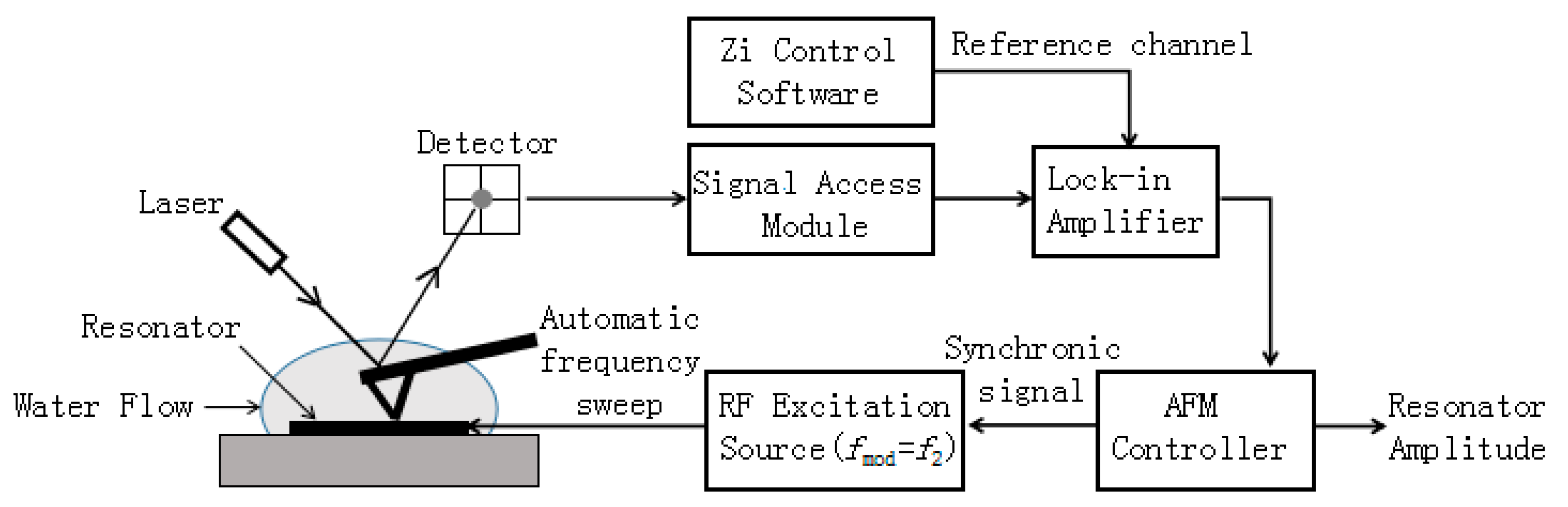
2.2. Experimental Setup and Principle
3. Experimental Results and Analysis
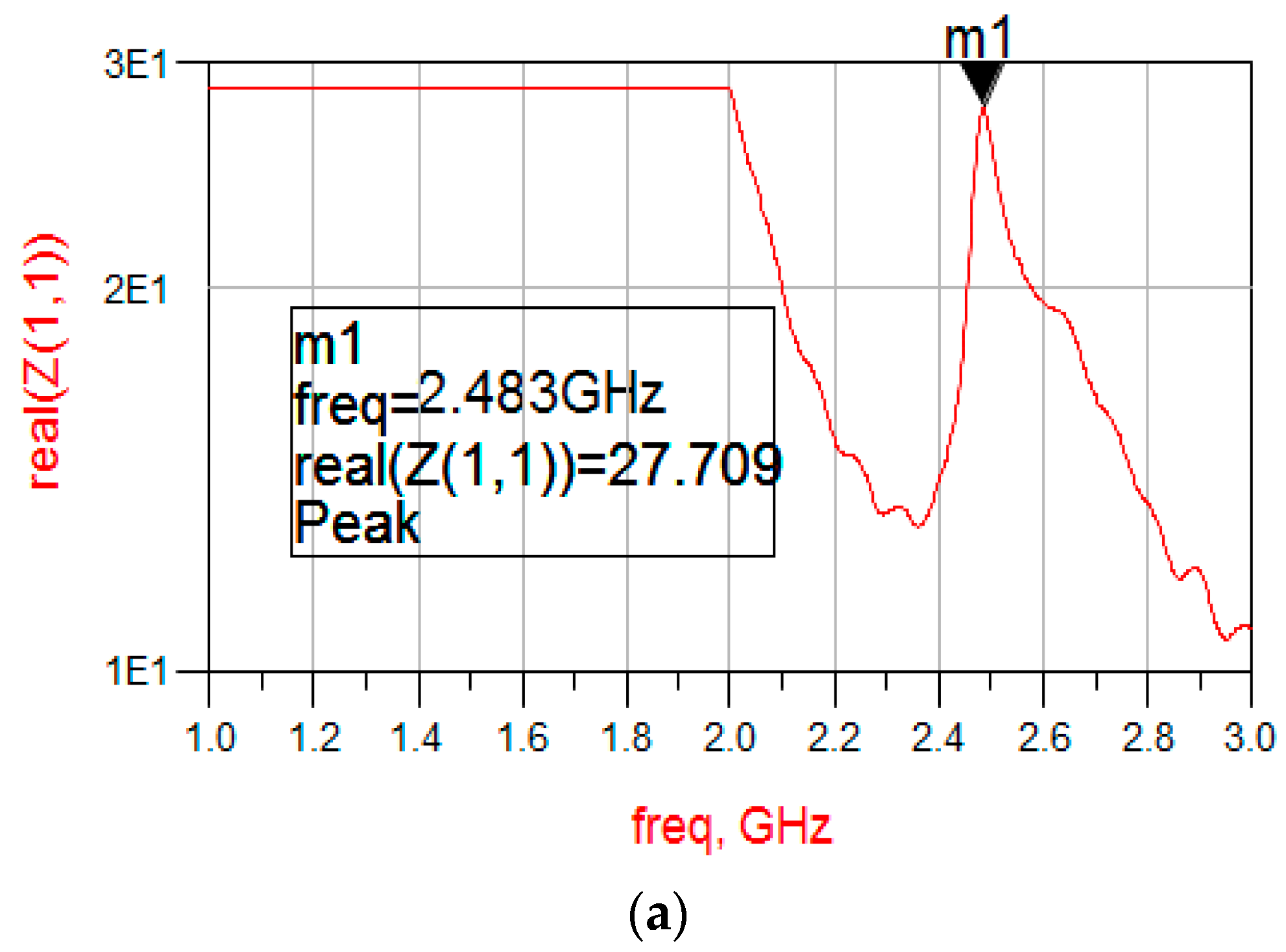
3.1. Amplitude-Frequency Curve of SMR in Air
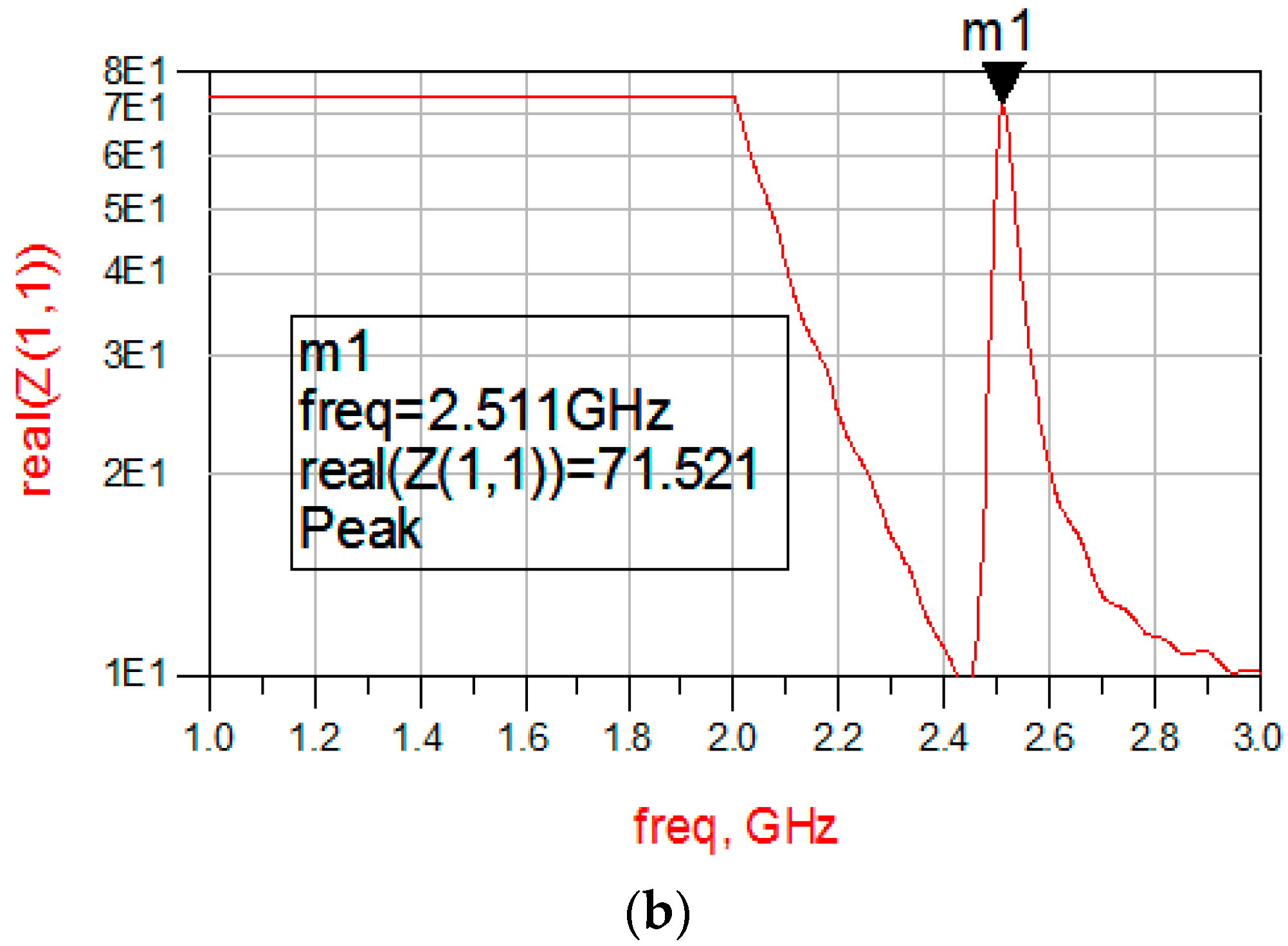
3.2. Amplitude-Frequency Curve of SMR in Fluid
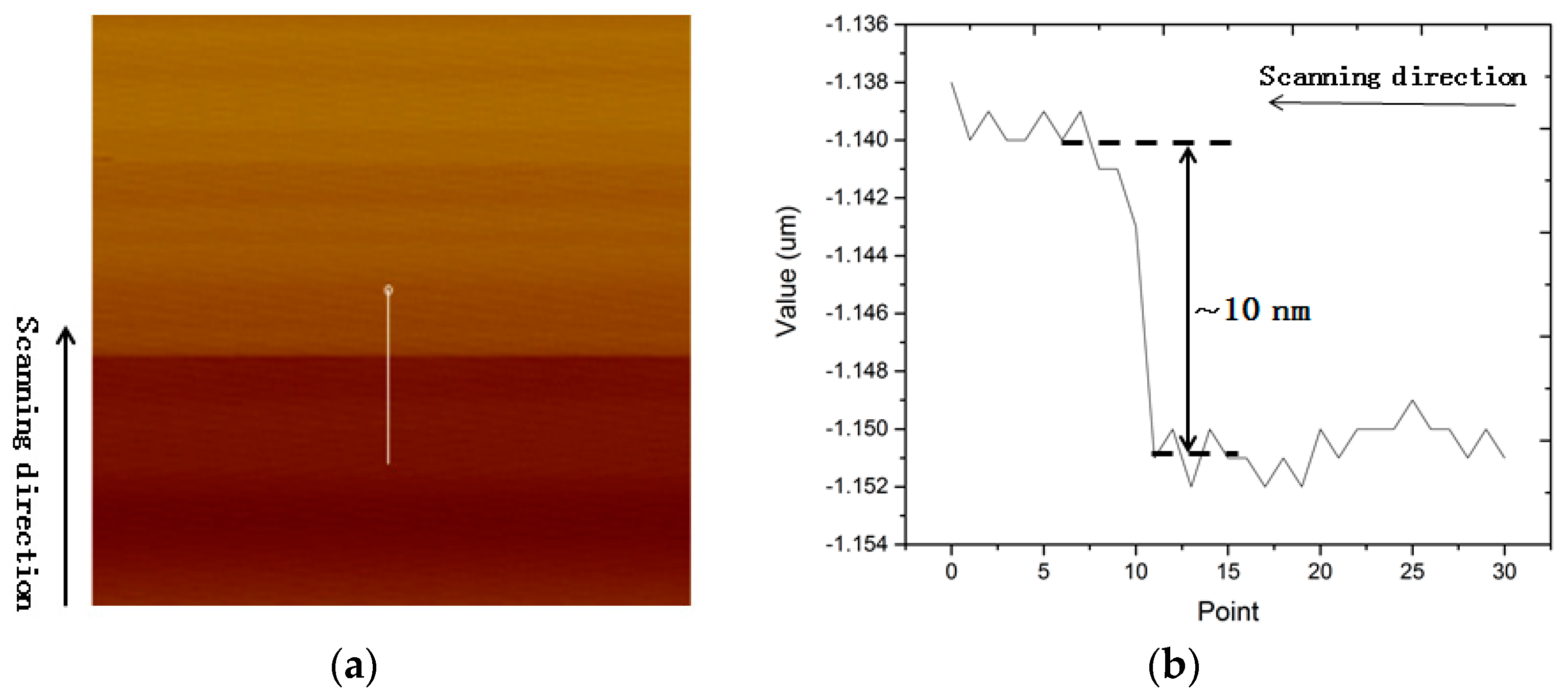
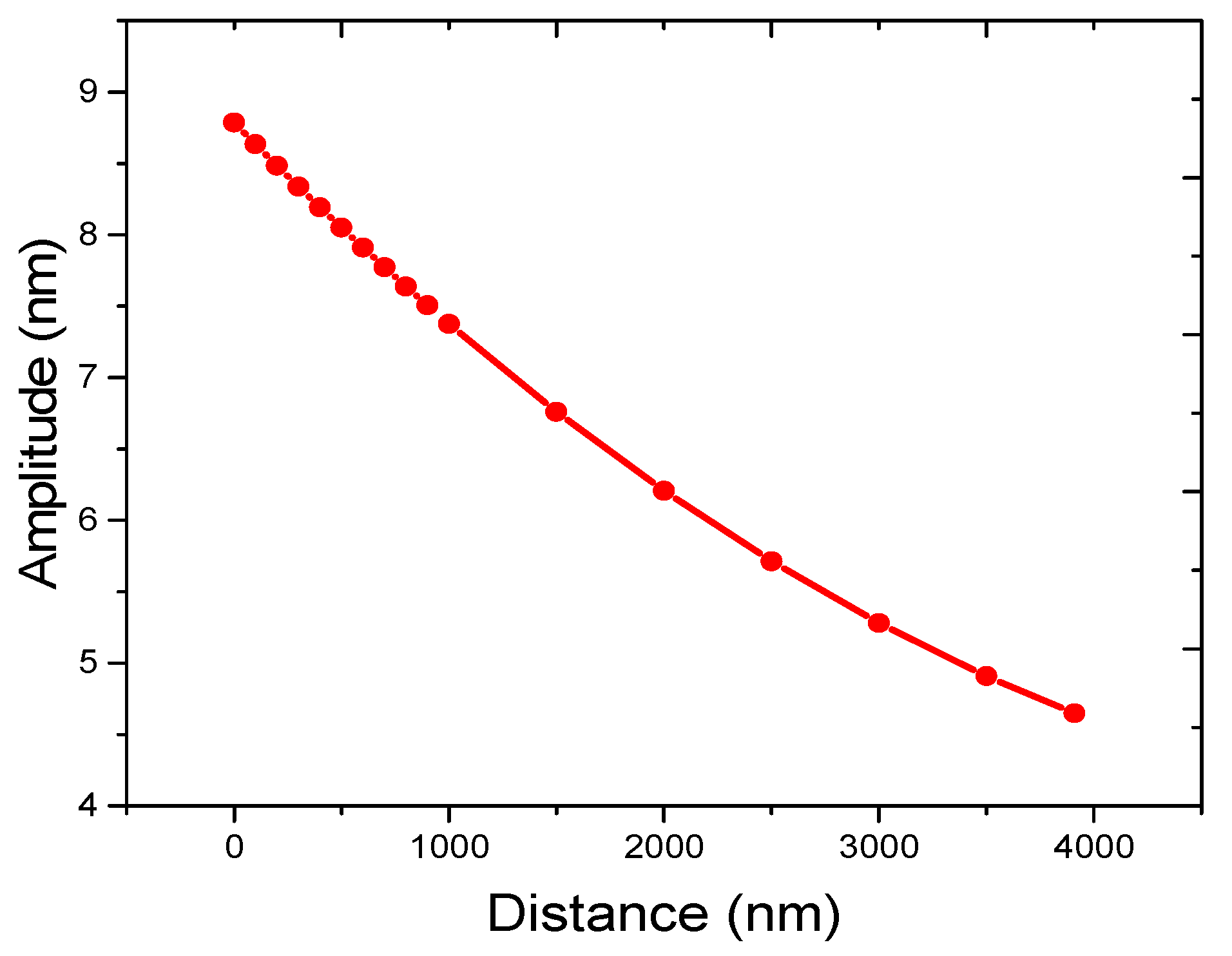
3.3. Measured Amplitudes at Different Heights above the Resonator in Tapping Mode
3.4. Amplitude-Frequency Curve of SMR in Contact Mode in Fluid
3.5. Measured Amplitude with Different Interaction Force between the AFM Tip and the Resonator in Contact Mode
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, J.; Liu, G.; Li, J.; Li, G. Recent developments of film bulk acoustic resonators (FBAR). Funct. Mater. Lett. 2016, 9, 1630002. [Google Scholar] [CrossRef]
- Moreira, M.; Bjurström, J.; Katardjev, I.; Yantchev, V. Aluminum scandium nitride thin-film bulk acoustic resonators for wide band applications. Vacuum 2011, 86, 23–26. [Google Scholar] [CrossRef]
- Mastromatteo, U.; Villa, F.F. High sensitivity acoustic wave AlN/Si mass detectors arrays for artificial olfactory and biosensing applications: A review. Sens. Actuators B Chem. 2013, 179, 319–327. [Google Scholar] [CrossRef]
- Dong, S.R.; Bian, X.L.; Jin, H.; Hu, N.N.; Zhou, J.; Wong, H.; Deen, M.J. Electrically tunable film bulk acoustic resonator based on Au/ZnO/Al structure. Appl. Phys. Lett. 2013, 103, 062904. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Ren, W.; Li, X.; Wang, G.; Shi, P.; Ye, Z. Design and micro-machining fabrication of piezoelectric diaphragm chambers for biomaterial detection. Ceram. Int. 2015, 41, S612–S617. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Yu, H.; Abbaspour-Tamijani, A.; Chae, J. In-liquid quality factor improvement for film bulk acoustic resonators by integration of microfluidic channels. IEEE Electron Device Lett. 2009, 30, 647–649. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Xu, Y.; Liu, W. Label-free immunosensor based on micromachined bulk acoustic resonator for the detection of trace pesticide residues. Sens. Actuators B Chem. 2014, 190, 378–383. [Google Scholar] [CrossRef]
- Fattinger, G.G.; Kaitila, J.; Aigner, R.; Nessler, W. Thin film bulk acoustic wave devices for applications at 5.2 GHz. In Proceedings of the 2003 IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 5–8 October 2003; Volume 1, pp. 174–177. [Google Scholar]
- Sorokin, B.P.; Kvashnin, G.M.; Volkov, A.P.; Bormashov, V.S.; Aksenenkov, V.V.; Kuznetsov, M.S.; Gordeev, G.I.; Telichko, A.V. AlN/single crystalline diamond piezoelectric structure as a high overtone bulk acoustic resonator. Appl. Phys. Lett. 2013, 102, 113507. [Google Scholar] [CrossRef]
- Flewitt, A.J.; Luo, J.K.; Fu, Y.Q.; Garcia-Gancedo, L.; Du, X.Y.; Lu, J.R.; Zhao, X.B.; lobrra, E.; Ramos, M.; Milne, W.I. ZnO based SAW and FBAR devices for bio-sensing applications. J. Non-Newton. Fluid Mech. 2015, 222, 209–216. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shih, W.C.; Chang, W.T.; Yang, C.H.; Kao, K.S.; Cheng, C.C. Biosensor for human IgE detection using shear-mode FBAR devices. Nanoscale Res. Lett. 2015, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Zhao, H.; Kim, E.S.; Zhang, H.; Yu, H.; Hu, X. Piezoelectric microelectromechanical resonant sensors for chemical and biological detection. Lab Chip 2012, 12, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Ryder, S.; Lee, K.B.; Meng, X.; Lin, L. Characterization of out-of-plane high frequency microresonators by AFM. In Proceedings of the 12th International Conference on TRANSDUCERS, Solid-State Sensors, Actuators and Microsystems, Boston, MA, USA, 8–12 June 2003; Volume 1, pp. 424–427. [Google Scholar]
- Agache, V.; Legrand, B.; Nakamura, K.; Kawakatsu, H.; Buchaillot, L.; Toshiyoshi, H.; Collard, D.; Fujita, H. Characterization of vertical vibration of electrostatically actuated resonators using atomic force microscope in non-contact mode. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Digest of Technical Papers 2005, Seoul, Korea, 5–9 June 2005; Volume 2, pp. 2023–2026. [Google Scholar]
- Garcia-Sanchez, D.; San-Paulo, A.; Esplandiu, M.J.; Perez-Murano, F.; Forró, L.; Aguasca, A.; Bachtold, A. Mechanical detection of carbon nanotube resonator vibrations. Phys. Rev. Lett. 2007, 99, 088501. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, D.; van-der-Zande, A.M.; San-Paulo, A.; Lassagne, B.; McEuen, P.L.; Bachtold, A. Imaging mechanical vibrations in suspended graphene sheets. Nano Lett. 2008, 8, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- San-Paulo, A.; Black, J.P.; White, R.M.; Bokor, J. Detection of nanomechanical vibrations by dynamic force microscopy in higher cantilever eigenmodes. Appl. Phys. Lett. 2007, 91, 053116. [Google Scholar] [CrossRef]
- Pan, S.; Zhang, H.; Liu, W.; Wang, Y.; Pang, W.; Duan, X. Biofouling Removal and Protein Detection Using a Hypersonic Resonator. ACS Sens. 2017. [Google Scholar] [CrossRef] [PubMed]
- San-Paulo, A.; Quevy, E.; Black, J.; Howe, R.T.; White, R.; Bokor, J. Mode shape imaging of out-of-plane and in-plane vibrating RF micromechanical resonators by atomic force microscopy. Microelectron. Eng. 2007, 84, 1354–1357. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Zhang, H.; Tang, Z.; Liu, W.; Lu, Y.; Wang, Z.; Yang, H.; Pang, W.; Zhang, H.; et al. Hypersonic Poration: A New Versatile Cell Poration Method to Enhance Cellular Uptake Using a Piezoelectric Nano-Electromechanical Device. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Y.; Li, D.C.; Fu, X.; Hu, X.T. Characterization of the vibration behavior of nanobeam-resonator using AFM dynamic mode. J. Harbin Inst. Technol. 2011, 43, 393–397. [Google Scholar]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Guo, X.; Xu, L.; Duan, X.; Zhang, H.; Pang, W.; Fu, X. Mechanical Vibration Measurement of Solidly Mounted Resonator in Fluid by Atomic Force Microscopy. Micromachines 2017, 8, 244. https://doi.org/10.3390/mi8080244
Xu F, Guo X, Xu L, Duan X, Zhang H, Pang W, Fu X. Mechanical Vibration Measurement of Solidly Mounted Resonator in Fluid by Atomic Force Microscopy. Micromachines. 2017; 8(8):244. https://doi.org/10.3390/mi8080244
Chicago/Turabian StyleXu, Fei, Xinyi Guo, Linyan Xu, Xuexin Duan, Hao Zhang, Wei Pang, and Xing Fu. 2017. "Mechanical Vibration Measurement of Solidly Mounted Resonator in Fluid by Atomic Force Microscopy" Micromachines 8, no. 8: 244. https://doi.org/10.3390/mi8080244




